Abstract
The microbiota–gut–brain axis (MGBA) involves bidirectional communication between intestinal microbiota and the gastrointestinal (GI) tract, central nervous system (CNS), neuroendocrine/neuroimmune systems, hypothalamic–pituitary–adrenal (HPA) axis, and enteric nervous system (ENS). The intestinal microbiota can influence host physiology and pathology. Dysbiosis involves the loss of beneficial microbial input or signal, diversity, and expansion of pathobionts, which can lead to loss of barrier function and increased intestinal permeability (IP). Colostrum, the first milk from mammals after birth, is a natural source of nutrients and is rich in oligosaccharides, immunoglobulins, growth factors, and anti-microbial components. The aim of this study was to investigate if bovine colostrum (BC) administration might modulate intestinal microbiota and, in turn, behavior in two mouse models, wild-type (WT) and Zonulin transgenic (Ztm)—the latter of which is characterized by dysbiotic microbiota, increased intestinal permeability, and mild hyperactivity—and to compare with control mice. Bioinformatics analysis of the microbiome showed that consumption of BC was associated with increased taxonomy abundance (p = 0.001) and diversity (p = 0.004) of potentially beneficial species in WT mice and shifted dysbiotic microbial community towards eubiosis in Ztm mice (p = 0.001). BC induced an anxiolytic effect in WT female mice compared with WT female control mice (p = 0.0003), and it reduced anxiogenic behavior in Ztm female mice compared with WT female control mice (p = 0.001), as well as in Ztm male mice compared with WT BC male mice (p = 0.03). As evidenced in MGBA interactions, BC supplementation may well be applied for prophylactic approaches in the future. Further research is needed to explore human interdependencies between intestinal microbiota, including eubiosis and pathobionts, and neuroinflammation, and the potential value of BC for human use. The MGH Institutional Animal Care and Use Committee authorized the animal study (2013N000013).
1. Introduction
Over recent decades, the fields of neuroscience and microbiology have become more entwined [1,2,3,4]. The gut–brain axis (GBA) involves bidirectional communication between the gastrointestinal (GI) tract and the central nervous system (CNS), and its importance in maintaining host immune and metabolic homeostasis has long been appreciated [1,5,6,7,8]. Research has shown that the commensal microorganisms in the intestinal lumen can influence host physiology and pathology. Furthermore, a growing body of research has been exploring the intestinal microbiota’s possible role in neurobehavioral, neurodevelopmental, and mental and neurodegenerative disorders [9,10,11].
The more recent concept of the microbiota–gut–brain axis (MGBA), therefore, includes the role of the intestinal microbiota and the CNS, neuroendocrine system, neuroimmune system, the hypothalamic–pituitary–adrenal (HPA) axis, sympathetic and parasympathetic arms of the autonomic nervous system (ANS), and the enteric nervous system (ENS) [3,12,13]. Changes in the intestinal environment appear to impact brain function and behavior and play a role in the development of inflammatory diseases in the intestinal tract, as well as the brain [14].
An imbalance of the intestinal microbiota, i.e., intestinal dysbiosis [15], involves loss of beneficial microbial input or signal, decreased microbial diversity, and an expansion of pathogenic microbes (pathobionts) [16,17,18]. As a consequence, dysbiosis can lead to loss of barrier function and increased intestinal permeability (IP) [19,20]. Zonulin is a family of structurally and functionally related peptides whose archetype member is pre-haptoglobin-2 [21,22]. Zonulin has been found to reversibly regulate intestinal permeability by modulating intercellular tight junctions (TJs) between epithelial cells, such as in the small intestine. The release of zonulin from the intestinal mucosa is triggered by several environmental stimuli, including intestinal dysbiosis, which is thought to be one of the mechanisms by which the intestinal microbiota can trigger inflammation in the CNS [23,24,25].
The mucosal barrier is of physical, biochemical, and immune nature, and the microbiota can be considered as part of this system because of the mutual influence occurring between the host and the luminal microorganisms. Different species of the intestinal microbiota seem to be able to impair and/or promote, or even reconstruct, the intestinal barrier function [19,26,27,28,29]. Increased antigen trafficking through a dysfunctional intestinal barrier due to dysbiosis allows harmful substances from the intestinal lumen to enter the bloodstream [19,25], subsequently triggering immune cell activation that may lead to systemic inflammation [30,31,32,33,34]. Defects in the intestinal barrier function, including dysbiosis, have been found in several psychiatric disorders, such as autism spectrum disorder (ASD) [35,36,37], schizophrenia [35,38,39,40], and anxiety disorders [41,42,43], which have been associated with increased inflammation [40,44,45,46,47,48,49]. Furthermore, a dysfunctional MGBA associated with neuroinflammation has been reported in ASD and attention deficit hyperactivity disorder (ADHD) [12,50,51,52,53,54,55]. More importantly, neuroinflammatory responses do not solely occur in the presence of local insult but can develop when normal functioning of CNS is challenged by distally occurring pathological events [56].
Overall, research suggests that dysbiosis of the intestinal microbiota and increased intestinal permeability (commonly, but not exclusively, due to dysbiosis) can affect brain function, mental health, and behavior [3,16,31,35,57,58,59,60,61,62,63,64,65,66].
Research shows that diet can both modulate the intestinal microbiota for maintaining or boosting/building eubiosis, or in the direction of developing dysbiosis [67,68,69,70,71,72,73,74,75,76,77]. Currently, the best-studied environmental effectors in microbiome variation are diet and antibiotic treatment [67,73,78,79,80,81,82,83,84,85]. However, understanding intestinal microbiota active modulation through external exposome and its impact on behavior or disease propensity is still in its infancy [15].
Colostrum, the first milk that the mammal produces after birth, is a natural source of both macro and micro-nutrients [86,87]. It is rich in immunoglobulins, growth factors, and anti-microbial components [87,88,89,90,91]. Bovine colostrum (BC) is important for the nutritional and immunological support, growth, and development of the newborn calf [92]. There is also increasing evidence that BC may be of value for treating various medical conditions and ailments in children and adults [92,93,94]. Moreover, extensive data show colostrum may have value for preventing and treating microbial infections, e.g., working via the host’s immune function by inactivating the microbes [87,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111].
Identifying and researching the MGBA can be done through interventions investigating effects on microbiota and behavior [1,65]. Although we do not yet fully understand the functional significance of the symbiotic relationship between the host and the microbiome, especially in the context of brain health, several tools and animal models have been invaluable in allowing researchers to constantly narrow the gaps in understanding of the MGBA. One of these is the zonulin-transgenic mouse (Ztm) model, which is characterized by increased intestinal permeability, dysbiotic intestinal microbiota, activation of the innate immune system, and mild hyperactivity [27,39]. A better understanding of the mechanism of the MGBA involving the neuro-immune system might offer a new approach to the treatment of mental disorders. Therefore, targeting the microbiota with nutritional and therapeutic strategies could be a novel approach for improved brain health and well-being.
Due to dysbiosis and increased trafficking of pro-inflammatory bacterial species and their products into the systemic circulation, the Ztm are predisposed to inflammation, including neuroinflammation. The aim of this study is to investigate if BC application modulates the intestinal microbiota and behavior in two mouse models, i.e., wild-type (WT) and Ztm, compared with control mice (WT Ctr and Ztm Ctr). This is the first study investigating the potential effects of BC using the unique zonulin-expressing mouse.
2. Materials and Methods
2.1. Animals
A colony of C57Bl/6 WT mice was maintained at the Massachusetts General Hospital animal facility, and the zonulin transgenic mouse (Ztm) model was generated as reported previously [112]. Both colonies were housed in separate cages within the same facility for the duration of the study. After weaning at 4 weeks, both colonies were housed, raised, and maintained under standard settings, i.e., in a room with a 12 h light/dark cycle, standard temperature and humidity, and with ad libitum access to standard mice chow and water. Every effort was made to limit animal suffering and to utilize only the minimum number of animals required to obtain accurate results. The MGH Institutional Animal Care and Use Committee authorized the animal study (protocol code 2013N000013; 3 March 2021).
2.2. Experiment
We investigated if BC administration in drinking water ad libitum would modulate/affect: (1) microbiota in WT and Ztm and/or increase intestinal microbial eubiosis in Ztm mice and (2) behavior in WT and Ztm and/or ameliorate behavioral changes previously reported in Ztm mice [39].
For these experiments, 27 (11 females and 16 males) WT and 26 (11 females and 15 males) Ztm, were used (n = 53). Before the experiment, the animals were divided into four groups. Two groups of each genotype (WT and Ztm) were fed with fresh, unprocessed BC in their drinking water at a ratio of 1:1 v/v ad libitum. The remaining groups (control mice) had no BC added to their drinking water. All groups received regular chow and drinking fluid ad libitum throughout the duration of the experiment. The mice were weighed thrice weekly, and their fluid intake was thoroughly recorded daily. After four weeks, fresh stool samples from all mice were obtained for microbiota investigation to compare between all groups and to baseline from our previous experiments [27,39]. To assess anxiety-like, obsessive, compulsive, and repetitive-like behaviors, we performed behavioral assays, i.e., marble burying (MB) and the light-dark box (LDB) test, as previously reported [39,113,114,115,116,117,118]. The experimental timeline and flowchart are shown in Figure 1.
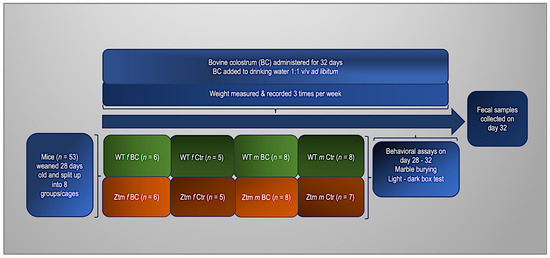
Figure 1.
Experimental timeline and flowchart. WT and Ztm mice in separate cages, females and males, receiving either BC added to drinking water (1:1 v/v) or water only ad libitum for 32 days. Behavioral assays performed on day 28–32 and fecal samples collected on day 32. BC = bovine colostrum treatment; WT = wild-type mice; Ztm = zonulin transgenic mice; Ctr = control mice, f = female; m = male; v/v = volume per volume, ad libidum = as much or as often as necessary or desired; n = number of mice in each group.
2.3. Bovine Colostrum (BC) Administration
Samples of BC were collected from authorized dairy farms in Iceland using robots, i.e., milking servers, and standard procedures were followed for the collection. BC was only collected from healthy dairy cows, and standard tests were performed to rule out infection or mastitis. Each farmer was required to register readings from the milking system and record information about the milking. A workbook was kept for methodology and frequency of cleaning and disinfecting the teats.
The first milking BC was collected in a sterile container, filtered, and refrigerated immediately at 4 °C. Then, the BC was transferred to one-liter sterile plastic bottles, selected in line with regulations regarding materials and objects intended to come in contact with food, within 12 h and placed immediately in a freezer at −20 °C until exported on dry ice (−80 °C) to the Mucosal Immunology and Biology Research Center at Massachusetts General Hospital.
During the experiment, an a priori calculated amount of BC was thawed daily and mixed with drinking water provided by the animal facility in a 1:1 v/v. The feeding took place at the same time every day, allowing for 2–3 h max flexibility. Water and BC were mixed thoroughly. To secure freshness, the BC was replaced daily, and all feeding bottles were cleaned thoroughly each time. The BC was well tolerated and did not induce any clinical symptoms in the animals.
Analyzing the raw material revealed the following per kg: water 80.7%, protein 9.8%, ash 1.1%, total fat 5.1%, and carbohydrates 4.1%, and the following minerals (in mg/kg ±20%): selenium 0.102, iron 1.33, copper 0.11, zinc 13.18, natrium 580, potassium 1.450, phosphorus 1.590, magnesium 290, chromium <100, and manganese 30.
2.4. Behavioral Assays
Two standard behavioral assays, MB [117,118] and the LDB [113,114,115,116] test (clarification in Section 2.4.1 and Section 2.4.2), were applied to investigate behavioral differences among the experimental groups. Behavioral evaluations were conducted when the mice were 8 weeks old (on day 28 of the experiment), over 4 days between 9:00 AM and 6:00 PM. To allow for behavioral habituation, the mice were brought into the experimental room at least thirty minutes prior to the start of the experiment. Males and females were never present in the testing room simultaneously and were always tested and acclimatized separately.
2.4.1. Marble Burying (MB)
When mice are put in a cage with marbles, they will bury the marbles. As a paradigm of the ethologically natural behavior of defensive burying, the MB test examines obsessive/compulsive/repetitive behavior [117,118]. The experimental mice had not been previously exposed to glass marbles, leading to the reasonable conclusion that the novelty of the marble triggers the response of marble burying. Standard housing cages (28 × 20 × 12 cm) were prepared in the standard way, filled with 7 cm of autoclaved and evenly distributed bedding material (SANI-CHIP®) without nesting material. Twenty marbles (Fisher Science EducationTM glass marbles #S04581, 1.42 cm) were equally spaced in four parallel lines with alternating black and blue colors. Cages were transferred to the testing faculty area, females and males separately, and one mouse was placed in the center of each cage, where it could freely interact with the marbles. After 20 min, the mice were carefully removed to avoid disturbing the bedding and/or marbles, and the number of buried marbles was counted. Marbles were considered buried when at least 2/3 of their volume was covered by bedding material.
2.4.2. Light Dark Box (LDB) Test
The LDB test may be useful to predict anxiolytic-like or anxiogenic-like activity in mice [116,119]. The LDB apparatus consists of two compartments, i.e., the light compartment makes up 2/3 of the box and is brightly lit and open, and the dark compartment comprises 1/3 of the entire box and is covered and dark. A 7 cm-wide door connects the 2 compartments of 35 cm-tall walls and is split into two 20 cm × 40 cm chambers. Rodents, including mice, prefer darker areas to lighter areas [113,114,115,116]. However, when in a new environment, the mice tend to explore. These two conflicting feelings lead to observable anxiety-like symptoms. No prior training is required for the LDB. There is no food or water deprivation, and only natural stressors, such as light, are used. Furthermore, an infrared beam sensor is attached to the ceiling allowing movement detection.
The mice were placed in the dark section of the unit and allowed to move around. Generally, the mice move around the perimeter of the compartment until they find the door. This process may take between 7 and 12 s. All four paws must be placed in the opposite compartment to be considered an entry. The distance each animal traveled in the light section, the total number of transitions, the time spent in each section, and the latency to enter the light section were recorded with Ethovision XT, an automated detection and quantification software. After each trial, all chambers were cleaned with super hypochlorous water to prevent a bias based on olfactory cues.
2.5. Microbiome Analysis; Sample Collection and DNA Extraction
Fresh fecal samples were collected from each individual mouse (n = 53) at eight weeks, after the behavioral tests were performed. The samples were flash-frozen and kept at −80 °C. Genomic DNA was extracted from each sample by applying the DNeasy powersoil extraction kit (Qiagen, Beverly, MA 01915, USA), following Qiagen’s instructions. To quantify the amount of DNA, Nanodrop was applied. Phylogenetic profiling was conducted by amplifying the hypervariable V4 region of the 16S rRNA gene by PCR, applying 5× prime master mix (MGH primer bank, Boston, MA 02114, USA). Reverse 806 primers were barcoded, and a unique forward 515 primer (Integrated DNA Technologies) was applied. Regular gel electrophoresis was run to confirm the correct amplification of the V4 regions. A QIAquick PCR purification kit (Qiagen, USA) was used for purifying PCR products. Concentration was measured by a Quant-iT Picogreen dsDNA kit, following the manufacturer’s instructions. The MGH NextGen Sequencing Core facility (Boston, USA) performed the sequencing of all samples, applying the Illumina system using the MiSeq v2 500 cycles reagent kit, following the manufacturer’s instructions. The system sequenced a total of 250 paired-end cycles for maximum coverage of the amplicon. The following primers were applied for the sequencing [120]:
read 1 (TATGGTAATT GT GTGYCAGCMGCCGCGGTAA)
read 2 (AGTCAGCCAGCCGGACTACNVGGGTWTCTAAT)
index (AATGATACGGCGACCACCGAGATCTACACGCT)
2.6. Bioinformatic Analysis of Microbiome Data
QIIME2 software package version 2018.2.0 was applied to process and analyze sequencing data [121]. Sequencing reads with low-quality scores (average Q < 25) were truncated to 240 bp and then filtered, applying the deblur algorithm (default settings) [122], and remaining high-quality reads were aligned to the reference library, applying mafft [123]. Next, the aligned sequences were masked to remove highly variable positions, and a phylogenetic tree was generated from the masked alignment by FastTree plugin [124]. Using default QIIME2 plugins [121], alpha and beta diversity metrics, and Principal Component Analysis, plots were generated [125,126,127,128,129,130]. To assign taxonomies to our sequences, we have used QIIME2’s feature-classifier plugin and pre-trained the Naïve Bayes classifier, which has been trained on the Silva 138 99% operational taxonomic units (OTUs) [131,132,133]. Differential abundance analysis of OTUs was performed using ANCOM [134].
2.7. Statistical Processing
Statistical analysis of the microbiome was performed, applying the Kruskal–Wallis test to assess statistical significance of abundance differences between groups. Alpha diversity analysis was performed with Kruskal–Wallis Rank-Sum and pairwise tests. For beta diversity analysis, permutation multivariate analysis of variance (PERMANOVA) with 1000 permutations was applied to test the significance of the community composition and structural differences among the groups. The Benjamini–Hochberg false discovery rate (FDR) was employed for multiple testing corrections, with a cutoff set at 0.05 (reported as q values) [135]. Statistical analyses for the behavioral experiments were performed in Graph Pad Prism 9, applying two-way ANOVA, and post-hoc analyses were carried out using Tukey’s HSD. Differences were considered statistically significant with p < 0.05.
3. Results
To investigate the modulating effect of colostrum on the intestinal microbial community, we analyzed the microbiome of eight-week-old WT and Ztm mice (n = 53). The composition of the intestinal microbiota of four-week-old mice is established, but still susceptible to fluctuations due to external factors, such as diet [136]. Therefore, this period (4–8 weeks) represents an excellent opportunity to influence the composition of the microbiota.
3.1. Alpha Diversity Analysis in Wild-Type (WT) and Zonulin Transgenic Mice (Ztm)
Microbial community structure can be assessed by alpha diversity, which includes measures of evenness (whether all species have a similar abundance within the community) and richness (the number of different bacterial species present). Alpha diversity was quantified using Pielou’s evenness, species richness, and Shannon diversity indexes, which relate OTU evenness and richness, the total number of observed species, and variation and complexity within the group. Alpha diversity analyses were performed with Kruskal–Wallis Rank-Sum and pairwise tests.
3.1.1. Wild-Type (WT) Groups
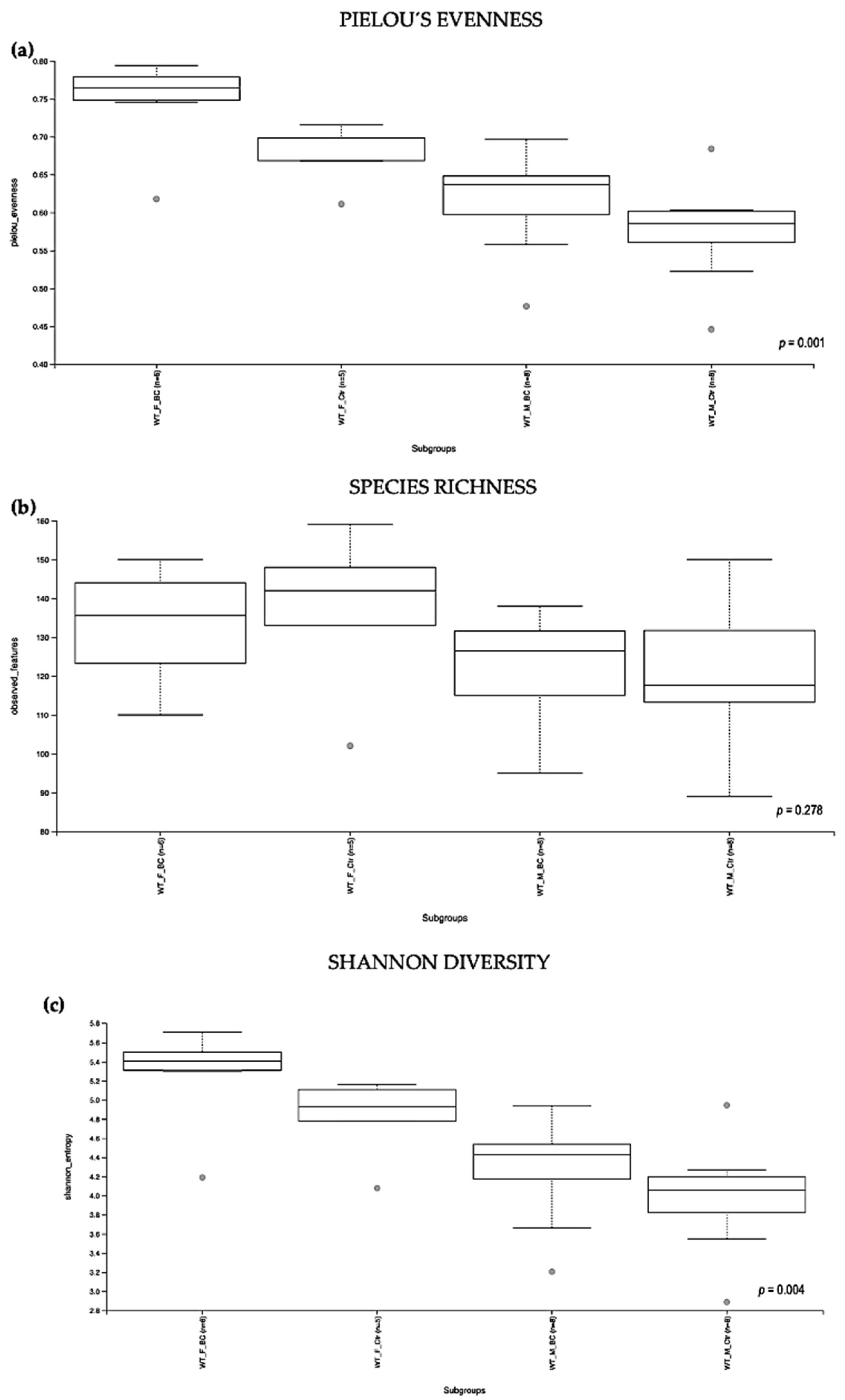
Evaluation of the relative abundance of different species of intestinal microbiota in WT female and male mice showed that the alpha diversity calculated using Pielou´s evenness index was significantly different between the groups (p = 0.001), applying the Kruskal–Wallis Rank-Sum test (Figure 2a). There was a significantly increased relative abundance in one of the subgroups, i.e., in WT f BC vs. WT f Ctr (Kruskal–Wallis pairwise (p = 0.04; Table 1). The significant difference between the rest of the subgroups is due to different baselines between WT female and male mice [27,39]. Evaluation of diversity in each WT sample, including the number of different species, using the species richness index, showed no significant difference between the groups (Kruskal–Wallis Rank-Sum p = 0.2; Figure 2b), nor any of the subgroups (Table 1).
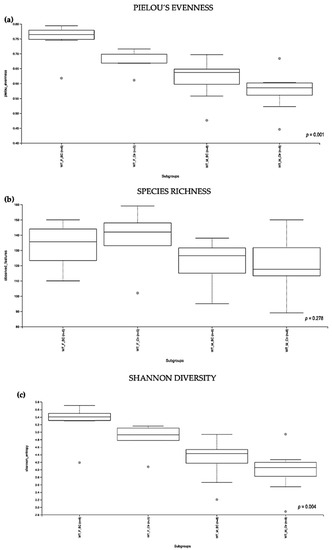
Figure 2.
Alpha diversity of the intestinal microbial communities between WT BC and control groups post BC treatment. Comparison of boxplots depicting Pileou´s evenness (a), species richness (b), and Shannon diversity (c). Diversity among WT f BC (n = 6), WT f Ctr (n = 5), WT m BC (n = 8), and WT m Ctr (n = 8) groups. p < 0.05 indicating that the alpha diversity between groups is significant. p values = Kruskal–Wallis rank-sum test (all groups). WT = wild-type mice; BC = bovine colostrum treatment; Ctr = control mice; f = female; m = male; n = number of mice in a group.

Table 1.
Alpha diversity of the intestinal microbiota in WT mice post BC treatment. Quantified using Pielou´s evenness, species richness, and Shannon diversity indexes, which relate OTU richness and evenness, the total number of observed species, and variation and complexity within the group. Alpha diversity analysis performed with Kruskal–Wallis rank-sum and pairwise tests. WT = wild- type mice; BC = bovine colostrum treatment; Ctr = control mice; f = female; m = male; n = number of mice in each group.
As indicated by the Shannon index, there was a significant difference in the diversity of microbial community abundances between the WT female and male groups (Kruskal–Wallis Rank-Sum p = 0.004; Figure 2c). When divided by sex, there was a significant difference between the subgroups, i.e., WT f BC vs. WT f Ctr (Kruskal–Wallis pairwise p = 0.04). Per contra, there was no difference between the WT male BC group when compared with the WT male control group (Kruskal–Wallis pairwise p = 0.1; Table 1).
Overall, a significant difference in the intestinal microbial community was observed in the WT female BC group when compared with the WT female control group. These findings suggest an increase in taxonomic abundance and diversity in the intestinal microbiota in WT female mice that underwent BC treatment, when compared with control mice.
3.1.2. Zonulin Transgenic Mice (Ztm) Groups
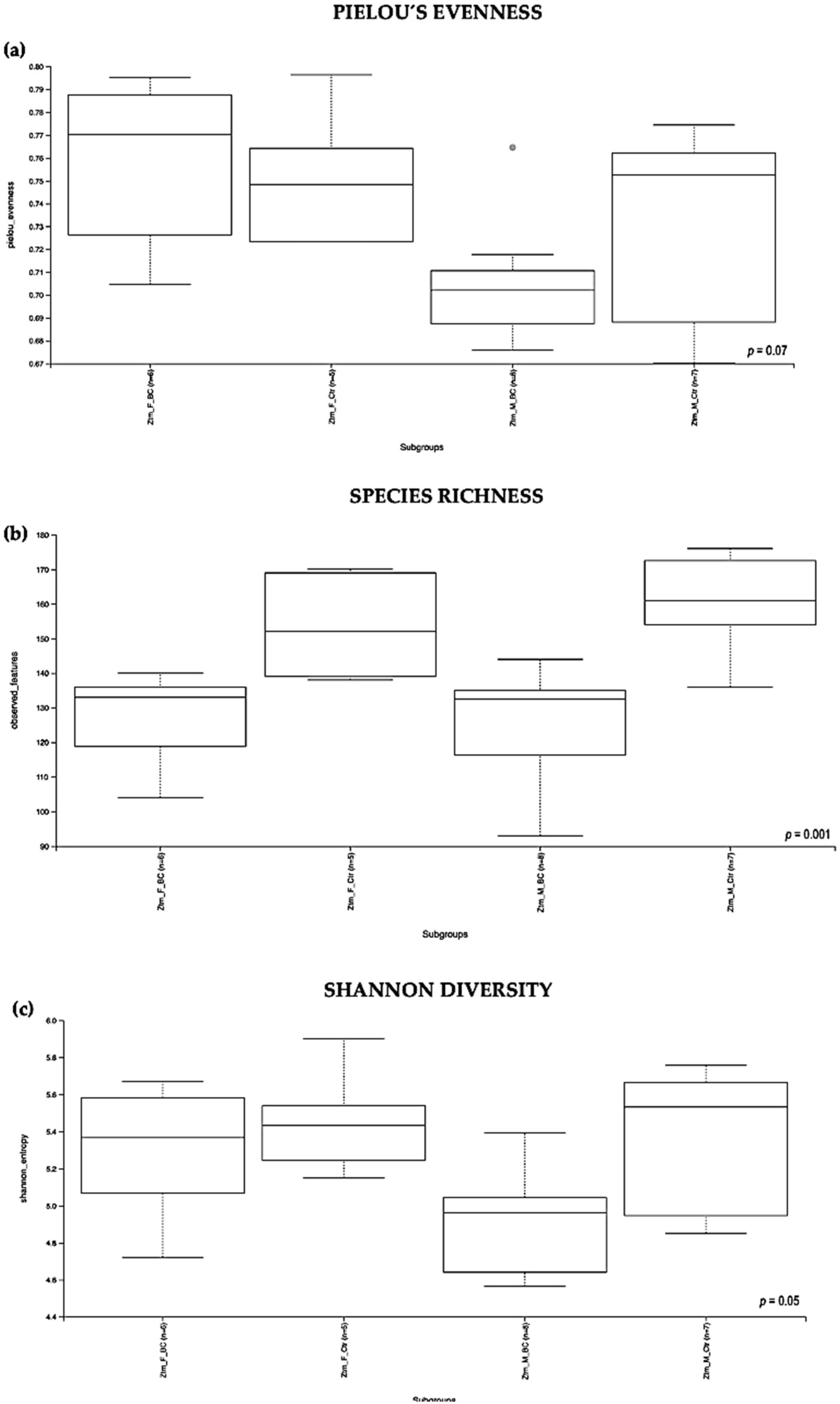
Evaluation of the relative abundance of different species’ intestinal microbiota in the Ztm female and male groups showed that, overall, the alpha diversity calculated using the evenness index was not significantly different in the BC groups when compared with the control groups (Kruskal–Wallis Rank-Sum p = 0.07; Figure 3a). No significant difference was found between the subgroups, i.e., Ztm f BC vs. Ztm f Ctr, nor Ztm m BC vs. Ztm m Ctr (Table 2).
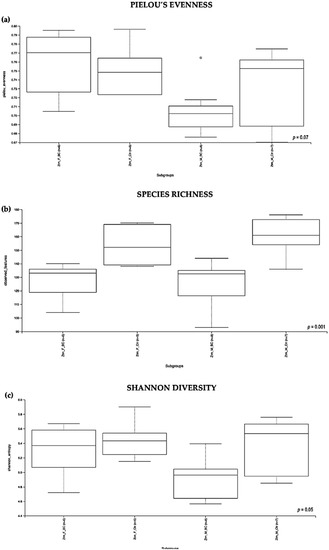
Figure 3.
Alpha diversity of the intestinal microbial communities between Ztm BC and control groups post BC treatment. Comparison of boxplots depicting Pileou´s evenness (a), species richness (b), and Shannon diversity (c). Diversity among Ztm f BC (n = 6), Ztm f Ctr (n = 5), Ztm m BC (n = 8), and Ztm m Ctr (n = 7) groups. p < 0.05 indicating that the alpha diversity between groups is significant. p values = Kruskal–Wallis rank-sum test (all groups). WT = wild-type mice; BC = bovine colostrum treatment; Ctr = control mice; f = female; m = male; n = number of mice in a group.

Table 2.
Alpha diversity of the intestinal microbiota in Ztm mice post BC treatment. Quantified using Pielou´s evenness, species richness, and Shannon diversity indexes, which relate OTU richness and evenness, the total number of observed species, and variation and complexity within the group. Alpha diversity analysis were performed with Kruskal–Wallis rank-sum and pairwise tests. Ztm = zonulin transgenic mice; BC = bovine colostrum treatment; Ctr = control mice; f = female; m = male; n = number of mice in each group.
Evaluation of the diversity in each sample, including the number of different species, using the species richness index showed significant difference between the Ztm treatment and control groups (Kruskal–Wallis Rank-Sum p = 0.001; Figure 3b), and in the subgroups, significant difference between Ztm f BC vs. Ztm f Ctr (Kruskal–Wallis pairwise p = 0.01) and between Ztm m BC vs. Ztm m Ctr (Kruskal–Wallis pairwise p = 0.002; Table 2).
As indicated by the Shannon index, there was a significant difference in microbial community diversity between the Ztm groups (Kruskal–Wallis Rank-Sum p = 0.05; Figure 3c). However, there was no significant difference between the subgroups, i.e., Ztm f BC vs. Ztm f Ctr (Kruskal–Wallis pairwise p = 0.5), nor Ztm m BC vs. Ztm m Ctr (Kruskal–Wallis pairwise p = 0.08; Table 2).
Overall, a significant difference in the intestinal microbial community was observed between the Ztm treatment and control groups, as well as between the female and male subgroups (Ztm f BC vs. Ztm f Ctr), suggesting an increase in taxonomic richness and diversity characterizes the intestinal microbiota in Ztm female and male mice that received BC.
3.2. Beta Diversity Analysis
Beta diversity (the degree of pair-wise similarity in species composition among the groups) was assessed by applying Bray–Curtis and Jaccard indices. Similarity measures of abundance, presence, and absence data at the level of species, genera, families, orders, classes, and phyla were assessed in the four groups of mice (f & m), i.e., WT BC, WT Ctr, Ztm BC, and Ztm Ctr (Table 3; Figure 4).

Table 3.
Beta diversity of the intestinal microbiota. Beta diversity showing the degree of pair-wise similarity in species composition between the groups assessed by Bray–Curtis and Jaccard matrices. Similarity measures of abundance, presence, and absence data of species, genera, families, orders, classes, and phyla was assessed in the four groups, WT (BC & Ctr) and Ztm (BC & Ctr), post BC treatement. WT = wild-type mice; BC = bovine colostrum treatment; Ctr = control mice; f = female; m = male; n = number of mice in each group.
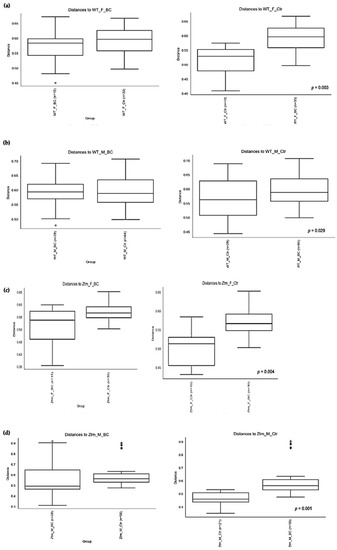
Figure 4.
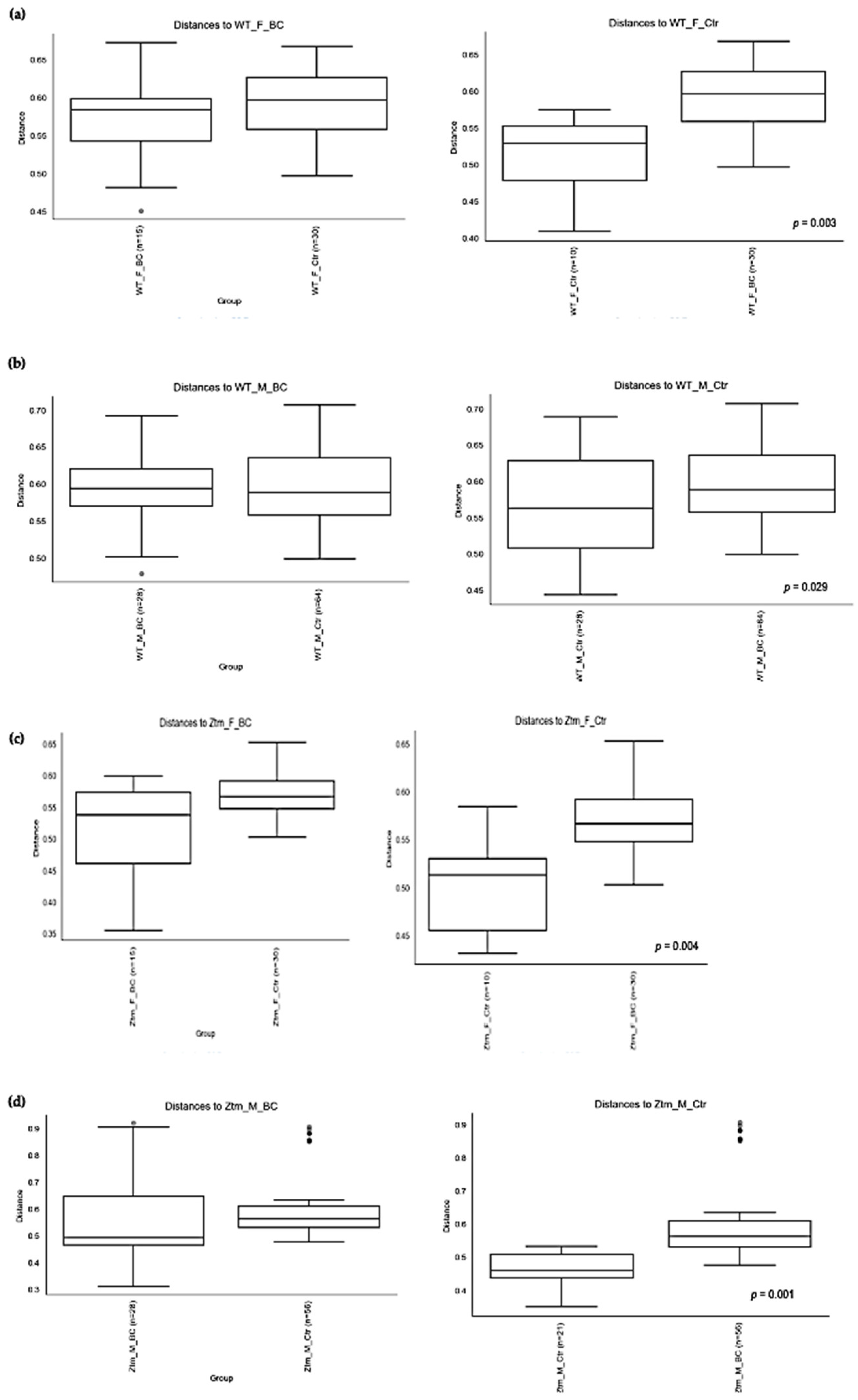
Beta diversity of the intestinal microbial communities between WT f BC and WT f control groups (a), WT m BC and WT m control groups (b), Ztm f BC and Ztm f control groups (c), and Ztm m BC and Ztm m control groups. (d) Box plot showing Jaccard distance between groups and within groups, p < 0.05 indicating beta diversity between groups is significant. BC = bovine colostrum treatment; Ctr = control mice; WT = wild-type mice; Ztm = zonulin transgenic mice; f = female; m = male; n = number of mice in a group. p = pairwise PERMANOVA results.
PERMANOVA tests demonstrated significant differences in intestinal microbiota between the groups and within the groups. The Bray–Curtis beta diversity index showed the overall dissimilarity of bacterial communities was significantly different between the WT BC groups compared with the WT control groups (p = 0.03; Table 3) and between Ztm BC groups compared with Ztm control groups (p = 0.001; Table 3). Within the groups, there was a significant difference within the WT male group, i.e., WT m BC vs. WT m Ctr (p = 0.03; Table 3) and within the Ztm female and male treatment groups, i.e., Ztm f BC vs. Ztm f Ctr (p = 0.01; Table 3) and Ztm m BC vs. Ztm m Ctr (p = 0.001; Table 3).
Similarity in species composition between the groups assessed by Jaccard indices demonstrates significant differences between all WT groups (p = 0.04; Table 3) and within all groups. In the subgroups, i.e., WT female and male BC groups compared with control groups, differences in the abundance and presence/absence of taxa were observed, indicating that the beta diversity is significant (WT f, p = 0.003; WT m, p = 0.02; Table 3; Figure 4a,b). Within the Ztm groups, similar differences were found in the abundance and presence/absence of taxa, indicating beta diversity significance between the groups (p = 0.001; Table 3). In the subgroups, i.e., the Ztm female and male BC groups, the abundance and presence/absence were significantly higher than in the control groups, i.e., Ztm f, p = 0.004; Ztm m, p = 0.001 (Table 3; Figure 4c,d).
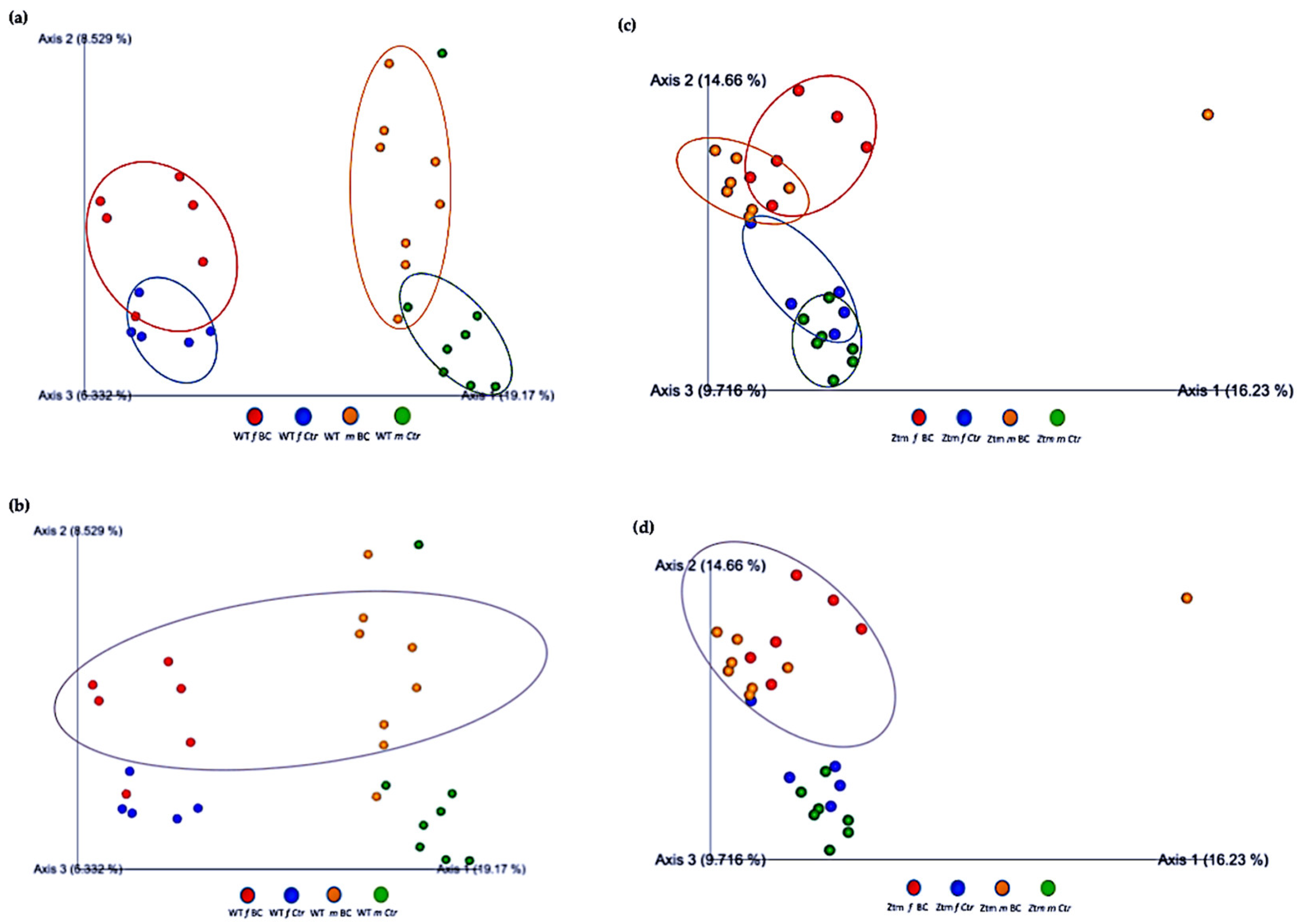
Furthermore, the relationships between groups of microbiota samples were assessed using principal component analysis (PCA), based on the Jaccard beta diversity index, as a measure of the overall dissimilarity of bacterial communities within and between the groups (Figure 5a–d). PCA of the intestinal microbial communities revealed a trend for partially overlapping sample groups in the WT (BC vs. Ctr) microbiome and distinct clustering of Ztm (BC vs. Ctr) microbiome profiles.
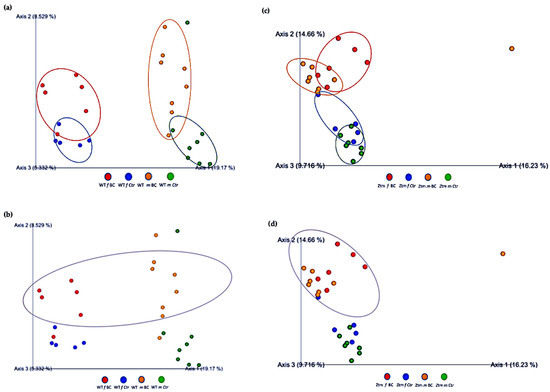
Figure 5.
Beta diversity of intestinal microbial communities between WT BC and control groups, and Ztm BC and control groups. PCA plot based on Jaccard distance. Each dot on the plot represents one sample from each group. The Jaccard patterns indicate BC and Ctr representing; (a): WT BC (f & m) vs. WT Ctr (f & m) partially overlapping; (b): WT BC groups (f & m); (c): Ztm BC (f & m) vs. Ztm Ctr (f & m) distinct clustering; (d): Ztm BC groups (f & m). The distance between dots indicates the degree of similarity of taxonomic composition of samples. BC = bovine colostrum treatment; Ctr = Control mice; WT = wild-type mice; Ztm = zonulin transgenic mice; f = female; m = male; PCA = principal component analysis.
3.3. Species Composition
3.3.1. Phylum Level
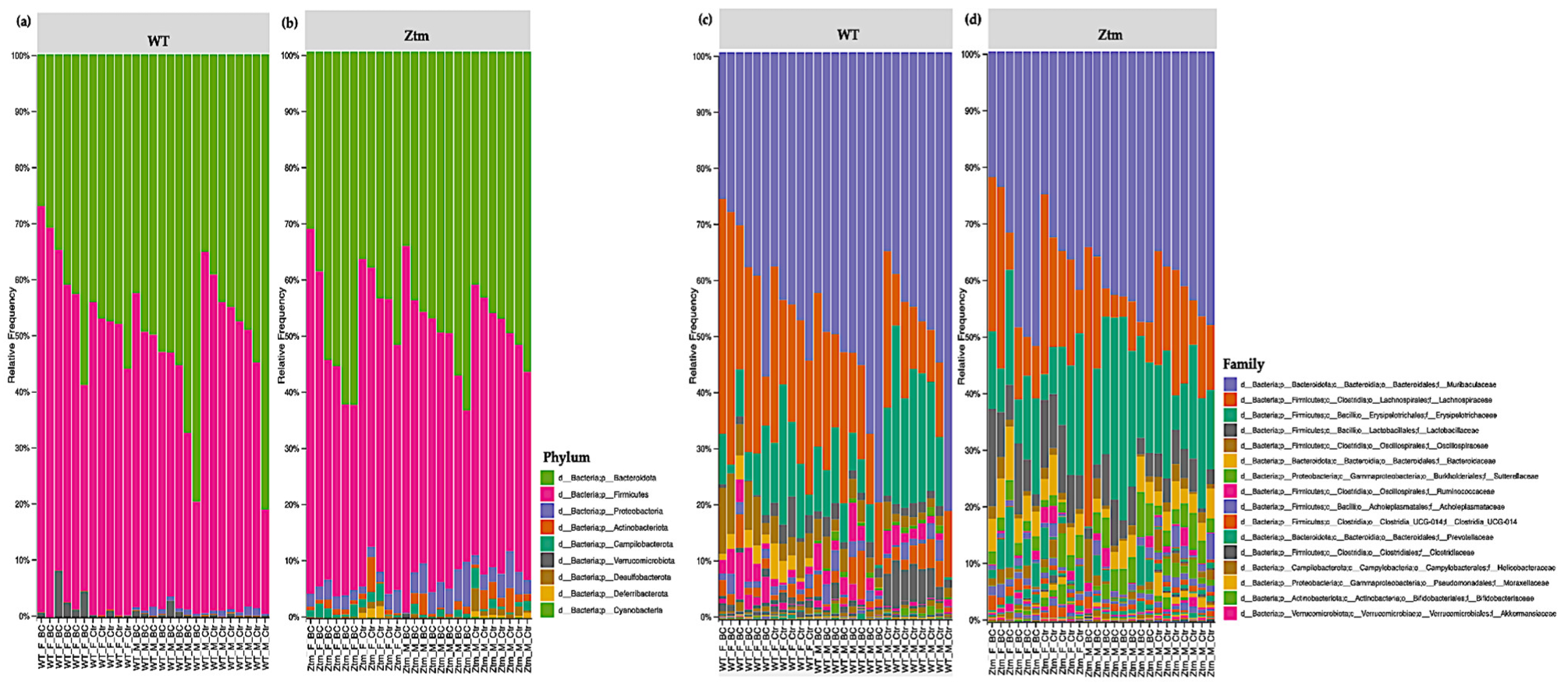
At the phylum level, Firmicutes and Bacteroidetes prevailed in all groups, i.e., Firmicutes 51.5% (WT f Ctr) and 49.4% (WT m Ctr), and 50.3% (Ztm f Ctr) and 42.9% (Ztm m Ctr), and Bacteroidota were 47.7% (WT f Ctr) and 49.2% (WT m Ctr), and 42.6% (Ztm f Ctr) and 47.9% (Ztm m Ctr) (Figure 6a,b).
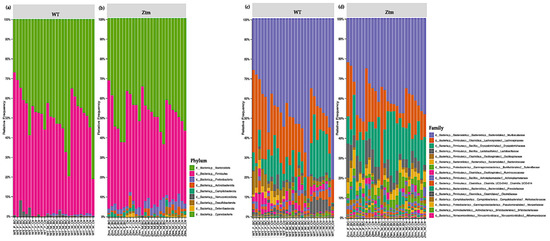
Figure 6.
Taxonomy representation at phyla (a), (b) and family (c), (d) level. The abscissa represents the groups, and the ordinate represents the relative abundance of intestinal bacteria in WT and Ztm mice (f & m). (a): Relative percentage of most abundant phyla in each sample between WT BC groups (n = 14) and WT control groups (n = 13). (b): Relative percentage of most abundant phyla in each sample between Ztm BC groups (n = 14) and Ztm control groups (n = 12). (c): Relative abundance of bacteria at family level in WT BC and control groups. (d): Relative abundance of bacteria at the family level in Ztm BC and control groups. n = 53. WT = wild-type mice; Ztm = zonulin transgenic mice; BC = bovine colostrum treatment; Ctr = control mice; F = female; M = male.
However, there was a shift in Firmicutes abundance in all the BC groups when compared with the control groups. The WT female BC group harbored an increased abundance (56.8%), the WT male BC group harbored a decreased abundance (43.8%), and the Ztm female BC group harbored a decreased abundance (43.5%) when compared with the control groups (51.5%; 49.4%; 50.3%). The Ztm male BC group harbored an increased abundance (43.4%) compared with the control group (42.9%). Furthermore, there was a shift in Bacteroidota within the BC groups when compared with the control groups. The WT female BC group harbored a decreased abundance (40.1%) and the WT male BC group harbored an increased abundance (54.4%) compared with the control groups (47.7%; 49.2%). However, the Ztm female and the Ztm male BC groups harbored an increased abundance of (51.5%) and (49.6%) compared with the control groups (42.6% and 47.9%) (Table S1).
Moreover, the WT BC groups harbored a decreased abundances of Proteobacteria compared with the control groups, but the Ztm BC groups harbored an increased abundance compared with the Ztm control groups. All BC groups harbored a decreased abundance of Actionbacteriota compared with the control groups, and all the BC groups, except one (Ztm f BC), harbored an increased abundance of Verrucomicrobiota. Campylobacterota was only detected in the Ztm groups, and the relative abundance was increased in the Ztm female BC group, but decreased in the Ztm male BC group compared with the control groups (Figure 6a,b; Table S1). Deferribacterota and Desulfobacterota were only found in the Ztm groups, and both BC groups harbored a reduced relative abundance, i.e., females and males. Cyanobacteriota was only detected in the Ztm groups and was increased in the Ztm female BC group, but reduced in the Ztm male BC group (Table S1).
Overall, there was a shift in all phyla in all treatment groups.
3.3.2. Family Level
At the family level, the intestinal microbiota of all groups contained high levels of Muribaculaceae, Lachnospiraceae, and Erysipelotrichaceae (Figure 6c,d; Table S1). However, there was a shift within the BC groups, and each comparison exhibited a different signature. The WT female BC group harbored a reduced abundance of Muribaculaceae (37.4%) compared with the WT female control group (44.7%), and the WT male BC group harbored an increased abundance (54.3%) compared with the WT male control group (49.2%). In the Ztm BC groups, the Ztm females harbored an increased abundance (38.6%) and the Ztm male BC group harbored an increased abundance (42.2%) compared with the Ztm control groups (34 % and 41.5 %), respectively (Table S1).
Within the treatment groups, the WT groups harbored an increased abundance of Lachnospiraceae (WT f BC = 29.7% and WT m BC = 19.5%) compared with the control groups (22.4% and 13.6%), respectively. The Ztm treatment groups harbored a reduced abundance (Ztm f BC = 14.8% and Ztm m BC = 10.9%) compared with the control groups (19% and 15.3%), respectively. Decreased abundance of Erysipelotrichaceae was found in all the treatment groups (WT f BC = 8.0% and WT m BC = 7.9%, and Ztm f BC = 13.9%), except for the Ztm male BC group, as they harbored an increased abundance (21.6%) compared with the control groups (WT f Ctr = 14.8%; WT m Ctr =17.6%; Ztm f Ctr = 14.7%; Ztm m Ctr 15.6%), respectively (Table S1).
The WT and Ztm female BC groups harbored a reduced abundance of Lactobacillaceae (0.6% and 8.3%) compared with the WT and Ztm female control groups (3.2% and 9.5%), respectively. However, the WT and Ztm male BC groups harbored an increased abundance (2% and 4.8%) when compared with the WT and Ztm male control groups (1.6% and 4%), respectively (Table S1). Clostridiaceae abundance was reduced in both WT BC groups (0.5% and 0.4%) compared with the WT control groups (1% and 4.8%), and in the Ztm BC groups (0.04% and 0.05%) when compared with the Ztm control groups (0.1% and 0.1%). All BC groups harbored an increased abundance of Oscillospiraceae when compared with the control groups.
Akkermansiaceae was only found in the WT groups, and both WT BC groups harbored an increased abundance (2.9% and 0.8%) compared with the control groups (0.4% and 0.2%). There was a slight expansion in the Ztm male BC group (0.07%) compared with the Ztm male control group (0%). (Table S1).
Clostridia UCG-014 relative abundance was increased in the WT female BC group (2.4%), in the Ztm female BC group (0.06%), and in the Ztm male BC group (0.2%), when compared with the control groups (1.1%; 0.05%; 0.1%). However, the relative abundance was reduced in the WT male BC group (2.7%) when compared with the control group (3.3%) (Table S1).
Ruminococcaceae abundance was increased in the WT female BC group (3.3%), in the WT male BC group (3.2%), and in the Ztm male BC group (0.9%), when compared with the control groups (1%; 2.1%; 0.8%). However, the Ztm female BC group harbored a reduced abundance (0.4%) compared with the control group (1.6%) (Table S1).
Overall, there was a shift in the microbial abundance in all bacterial families within all treatment groups, except for Prevotellaceae in the WT groups, Bacteriodaceae in the WT male group, and Akkermansiaceae in the Ztm female group.
3.3.3. Genus Level
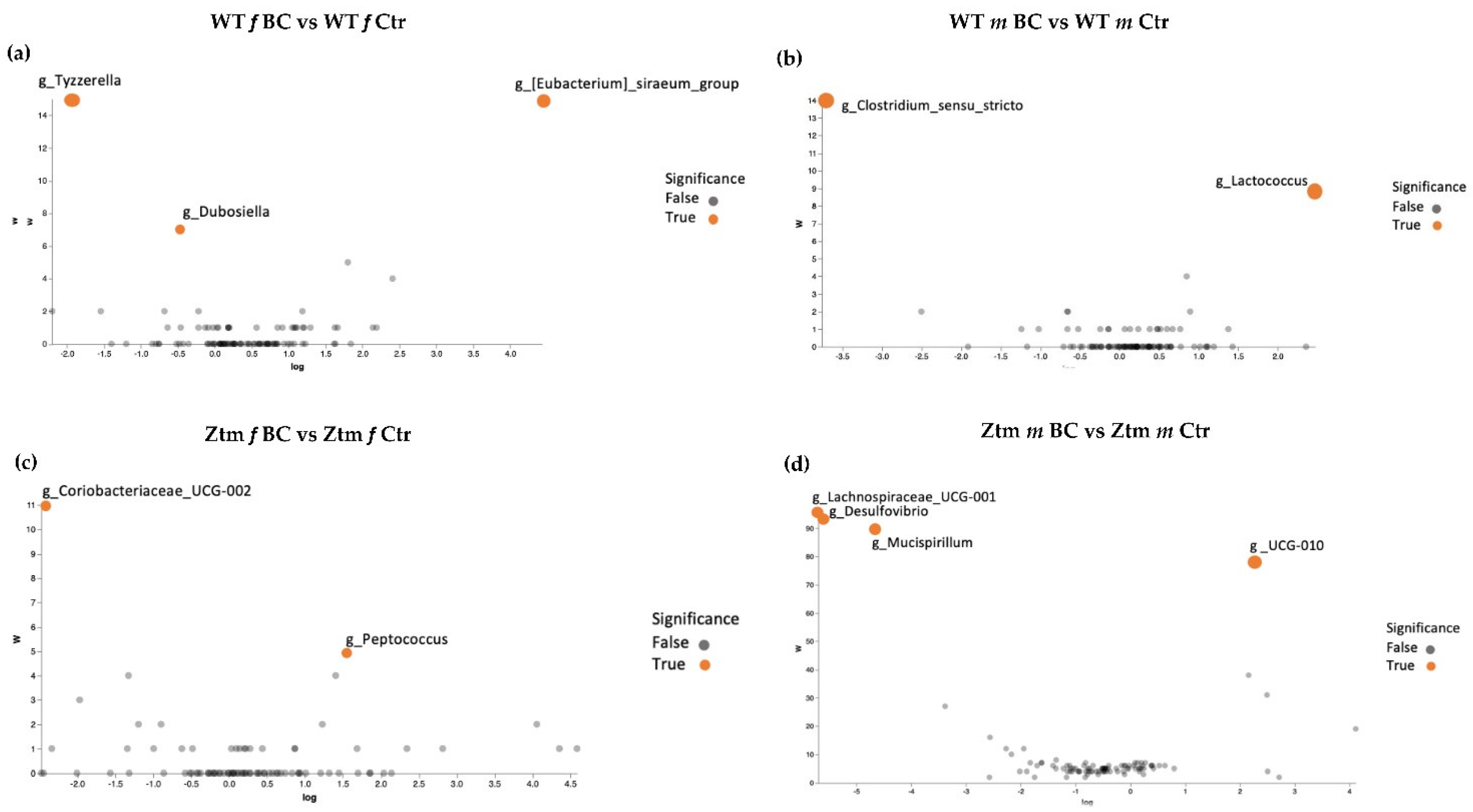
Volcano plot analysis showed significant differences between all the BC groups compared with the control groups (Figure 7a–d). The WT female BC group harbored a significantly increased abundance of Eubacterium (Ruminococcaceae family) and a significantly reduced abundance of Tyzzerella (Lachnospiraceae family) and Dubosiella (Erysipelotrichaceae family) (Figure 7a).
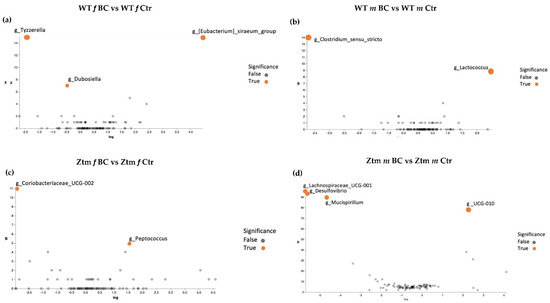
Figure 7.
ANCOM volcano plot of statistical differences between treatment groups (WT BC & Ztm BC) and control groups (WT Ctr & Ztm Ctr) at genus level. (a): WT f BC harbor a significant increased abundance of Eubacterium (Ruminococcaceae family) and a significantly reduced abundance of Tyzzerella (Lachnospiraceae family) and Dubosiella (Erysipelotrichaceae family) compared with control group. (b): WT m BC harbor a significantly increased abundance of Lactococcus (Streptococcaceae family) and a significantly reduced abundance of Clostridium sensu stricto 1 (Clostridiaceae family) compared with control group. (c): Ztm f BC harbor a significantly reduced abundance of Coriobacteriaceae UCG-002 (Atopobiaceae family) and Peptococcus (Peptococcaceae family) compared with control group. (d): Ztm m BC harbor a significantly reduced abundance of Lachnospiraceae UCG-001 (order Clostridia), Desulfovibrio (Desulfovibrionacea family), and Mucispirillum (Deferribacteraceae family) and a significant increased abundance of UCG-010 (order Oscillospirales) compared with control group. WT = wild-type mice; Ztm = zonulin transgenic mice; Ctr = control mice; BC = bovine colostrum treatment; f = female; m = male.
The WT male BC group harbored a significantly increased abundance of Lactococcus (Streptococcaceae family) and a significantly reduced abundance of Clostridium sensu stricto 1 (Clostridiaceae family) (Figure 7b). The Ztm female BC group harbored a significantly increased abundance of Coriobacteriaceae UCG-002 (Atopobiaceae family) and a reduced abundance of Peptococcus (Peptococcaceae family) (Figure 7c). The Ztm male BC group harbored a significantly reduced abundance of Lachnospiraceae UCG-001 (order Clostridia), Desulfovibrio (Desulfovibrionacea family), and Mucispirillum (Deferribacteraceae family), but an increased abundance of UCG-010 (order Oscillospirales) (Figure 7d).
At the genus level, the intestinal microbial communities of all groups harbored a high abundance of Lachnospiraceae NK4A136_group (Table S1). By contrast, the WT groups (BC and Ctr) were characterized by a higher abundance of Turibacter compared with the Ztm groups (BC and Ctr), and the Ztm groups (BC and Ctr) were characterized by a higher abundance of Faecalbacaculum, Dubosiella, and Parasuttella compared with the WT groups (BC and Ctr). Both female groups, i.e., WT f (BC and Ctr) and Ztm f (BC and Ctr), were characterized by a higher abundance of Roseburia compared with the male groups (BC and Ctr; Table S1).
The WT female BC group harbored an increased abundance of the Ruminococcus and Eubacterium Xylanophilum groups when compared with the WT control group. The WT male BC group harbored an increased abundance of Oscillibacter, Eubacterium Xylanophilum group A2 when compared with the WT male control group (Table S1).
The Ztm female BC group harbored a reduced abundance of Turicibacter, Bifidobacterium, Desulfovibrio, Incertae Sedis, Rombutsia, and Blautia when compared with the Ztm female control group. In the Ztm male BC group, Dubosiella, Clostridia UCG-014, and Ruminococcus abundance were increased, but Roseburia, Helicobacter, Bifidobacterium, and Desulfovibrio abundance were reduced compared with the Ztm male control group (Table S1).
In the total of 69 genera, there was a shift in the abundance of 44 genera within the WT treatment groups and of 61 genera withing the Ztm treatment groups.
3.3.4. Analysis of Differential Species Abundances
A deeper comparison of the microbiota using the ANCOM method [134] confirmed a significantly increased abundance of the Eubacterium siraeum group; uncultured bacterium, Acutalibacter muris sp. (Rumnococcaceae family) and a significant reduction of Tyzzerella; uncultured bacterium (Lachnospiraceae family) and Dubosiella; uncultured bacterium (Erysipelotrichaceae family) in the WT female BC group compared with the WT female control group (Figure 6c and Figure 7a). There was a significantly increased abundance of uncultured species of the Lactococcus genus (Streptococcaceae family) and a significantly reduced abundance of uncultured bacterium (Peptococcaceae family) and Clostridium sensu stricto 1 sp. (Clostridiaceae family) in the WT male BC group when compared with the WT male control group (Figure 7b). In the Ztm female BC group, a significantly reduced abundance of Coriobacteriaceae_UCG-002 and Peptococcus genera (Peptococcaceae family) were found compared with the Ztm female control group (Figure 7c). In the Ztm male BC group, a significant reduction of the genera Lachnospiraceae_UCG-001, Desulfovibrio (Desulfovibrionaceae family), and Mucispirillum schaedleri sp. (Deferribacteraceae family), and a significantly increased abundance of UCG-010 genus (Ruminococcaceae family) was found compared to the Ztm male control group (Figure 7d).
Overall, there was an increase of species abundance in all treatment groups, except in the Ztm female group, where there was only a reduction.
3.4. Behavioral Assays
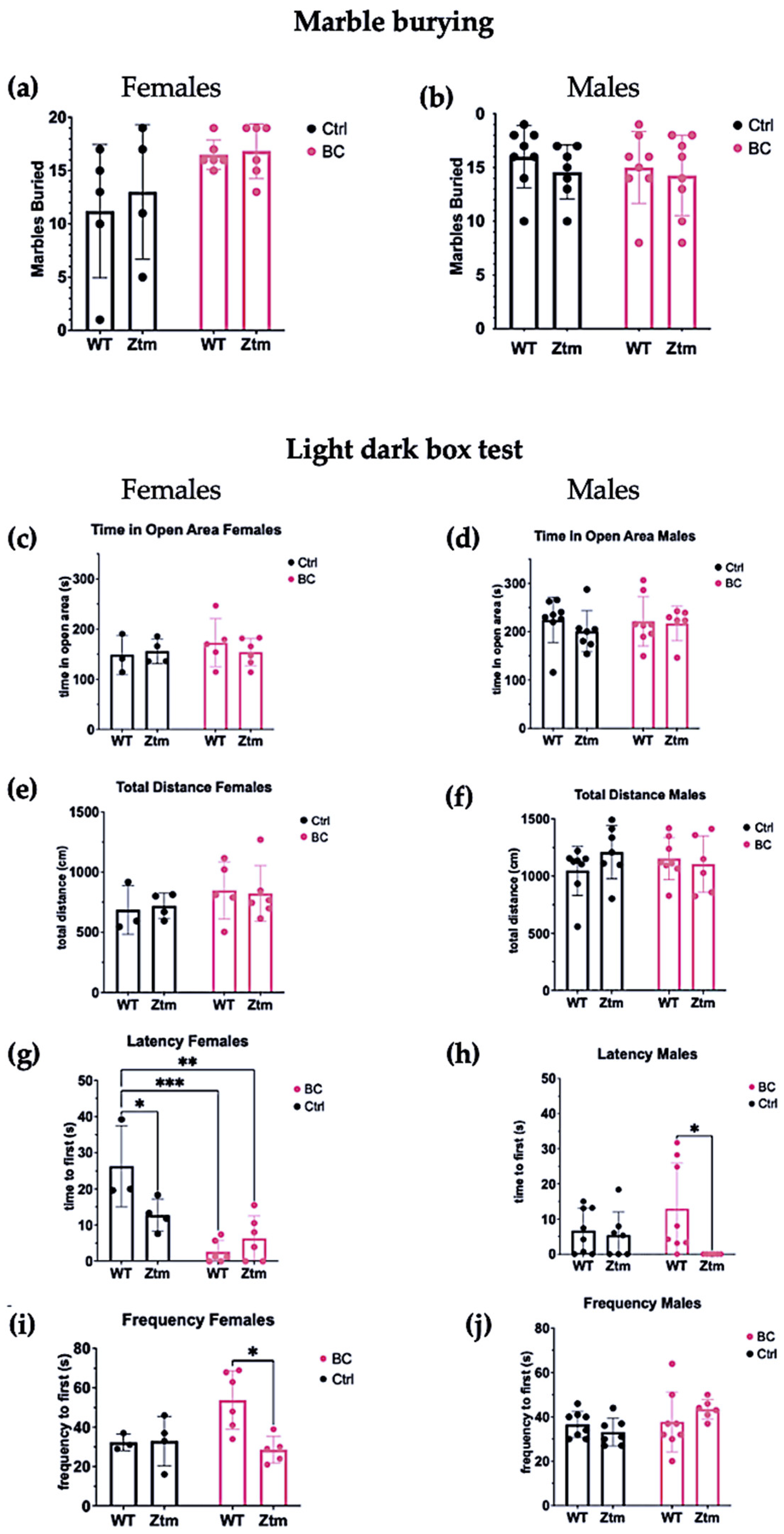
To assess behavioral patterns in the studied animals, we tested for repetitive and anxiogenic behavior in all groups by applying both MB and LDB tests (Figure 8). No significant difference was observed between any of the groups for the number of marbles buried (Figure 8a,b).
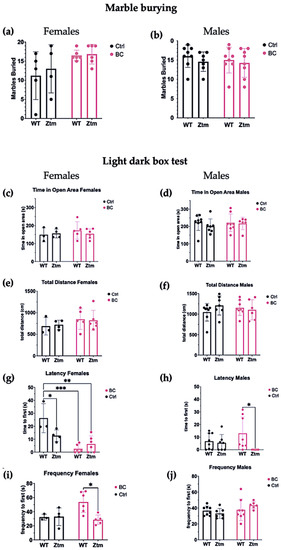
Figure 8.
A behavior profile of WT and Ztm mice measuring treatment effects post BC application. Marble burying (a,b): No significant difference was observed in total number of marbles buried between the groups. Light dark box (c–j): No significant difference was observed for time spent in open area between any of the groups (c,d). No significant difference was observed in total distance traveled between the groups (e,f).Within the female groups, latency to enter the light section was significantly different between WT BC and WT control groups (p = 0.0003), and between Ztm BC and WT control groups (p = 0.001) (g). A significant difference was observed in latency to enter the light section between WT male BC and Ztm male BC groups (p = 0.03) (h). There was a significant difference in frequency of transitions between WT female BC and Ztm BC groups (p = 0.01) (i). No significant difference was observed in frequency of transitions between any of the WT or Ztm male groups (j). n = 53. WT = wild-type mice; BC = bovine colostrum treatment; Ctrl = control mice; Ztm = zonulin transgenic mice; n = total number of mice. * p < 0.05; ** p < 0.005; *** p < 0.0005; p values adjusted with Tukey´s HSD.
In the LDB test, there was no significant difference between any of the groups for time spent in open area (Figure 8c,d) or the total distance each animal traveled in the light section (Figure 8e,f).
Significant differences were found in the mean value of latency to first enter the light section (Interaction effect (F(1,15) = 8.8, p = 0.009)) and (Treatment effect (F(1,15) = 26.8, p = 0.0001))) in both WT and Ztm female BC groups when compared with the WT female control group (Figure 8g). Post hoc testing revealed significantly reduced latency to enter the light section, i.e., WT f BC vs. WT f Ctr (p = 0.0003, 95% C.I. = 0.57, 25.32), and Ztm f BC vs. WT f Ctr (p = 0.001, 95% C.I. = 7.56, 32.27).
The mean value of latency to first enter the light section was significantly different between the WT m BC group and the Ztm m BC group (Genetic background effect (F(1,25)) = 5.2, p = 0.03))), and post hoc testing revealed a reduced latency to enter the light section (p = 0.03, 95% C.I. = 11.31, 36.02; Figure 8h).
A significant difference was observed in the frequency of transitions between the WT female BC group and the Ztm female BC group (Interaction effect (F(1,14) = 5.4, p = 0.03; and Genetic Background effect (F(1,14) = 4.9, p = 0.04)). Post-hock analysis revealed increased transitions, i.e., WT f BC compared with Ztm f BC (p = 0.01, 95% C.I. = 5.25, 45.22; Figure 8i). No significant difference was observed in the frequency of transitions between any of the WT or Ztm groups (Figure 8j).
Overall, a significant difference in latency to enter the light compartment was observed within the WT female and Ztm female and male BC groups, and for frequency of transitions within the WT and Ztm female BC groups.
4. Discussion
The zonulin-expressing mouse model is a relevant model for studying the crosstalk between the microbiome, the intestinal tract, and the brain in the context of neurobehavioral and neuroinflammatory disorders [27,39]. In the present study, we investigated the treatment effects of BC in WT and Ztm mice (Ztm mice characterized by increased intestinal permeability and mild hyperactivity) and compared with WT and Ztm control mice.
Our aim was to study the effect of BC by analyzing the intestinal microbiota and behavior in both colonies. We hypothesized that pursuant to the BC application, intestinal microbial eubiosis could be increased in the Zonulin-expressing mice, which synergize with a dysbiotic, pro-inflammatory microbial community, and, in turn, ameliorate behavioral changes previously reported in Ztm mice [39].
Miranda-Ribera et al. [27,39] previously reported that Ztm has an intrinsic increased intestinal permeability in the small intestine, associated with altered blood–brain barrier integrity at baseline, that influences the development of the immune system. Due to dysbiotic microbial community composition and increased trafficking of pro-inflammatory bacterial species and their products into the systemic circulation, the Ztm mice (female and male) are, therefore, predisposed to inflammation, including neuroinflammation. Moreover, antibiotic depletion of the intestinal microbiota downregulated brain inflammatory markers, ameliorating some anxiety-like behavior in the Ztm mice [27,39].
In the present study, BC administration demonstrated anxiolytic potential and moderately reduced anxiogenic behavior in both WT and Ztm mice. Our findings on latency suggest that BC application reduced aversion to moving from the dark section to the bright-lighted area within the WT female and the Ztm female and male BC groups, and it reduced the frequency of transitions within the Ztm female BC group. The BC treatment may have induced anxiolytic potential, causing reduced latency by increased mobility entering the light compartment, as well as reduced transitions. However, in both cases, we are comparing genotypes with different baselines, except for the significantly reduced latency between the WT female BC and WT female control groups.
These findings correlate with significant differences in the increased abundance and taxonomic diversity of the intestinal microbial community in the WT female mice and taxonomic richness in the Ztm groups (females and males) exposed to BC, compared with the control groups.
4.1. Microbiota, Short Chain Fatty Acids, and Behavior in Wild-Type (WT) Mice
The alpha and beta diversity were significantly increased in the WT female mice that underwent BC treatment. Interestingly, many of the increased members of the microbiota produce short-chain fatty acids (SCFAs) in the colon.
At the phylum level, we observed a shift in the Firmicute Bacteroidetes ratio (F/B ratio) within all the BC treatment groups, with an increased F/B ratio in the WT BC females. Among Firmicutes, the Lachnospiraceae, Lactobacillaceae, and Ruminococcaceae species produce butyrate and other SCFAs. The three major SCFAs produced by intestinal bacteria are butyrate, propionate, and acetate, which can exert several beneficial effects on human metabolism and cognitive function [137,138,139,140,141,142]. Moreover, SCFAs may positively affect intestinal barrier integrity and the host’s immunity [143,144]. Butyrate is produced by various genera (e.g., Blautia, Lachnospira, Roseburia) belonging to the Lachnospiraceae family, and taxa of this family have repeatedly shown their ability to produce beneficial metabolites for the host [138,145]. Butyrate is absorbed/metabolized by the epithelium and may stabilize intestinal barrier protection [145]. Akkermansiaeae, which belongs to the phylum Verrucomicrobia, known to promote intestinal health, and produces both propionate and acetate [142], was found to be increased in the WT female BC group.
Accumulating evidence suggests that these SCFAs can enter the CNS, providing neuroactive properties, though the mechanisms involved in the action of SCFAs on the CNS remain largely unknown [146]. Pre-clinical studies demonstrate that SCFAs exert widespread influence on key neurological and behavioral processes and may be involved in critical phases of neurodevelopmental and neurodegenerative disorders [147,148,149,150,151]. Moreover, SCFAs might directly influence neural function by reinforcing blood–brain barrier integrity, modulating neurotransmission, influencing levels of neurotrophic factors, and promoting memory consolidation. Increased evidence suggests a potential key role of SCFAs in gut–brain axis signaling [146]. However, translating these promising pre-clinical benefits to human neurodevelopmental disorders is challenging [152].
4.2. Dysbiosis, Intestinal Barrier, and Behavior in Zonulin Transgenic Mice (Ztm)
Altered microbial composition generally involves decreased abundance and diversity of species and their metabolites, breakdown of the intestinal barrier integrity, and loss of goblet cells. This may result in reduced mucus secretion, thinning of the mucus layer, and, in turn, translocation of pathobionts and toxic metabolites into the blood circulation, which can lead to local and systemic inflammatory responses [23,25,31].
As indicated in the Shannon index, there was a significant difference in microbial community abundances between the Ztm treatment and control groups. PERMANOVA demonstrated significant differences between all groups and within the groups. We observed a decrease in the F/B ratio in the Ztm female BC group compared with the Ztm female control group. Studies reveal that members of the Bacteroidetes and Firmicutes occupy different functional niches in the gut ecosystem, and, therefore, differences between individuals in their relative proportion may lead to large differences in function with relevance for host health [153,154]. The members of the phylum Bacteroidota play an essential role in maintaining the integrity of the interbacterial bonds in the intestines, produce SCFAs (butyrate, propionate, acitate), and are involved in the metabolism of bile acids and the transformation of toxic compounds [142,153,154,155,156]. The Prevotellaceae family, belonging to the Bacteroidota phylum, was increased in the Ztm BC groups. The presence of Prevotella in the intestinal microbiota in humans has been inversely correlated with Parkinson’s disease [157,158,159,160]. However, Prevotella is a gram-negative bacterium and, therefore, contains lipopolysaccharide (LPS) known to be able to impact human health, primarily through interactions with the immune system, by inducing an innate immune response, specifically through Toll-like receptors (TLRs) [161,162].
Deferribacterota and Desulfobacterota phyla were only found in the Ztm groups, and both BC groups harbored a significantly reduced relative abundance, i.e., Ztm female and male groups. Deferribacterota and Desulfobacterota phyla are involved in the activation of systemic inflammation in the host organism and can cause inflammatory damage and exacerbate energy metabolism disorders [137,163].
4.3. Dysbiosis, Behavior, Mental Disorders, and Neuroinflammation
Animal and human studies have demonstrated a clear correlation between altered microbial composition and altered behavior [164,165,166,167,168,169,170,171,172], mental disorders [173,174,175], and the development of neuroinflammation [158,176,177,178,179,180,181]. The intestinal microbiota communicate with the brain via the neural, immune, and metabolic pathways and impact neuronal plasticity and cognition [66,182,183]. This communication takes place either directly via the vagus nerve or indirectly via microbial-derived metabolites, as well as intestinal-derived metabolites, intestinal-derived hormones, and endocrine peptides [3,184].
Human studies have shown that altered microbial composition may affect neurochemical signaling and, therefore, initiate the cascade of pro-inflammatory pathways, which have been linked with depressive outcome [185,186]. Associations between abnormal intestinal microbial commensal compositions and anxiety disorders are well established [41,187]. Several reports show that experimental manipulations altering the intestinal microbiota impact anxiety-like behavior related to inflammatory status [177,188,189,190,191,192,193,194,195,196,197].
Chen et al. showed that Lachnospiraceae and Ruminococcaceae were less abundant in subjects diagnosed with anxiety disorders compared with healthy controls [41]. Both taxa are butyrate producers and may enhance the intestinal barrier by suppressing inflammation [198]. In the present study, the taxa, as mentioned above, were significantly increased in both WT female and male BC groups, and Ruminococcus was increased in both Ztm female and male BC groups.
Alzheimer’s disease (AD) has been associated with a reduction in Bacteroidetes and an increase in the F/B ratio [199,200], and a higher level of Firmicutes has been reported in patients with mild cognitive impairment (MCI) [201]. Inconsistent with this evidence, a decrease in Firmicutes in AD [202,203] has also been reported. Leu and coworkers [202] reported an increased abundance of Bacteroidetes in amnestic MCI, yet no difference was recorded when comparing AD patients and healthy controls. These inconsistent findings might reflect the dynamic changes of the intestinal microbiota during each stage of cognitive dysfunction.
An increase in some bacteria belonging to the phylum Firmicutes, including Ruminococcaceae and Enterococcaceae, has been correlated with neuroinflammation [200,201]. However, there is evidence of an abundance of Clostridiaceae, Ruminococcaceae, Eubacteriaceae, and Veillonellaceae in subjects absented from neuroinflammation, in conjunction with normal cognitive function [200,202,204]. An increase in Enterobacteriaceae, belonging to the Proteobacteria phylum, has been shown to correlate with cognitive impairment in several studies [201,202,204]. However, since many classes of bacteria with contrary characteristics are subordinated to one phylum, unambiguous results on the phylum level are scarce.
Findings from our study reveal that BC treatment modulates the intestinal microbial communities in WT mice towards a preponderance of potentially beneficial species. These microbial shifts possibly prevented anxiogenic behavior in the WT female mice. According to our data, the WT female BC group harbored a significantly increased abundance of the anti-inflammatory bacteria Eubacterium [205], an increased abundance of the anti-inflammatory bacteria Ruminococcus (research has shown reduced abundance in major depressive disorder (MDD) [206,207,208,209] and in Parkinson’s Disease (PD) [210])), increased abundance of the Eubacterium Xylanophilum group (reduced abundance has been found in ASD [211,212]), and significantly reduced abundance of the pro-inflammatory bacteria Tyzzerella [213,214], when compared with the WT female control group. Animal and human research show that the relative abundances of the genera Tyzzerella correlates with circulating pro-inflammatory cytokines (IL-1β) and certain behavioral outcomes, such as lethargy and anxiety-like behavior in chemotherapy-induced inflammation in female mice [213], as well as increased abundance by eightfold in neurogenerative diseases, such as in pediatric multiple sclerosis in humans [214].
Moreover, the BC treatment shifted the dysbiotic microbial community towards eubiosis in the Ztm mice, i.e., increased the balance within the intestinal microbial ecosystem by reducing the abundance of potentially pathogenic species. The increased eubiosis in the Ztm female BC group was paralleled by the BC anxiolytic effects. The group harbored a significantly reduced abundance of the pro-inflammatory genera Peptococcus [215,216,217] and Bifidobacterium (research shows an increased abundance of Bifidobacteria in ADHD [55,218,219]; in MDD [220,221,222]; and in bipolar (BP) [220,223]; in Schizophrenia [224,225]; yet reduced abundance in AD [52,211,226,227,228,229])). The Ztm female BC group harbored a significantly reduced abundance of Desulfovibrio (an increased abundance is known in BP [220,223], and in ASD [52,211,229,230]), reduced abundance of Blautia (reduced abundance known in PD [210], yet an increased abundance in MDD [205,209,231]) when compared with the Ztm female control group.
The increased abundance of the eubiotic intestinal microbial community within the Ztm male BC group was likewise paralleled by moderate anxiolytic effects. The BC group harbored a significantly reduced abundance of Desulfovibrio (associated with the severity of symptoms in ASD [232]) and increased abundance of Ruminococcus, an important contributor to the intestinal ecosystem [233]. An eubiotic microbiome [234] has been shown to decrease intestinal hyperpermeability and mucosal inflammatory markers in both mice [235] and humans [236]. Relieving dysbiosis and eliminating pathobionts by increasing intestinal eubiosis can elevate the production of favorable metabolites, mucus production, and protection against inflammation levels [237].
4.4. Bovine Colostrum, Oligosaccharides, Short-Chain FattyAacids (SCFAs), and Eubiosis
BC has been researched for its main constituents [87] in gastrointestinal health and disease [99], for supporting immune and digestive health [105], its effects on enteric bacteria [238], and in pediatrics [102]. Moreover, BC is rich in prebiotic components, such as oligosaccharides and glycans [87,89,239]. Prebiotics are substrates that are selectively utilized by the host’s eubiotic commensal bacteria and confer a health benefit [240]. Oligosaccharides and glycans provide the host with favorable metabolites, such as SCFAs [241,242,243,244].
Oligosaccharides have been demonstrated to reduce stress responsiveness and anxiety and facilitate changes in hippocampal synaptic efficacy [245,246,247,248]. Research has shown that oligosaccharides can significantly increase Lactobacillus, Bacteroides, and Bifidobacterium spp. [249,250,251]. Moreover, research shows the above taxa can reduce the production of proinflammatory cytokines and increase anti-inflammatory cytokines, which may attenuate post-inflammatory anxiety [3]. Oligosaccharides are known to prevent an LPS-mediated increase in cortical 5-HT2A receptor and IL1-β levels in mice [3]. Administration of oligosaccharides has been known to induce suppression of the neuroendocrine stress response and to increase the processing of positive versus negative attentional vigilance, thus resulting in an early anxiolytic-like phenotype [3].
γ-Aminobutyric acid (GABA) is a major inhibitory neurotransmitter of the vertebrate central nervous system [252] and the main inhibitory neurotransmitter in the brain [253]. Dysfunctions in the GABA system are implicated in anxiety disorders [252]. According to Berding et al. [65], dietary intervention applying prebiotic foods improved perceived stress in a healthy population, while eliciting specific metabolic changes in the intestinal microbiota. A transcriptome analysis of human fecal samples from healthy individuals showed that GABA-producing pathways are actively expressed by Bacteroides [254], and research shows that certain strains of Lactobacillus secrete GABA [255]. The extent to which pre- and probiotic [256] foods might be therapeutically useful in patients with clinically recognized anxiety disorders is presently unknown, which provides a reasonable rationale for exploring their potential value further [257].
In a randomized, double-blind, placebo-controlled trial, the effect of early enteral BC supplementation on intestinal permeability in critically ill patients was assessed. Plasma endotoxin concentration decreased significantly in the treatment group (p < 0.05), and zonulin plasma levels reduced significantly compared with the placebo group (p < 0.001) [109]. In another systematic review, the BC supplementation effect on increased intestinal permeability in athletes was assessed and showed beneficial effects for reverting intestinal hyperpermeability [258].
In a small pilot study, a probiotic/colostrum supplementation was tested on intestinal function in children diagnosed with ASD [259]. All study participants experienced a reduction in at least one gastrointestinal symptom in at least one treatment arm of the study, and some of the participants reported a reduced frequency of specific gastrointestinal symptoms, as well as of the occurrence of aberrant behaviors. However, no treatment effect on any specific microbial genera was found, as the participants’ microbiota baseline did not shift much throughout the study period. No significant changes were observed for any of the urine or serum metabolites.
In conclusion, further investigation is needed to examine the interdependencies among the components of MGBA and the potential value of BC for human use. The main limitations of this study were the small sample size in each group and limited analysis of the raw material, such as basic chemical composition and no microbiological analysis performed.
4.5. Future Perspectives
Studies showing the prebiotic influence on brain physiology and behavior are primarily descriptive. Further studies are warranted to understand mechanisms. The focus should be on which commensal microbial-derived metabolites are involved, as well as on the pathways by which these effects can be mediated. Moreover, chemical and microbiological analyses are of importance, as differences in the BC collection period may cause bioactive variability, as well as variation in processing, pasteurization, and storage conditions. When researching BC as a therapeutic agent for a medical condition, consistency of the product is vital. Based on our findings and the considerations outlined above, original research on BC application is needed from well-conducted, clinical, randomized control trials on human/clinical use in order to evaluate long-term safety/efficacy and to determine the optimal dose. Benefits and challenges need to be addressed to fully understand the potential value of BC for both prophylactic and clinical use.
5. Conclusions
In conclusion, we found significant differences in the microbiota in all BC groups compared with control groups. Moreover, our findings reveal that BC treatment modulates the intestinal microbial communities in WT and Ztm mice towards a preponderance of potentially beneficial species, and it shifts the dysbiotic microbial community towards eubiosis by reducing the abundance of potentially pathogenic species. The increased microbial ecosystem balance seemed to create a mild anxiolytic effect, and it also possibly prevented anxiogenic effect in the WT female group and reduced anxiogenic behavior in Ztm female and male mice. Colostrum application seems to support a healthy F/B ratio and significantly increase the abundance of anti-inflammatory microbial commensal bacteria, such as Lachnospiraceae, Prevotellaceae, Ruminococcaceae, Akkermansiaceae, the Eubacterium xylanophilum group, and Lactococcus. Moreover, BC application seems to significantly reduce various pro-inflammatory bacterial species, such as Deferribacterota, Desulfobacterota, Tyzzerella, Peptococcus, and Enterococcaceae. Regarding the evidence of MGBA interactions, BC treatment could be considered for prophylactic approaches in the future. However, further research is needed to explore the interdependencies among the intestinal microbiota, eubiosis, pathobionts, and neuroinflammation, as well as the potential value of BC for human use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11010091/s1, Table S1: Taxa.
Author Contributions
Conceptualization, B.A., A.F., M.F. and A.M.-R.; methodology, B.A., A.F., M.F. and A.M.-R.; formal analysis, B.A., M.C. and R.I.S.; investigation, B.A., A.M.-R., T.K. and J.L.; data curation, B.A.; writing—original draft preparation, B.A.; writing—review and editing, B.A., A.F., B.E.B., A.M.-R., M.F., B.L., L.S.G., M.G., R.I.S., M.C., J.L. and T.K.; visualization, B.A. and A.F.; supervision, A.F.; validation, A.F.; project administration, A.F.; B.A.; A.M.-R., M.F., T.K. and J.L.; resources, A.F., A.M.-R. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by the Spector Family Foundation to M.F.; the MGH ECOR Feasibility Grant to A.M.R.; European Commission, award number 825033 “GEMMA project” funded on Horizon 2020 program (call H2020-SC1-BHC -03-2018) to A.F.; the Fulbright Foundation, the Leifur Eiríksson Foundation, University of Iceland Research Fund, and the Nutricia Research Foundation to B.A.; the Uehara Memorial Foundation Overseas Postdoctoral Fellowships and the Ito Foundation for the Promotion of Medical Science Travel Grants for Overseas Exchange to T.K.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (Institutional Animal Care and Use Committee) of Massachusetts General Hospital (protocol code 2013N000013; 3 March 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable. All data generated or analyzed during this study are included in this published article.
Acknowledgments
We thank Arnar Bjarni Eiríksson from Gunnbjarnarholt, Ragnhildur Sævarsdóttir from Hjálmstaðir, and Sigurður Jónsson from Hánefsstaðir, in Iceland, for generously providing the colostrum samples, and for providing technical information regarding the raw material, and other practical aspects.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Alzheimer’s disease |
| ADHD | attention deficit hyperactivity disorder |
| ANS | autonomic nervous system |
| ASD | autism spectrum disorder |
| BC | bovine colostrum |
| BP | bipolar |
| CNS | central nervous system |
| Ctr | control mice |
| ENS | enteric nervous system |
| F/B ratio | Firmicute Bacteroidetes ratio |
| FDR | false discovery rate |
| GABA | γ-Aminobutyric acid |
| GBA | gut–brain axis |
| GI | gastrointestinal |
| HSD | honestly significant difference |
| HPA | hypothalamic-pituitary-adrenal |
| IP | intestinal permeability |
| LDB | light-dark box |
| LPS | lipopolysaccharide |
| MB | marble burying |
| MCI | mild cognitive impairment |
| MDD | major depressive disorder |
| MGBA | microbiota–gut–brain axis |
| OTUs | operational taxonomic units |
| PD | Parkinson’s Disease |
| PCA | principal component analysis |
| PERMANOVA | permutation multivariate analysis of variance |
| SCFAs | short-chain fatty acids |
| TJs | tight junctions |
| TLRs | Toll-like receptors |
| WT | wild-type |
| Ztm | zonulin transgenic mouse |
References
- Borghi, E.; Vignoli, A.; D’Agostino, A. The Contribution of Microbiology to Neuroscience: More Complex than It Seems? Behav. Brain Sci. 2019, 42, e67. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The Progress of Gut Microbiome Research Related to Brain Disorders. J. Neuroinflamm 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology From the Microbiota–Gut–Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-Induced Microbiome Depletion Alters Metabolic Homeostasis by Affecting Gut Signaling and Colonic Metabolism. Nat. Commun. 2018, 9, 2872. [Google Scholar] [CrossRef]
- Nash, M.J.; Frank, D.N.; Friedman, J.E. Early Microbes Modify Immune System Development and Metabolic Homeostasis—The “Restaurant” Hypothesis Revisited. Front. Endocrinol. 2017, 8, 349. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. Gut Microflora as a Target for Energy and Metabolic Homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 729–734. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Homeostatic Impact of Indigenous Microbiota and Secretory Immunity. Benef. Microbes 2010, 1, 211–227. [Google Scholar] [CrossRef]
- Hansen, B.H.; Oerbeck, B.; Skirbekk, B.; Petrovski, B.É.; Kristensen, H. Neurodevelopmental Disorders: Prevalence and Comorbidity in Children Referred to Mental Health Services. Nord. J. Psychiatry 2018, 72, 285–291. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Windows: Implications for Brain Disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-López, G.; Velázquez-Moctezuma, J.; Noesselt, T. The Microbiota–Gut–Brain Axis: Neurobehavioral Correlates, Health and Sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2019, 60, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Savu, O.; Mihaescu, G.; Vrancianu, O.; Chifiriuc, M.-C. Dysbiosis, Tolerance, and Development of Autoimmune Diseases. In Immunology of the GI Tract; IntechOpen: London, UK, 2022; ISBN 978-1-80356-087-8. [Google Scholar]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Al-Rashidi, H.E. Gut Microbiota and Immunity Relevance in Eubiosis and Dysbiosis. Saudi J. Biol. Sci. 2022, 29, 1628–1643. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Fasano, A.; Shea-Donohue, T. Mechanisms of Disease: The Role of Intestinal Barrier Function in the Pathogenesis of Gastrointestinal Autoimmune Diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal Permeability and Its Regulation by Zonulin: Diagnostic and Therapeutic Implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Vermeire, S.; Cleynen, I. The Role of Haptoglobin and Its Related Protein, Zonulin, in Inflammatory Bowel Disease. Tissue Barriers 2013, 1, e27321. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin, Regulation of Tight Junctions, and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.S.; Sturgeon, C.; Fasano, A.; Cascella, N.G.; Eaton, W.W.; McMahon, R.P.; Kelly, D.L. Elevated Zonulin, a Measure of Tight-Junction Permeability, May Be Implicated in Schizophrenia. Schizophr. Res. 2019, 211, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Miranda-Ribera, A.; Ennamorati, M.; Serena, G.; Cetinbas, M.; Lan, J.; Sadreyev, R.I.; Jain, N.; Fasano, A.; Fiorentino, M. Exploiting the Zonulin Mouse Model to Establish the Role of Primary Impaired Gut Barrier Function on Microbiota Composition and Immune Profiles. Front. Immunol. 2019, 10, 2233. [Google Scholar] [CrossRef]
- Yu, L.C.-H.; Wang, J.-T.; Wei, S.-C.; Ni, Y.-H. Host-Microbial Interactions and Regulation of Intestinal Epithelial Barrier Function: From Physiology to Pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Stewart, A.S.; Pratt-Phillips, S.; Gonzalez, L.M. Alterations in Intestinal Permeability: The Role of the “Leaky Gut” in Health and Disease. J. Equine. Vet. Sci. 2017, 52, 10–22. [Google Scholar] [CrossRef]
- Fasano, A. All Disease Begins in the (Leaky) Gut: Role of Zonulin-Mediated Gut Permeability in the Pathogenesis of Some Chronic Inflammatory Diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Alterations in Intestinal Permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Hill, I. Serum Zonulin, Gut Permeability, and the Pathogenesis of Autism Spectrum Disorders: Cause, Effect, or an Epiphenomenon? J. Pediatr. 2017, 188, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Asbjornsdottir, B.; Snorradottir, H.; Andresdottir, E.; Fasano, A.; Lauth, B.; Gudmundsson, L.S.; Gottfredsson, M.; Halldorsson, T.I.; Birgisdottir, B.E. Zonulin-dependent Intestinal Permeability in Children Diagnosed with Mental Disorders: A Systematic Review and Meta-analysis. Nutrients 2020, 12, 1982. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, J.; Zhang, J.; Liang, C.; Wang, Y.; Chen, B.; Zhao, C.; Wang, J.; Zhang, G.; Zhao, D.; et al. Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genom. Proteom. Bioinform. 2019, 17, 26–38. [Google Scholar] [CrossRef]
- Ravaccia, D.; Ghafourian, T. Critical Role of the Maternal Immune System in the Pathogenesis of Autism Spectrum Disorder. Biomedicines 2020, 8, 557. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood–Brain Barrier and Intestinal Epithelial Barrier Alterations in Autism Spectrum Disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef]
- Miranda-Ribera, A.; Serena, G.; Liu, J.; Fasano, A.; Kingsbury, M.A.; Fiorentino, M.R. The Zonulin-Transgenic Mouse Displays Behavioral Alterations Ameliorated via Depletion of the Gut Microbiota. Tissue Barriers 2021, 10, 2000299. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, J.; Wu, D.; Yu, S.; Qiang, X.; Bai, H.; Wang, H.; Peng, Z. Association between Fecal Microbiota and Generalized Anxiety Disorder: Severity and Early Treatment Response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic Administration Normalizes Lipopolysaccharide (LPS)-Induced Anxiety and Cortical 5-HT2A Receptor and IL1-β Levels in Male Mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased Human Intestinal Barrier Permeability Plasma Biomarkers Zonulin and FABP2 Correlated with Plasma LPS and Altered Gut Microbiome in Anxiety or Depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Wingo, A.P.; Gibson, G. Blood Gene Expression Profiles Suggest Altered Immune Function Associated with Symptoms of Generalized Anxiety Disorder HHS Public Access. Brain Behav. Immun. 2015, 43, 184–191. [Google Scholar] [CrossRef]
- Copeland, W.E.; Shanahan, L.; Worthman, C.; Angold, A.; Costello, E.J. Generalized Anxiety and C-Reactive Protein Levels: A Prospective, Longitudinal Analysis. Psychol. Med. 2012, 42, 2641–2650. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Beekman, A.T.F.; de Jonge, P.; Penninx, B.W.J.H. Anxiety Disorders and Inflammation in a Large Adult Cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef]
- Mochcovitch, M.D.; da Rocha Freire, R.C.; Garcia, R.F.; Nardi, A.E. A Systematic Review of FMRI Studies in Generalized Anxiety Disorder: Evaluating Its Neural and Cognitive Basis. J. Affect. Disord. 2014, 167, 336–342. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the Role of Inflammation in Mood and Anxiety-Related Disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.-K. Molecular Sciences Neuroinflammation-Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 6546. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the Gut-Brain-Axis: Implications for New Therapeutic Design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. A Clin. J. 2018, 17, 28. [Google Scholar]
- Kim, Y.-K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Patho-Physiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut Microbiome in ADHD and Its Relation to Neural Reward Anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Granziera, C.; Hooker, J.M.; Loggia, M.L. In Vivo Imaging of Human Neuroinflammation. ACS Chem. Neurosci. 2016, 7, 470–483. [Google Scholar] [CrossRef]
- Luca, M.; Chattipakorn, S.C.; Sriwichaiin, S.; Luca, A. Cognitive-Behavioural Correlates of Dysbiosis: A Review. Int. J. Mol. Sci. 2020, 21, 4834. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive Impairment by Antibiotic-Induced Gut Dysbiosis: Analysis of Gut Microbiota-Brain Communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From Gut Dysbiosis to Altered Brain Function and Mental Illness: Mechanisms and Pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Perrotta, G. The Intestinal Microbiota: Towards a Multifactorial Integrative Model. Eubiosis and Dysbiosis in Morbid Physical and Psychological Conditions. Arch. Clin. Gastroenterol. 2021, 7, 24–35. [Google Scholar] [CrossRef]
- Fasano, A. Physiological, Pathological, and Therapeutic Implications of Zonulin-Mediated Intestinal Barrier Modulation: Living Life on the Edge of the Wall. Am. J. Pathol. 2008, 173, 1243. [Google Scholar] [CrossRef]
- Foster, J.A.; Lyte, M.; Meyer, E.; Cryan, J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience. Int. J. Neuropsychopharmacol. 2016, 19, pyv114. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemigun, K.; Schnermann, M.E.; Schmid, M.; Cryan, J.F.; Nöthlings, U. A Prospective Investigation into the Association between the Gut Microbiome Composition and Cognitive Performance among Healthy Young Adults. Gut Pathog. 2022, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Bastiaanssen, T.F.S.; Moloney, G.M.; Boscaini, S.; Strain, C.R.; Anesi, A.; Long-Smith, C.; Mattivi, F.; Stanton, C.; Clarke, G.; et al. Feed Your Microbes to Deal with Stress: A Psychobiotic Diet Impacts Microbial Stability and Perceived Stress in a Healthy Adult Population. Mol. Psychiatry 2022, 1–10. [Google Scholar] [CrossRef]
- Esposito, P.; Ismail, N. Linking Puberty and the Gut Microbiome to the Pathogenesis of Neurodegenerative Disorders. Microorganisms 2022, 10, 2163. [Google Scholar] [CrossRef]
- Conlon, M.; Bird, A. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Kim, B.; Choi, H.-N.; Yim, J.-E. Effect of Diet on the Gut Microbiota Associated with Obesity. J. Obes. Metab. Syndr. 2019, 28, 216–224. [Google Scholar] [CrossRef]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Butterworth, P. Does Reverse Causality Explain the Relationship between Diet and Depression? J. Affect. Disord. 2015, 175, 248–250. [Google Scholar] [CrossRef]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef]
- O’Neil, A.; Quirk, S.E.; Housden, S.; Brennan, S.L.; Williams, L.J.; Pasco, J.A.; Berk, M.; Jacka, F.N. Relationship Between Diet and Mental Health in Children and Adolescents: A Systematic Review. Am. J. Public Health 2014, 104, e31–e42. [Google Scholar] [CrossRef]
- Heilskov Rytter, M.J.; Andersen, L.B.B.; Houmann, T.; Bilenberg, N.; Hvolby, A.; Mølgaard, C.; Michaelsen, K.F.; Lauritzen, L. Diet in the Treatment of ADHD in Children—A Systematic Review of the Literature. Nord. J. Psychiatry 2015, 69, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.L.; Dash, S.R.; Jacka, F.N. The Importance of Diet and Gut Health to the Treatment and Prevention of Mental Disorders. Int. Rev. Neurobiol. 2016, 131, 325–346. [Google Scholar]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Adalsteinsdottir, S.A.; Magnusdottir, O.K.; Halldorsson, T.I.; Birgisdottir, B.E. Towards an Individualized Nutrition Treatment: Role of the Gastrointestinal Microbiome in the Interplay Between Diet and Obesity. Curr. Obes. Rep. 2018, 7, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as Deep Modulators of Gut Microbiota: Between Good and Evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.M.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal Exposure to Antibiotics, Cesarean Section and Risk of Childhood Obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Blaser, M.J. Antibiotics in Early Life and Obesity. Nat. Rev. Endocrinol. 2015, 11, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.C.; Forrest, C.B.; Zhang, P.; Richards, T.M.; Livshits, A.; DeRusso, P.A. Association of Antibiotics in Infancy With Early Childhood Obesity. JAMA Pediatr. 2014, 168, 1063. [Google Scholar] [CrossRef]
- Kruis, W. Review Article: Antibiotics and Probiotics in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. Suppl. 2004, 20, 75–78. [Google Scholar] [CrossRef]
- Dinan, K.; Dinan, T. Antibiotics and Mental Health: The Good, the Bad and the Ugly. J. Intern. Med. 2022, 292, 858–869. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef]
- Godden, S. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Keech, A.M. Peptide Immunotherapy: Colostrum: A Physician’s Reference Guide, 2nd ed.; AKS Publishing: New Delhi, India, 2009; ISBN 0692002421. [Google Scholar]
- Playford, R.J. The Use of Bovine Colostrum in Medical Practice and Human Health: Current Evidence and Areas Requiring Further Examination. Nutrients 2021, 14, 92. [Google Scholar] [CrossRef]
- Struff, W.G.; Sprotte, G. Bovine Colostrum as a Biologic in Clinical Medicine: A Review. Part I: Biotechnological Standards, Pharmacodynamic and Pharmacokinetic Characteristics and Principles of Treatment. Int. J. Clin. Pharmacol. Ther. 2007, 45, 193–202. [Google Scholar] [CrossRef]
- Struff, W.G.; Sprotte, G. Bovine Colostrum as a Biologic in Clinical Medicine: A Review–Part II: Clinical Studies. Int. J. Clin. Pharmacol. Ther. 2008, 46, 211–225. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and Properties of Bovine Colostrum: A Review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Bagwe, S.; Tharappel, L.J.P.; Kaur, G.; Buttar, H.S. Bovine Colostrum: An Emerging Nutraceutical. J. Complement. Integr. Med. 2015, 12, 175–185. [Google Scholar] [CrossRef]
- Thapa, B.R. Health Factors in Colostrum. Indian J. Pediatr. 2005, 72, 579–581. [Google Scholar] [CrossRef]
- He, F.; Tuomola, E.; Arvilommi, H.; Salminen, S. Modulation of Human Humoral Immune Response through Orally Administered Bovine Colostrum. FEMS Immunol. Med. Microbiol. 2001, 31, 93–96. [Google Scholar] [CrossRef]
- Jones, A.W.; March, D.S.; Thatcher, R.; Diment, B.; Walsh, N.P.; Davison, G. The Effects of Bovine Colostrum Supplementation on in Vivo Immunity Following Prolonged Exercise: A Randomised Controlled Trial. Eur. J. Nutr. 2019, 58, 335–344. [Google Scholar] [CrossRef]
- Tacket, C.O.; Binion, S.B.; Bostwick, E.; Losonsky, G.; Roy, M.J.; Edelman, R. Efficacy of Bovine Milk Immunoglobulin Concentrate in Preventing Illness after Shigella Flexneri Challenge. Am. J. Trop. Med. Hyg. 1992, 47, 276–283. [Google Scholar] [CrossRef]
- Menchetti, L.; Traina, G.; Tomasello, G.; Casagrande-Proietti, P.; Leonardi, L.; Barbato, O.; Brecchia, G. Potential Benefits of Colostrum in Gastrointestinal Diseases. Front. Biosci. 2016, 8, 331–351. [Google Scholar] [CrossRef]
- Chandwe, K.; Kelly, P. Colostrum Therapy for Human Gastrointestinal Health and Disease. Nutrients 2021, 13, 1956. [Google Scholar] [CrossRef]
- Rathe, M.; Müller, K.; Sangild, P.T.; Husby, S. Clinical Applications of Bovine Colostrum Therapy: A Systematic Review. Nutr. Rev. 2014, 72, 237–254. [Google Scholar] [CrossRef]
- Buttar, H.S.; Bagwe, S.M.; Bhullar, S.K.; Kaur, G. Health Benefits of Bovine Colostrum in Children and Adults. In Dairy in Human Health and Disease across the Lifespan; Academic Press: London, UK, 2017; ISBN 9780128098691. [Google Scholar]
- Sangild, P.T.; Vonderohe, C.; Melendez Hebib, V.; Burrin, D.G. Potential Benefits of Bovine Colostrum in Pediatric Nutrition and Health. Nutrients 2021, 13, 2551. [Google Scholar] [CrossRef]
- Pakkanen, R.; Aalto, J. Review Paper Growth Factors and Antimicrobial Factors of Bovine Colostrum. In International Dairy Journal; Elsevier: Amsterdam, The Netherlands, 1997; Volume 7. [Google Scholar]
- Guberti, M.; Botti, S.; Capuzzo, M.T.; Nardozi, S.; Fusco, A.; Cera, A.; Dugo, L.; Piredda, M.; de Marinis, M.G. Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients 2021, 13, 2194. [Google Scholar] [CrossRef]
- Ghosh, S.; Iacucci, M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients 2021, 13, 3798. [Google Scholar] [CrossRef]
- An, M.J.; Cheon, J.H.; Kim, S.W.; Park, J.J.; Moon, C.M.; Han, S.Y.; Kim, E.S.; Kim, T.I.; Kim, W.H. Bovine Colostrum Inhibits Nuclear Factor ΚB–Mediated Proinflammatory Cytokine Expression in Intestinal Epithelial Cells. Nutr. Res. 2009, 29, 275–280. [Google Scholar] [CrossRef]
- Saad, K.; Abo-Elela, M.G.M.; El-Baseer, K.A.A.; Ahmed, A.E.; Ahmad, F.-A.; Tawfeek, M.S.K.; El-Houfey, A.A.; AboulKhair, M.D.; Abdel-Salam, A.M.; Abo-elgheit, A.; et al. Effects of Bovine Colostrum on Recurrent Respiratory Tract Infections and Diarrhea in Children. Medicine 2016, 95, e4560. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine Colostrum Modulates Cytokine Production in Human Peripheral Blood Mononuclear Cells Stimulated with Lipopolysaccharide and Phytohemagglutinin. J. Interferon Cytokine Res. 2009, 29, 37–44. [Google Scholar] [CrossRef]
- Eslamian, G.; Ardehali, S.H.; Baghestani, A.-R.; Vahdat Shariatpanahi, Z. Effects of Early Enteral Bovine Colostrum Supplementation on Intestinal Permeability in Critically Ill Patients: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrition 2019, 60, 106–111. [Google Scholar] [CrossRef]
- Marnila, P.; Rokka, S.; Rehnberg-Laiho, L.; Kärkkäinen, P.; Kosunen, T.U.; Rautelin, H.; Hänninen, M.L.; Syväoja, E.L.; Korhonen, H. A Novel Extract from Bovine Colostrum Whey Supports Anti-Bacterial and Anti-Viral Innate Immune Functions in Vitro and in Vivo: I. Enhanced Immune Activity in Vitro Translates to Improved Microbial Clearance in Animal Infection Models. Prev. Med. 2012, 54, S116–S123. [Google Scholar] [CrossRef]
- Batista da Silva Galdino, A.; do Nascimento Rangel, A.H.; Buttar, H.S.; Sales Lima Nascimento, M.; Cristina Gavioli, E.; de Oliveira, R.P.; Cavalcanti Sales, D.; Urbano, S.A.; Anaya, K. Bovine Colostrum: Benefits for the Human Respiratory System and Potential Contributions for Clinical Management of COVID-19. Food Agric. Immunol. 2021, 32, 143–162. [Google Scholar] [CrossRef]
- Levy, A.P.; Levy, J.E.; Kalet-Litman, S.; Miller-Lotan, R.; Levy, N.S.; Asaf, R.; Guetta, J.; Yang, C.; Purushothaman, K.R.; Fuster, V.; et al. Haptoglobin Genotype Is a Determinant of Iron, Lipid Peroxidation, and Macrophage Accumulation in the Atherosclerotic Plaque. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 134–140. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The Mouse Light/Dark Box Test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Takao, K.; Miyakawa, T. Light/Dark Transition Test for Mice. J. Vis. Exp. 2006, 13, 104. [Google Scholar] [CrossRef]
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the Light/Dark Test for Anxiety in Adult and Adolescent Male Rats. Behav. Brain Res. 2013, 256, 119. [Google Scholar] [CrossRef]
- Holmes, A.; Iles, J.P.; Mayell, S.J.; Rodgers, R.J. Prior Test Experience Compromises the Anxiolytic Efficacy of Chlordiazepoxide in the Mouse Light/Dark Exploration Test. Behav. Brain Res. 2001, 122, 159–167. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble Burying Reflects a Repetitive and Perseverative Behavior More than Novelty-Induced Anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- Lazic, S.E. Analytical Strategies for the Marble Burying Test: Avoiding Impossible Predictions and Invalid p-Values. BMC Res. Notes 2015, 8, 141. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Shepherd, J.K. Influence of Prior Maze Experience on Behaviour and Response to Diazepam in the Elevated Plus-Maze and Light/Dark Tests of Anxiety in Mice. Psychopharmacology 1993, 113, 237–242. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems 2017, 2, e00191-16. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Gonzalez, A.; Smarr, L.; McDonald, D.; Morton, J.T.; Navas-Molina, J.A.; Knight, R. Bringing the Dynamic Microbiome to Life with Animations. Cell Host Microbe 2017, 21, 7–10. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A Tool for Visualizing High-Throughput Microbial Community Data. Gigascience 2013, 2, 16. [Google Scholar] [CrossRef]
- Chen, J.; Bittinger, K.; Charlson, E.S.; Hoffmann, C.; Lewis, J.; Wu, G.D.; Collman, R.G.; Bushman, F.D.; Li, H. Associating Microbiome Composition with Environmental Covariates Using Generalized UniFrac Distances. Bioinformatics 2012, 28, 2106–2113. [Google Scholar] [CrossRef]
- Chang, Q.; Luan, Y.; Sun, F. Variance Adjusted Weighted UniFrac: A Powerful Beta Diversity Measure for Comparing Communities Based on Phylogeny. BMC Bioinform. 2011, 12, 118. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative Beta Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management for the Masses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Silva. Available online: https://www.arb-silva.de/ (accessed on 21 July 2022).
- Mandal, S.; van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing on JSTOR. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Schloss, P.D.; Schubert, A.M.; Zackular, J.P.; Iverson, K.D.; Young, V.B.; Petrosino, J.F. Stabilization of the Murine Gut Microbiome Following Weaning. Gut Microbes 2012, 3, 383–393. [Google Scholar] [CrossRef]
- Gryaznova, M.; Dvoretskaya, Y.; Burakova, I.; Syromyatnikov, M.; Popov, E.; Kokina, A.; Mikhaylov, E.; Popov, V. Dynamics of Changes in the Gut Microbiota of Healthy Mice Fed with Lactic Acid Bacteria and Bifidobacteria. Microorganisms 2022, 10, 1020. [Google Scholar] [CrossRef]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Arfan Ikram, M.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between Gut Microbiota and Circulating Metabolites in Population-Based Cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, P.; Jiang, Q.; Xu, Q.; Zheng, Y.; Yan, J.; Ji, H.; Ning, J.; Zhang, X.; Li, C.; et al. Depletion of Acetate-Producing Bacteria from the Gut Microbiota Facilitates Cognitive Impairment through the Gut-Brain Neural Mechanism in Diabetic Mice. Microbiome 2021, 9, 145. [Google Scholar] [CrossRef]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key Bacterial Taxa and Metabolic Pathways Affecting Gut Short-Chain Fatty Acid Profiles in Early Life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef]
- Müller, B.; Rasmusson, A.J.; Just, D.; Jayarathna, S.; Moazzami, A.; Novicic, Z.K.; Cunningham, J.L. Fecal Short-Chain Fatty Acid Ratios as Related to Gastrointestinal and Depressive Symptoms in Young Adults. Psychosom. Med. 2021, 83, 693–699. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of Microbiota-Derived Short-Chain Fatty Acids in Nervous System Disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Mariadason, J.M.; Barkla, D.H.; Gibson, P.R. Effect of Short-Chain Fatty Acids on Paracellular Permeability in Caco-2 Intestinal Epithelium Model. Am. J. Physiol. 1997, 272, G705–G712. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; de Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front. Neurosci. 2017, 11, 490. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut Instincts: Microbiota as a Key Regulator of Brain Development, Ageing and Neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Baxter, A.J.; Scott, K.M.; Ferrari, A.J.; Norman, R.E.; Vos, T.; Whiteford, H.A. Challenging the myth of an “epidemic” of common mental disorders: Trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress. Anxiety 2014, 31, 506–516. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and Metabolic Disease: Revisiting the Bacterial Phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Abdallah Ismail, N.; Ragab, S.H.; Abd Elbaky, A.; Shoeib, A.R.S.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in Gut Microbiota in Obese and Normal Weight Egyptian Children and Adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef]
- Greenhill, C. Gut Microbiota: Firmicutes and Bacteroidetes Involved in Insulin Resistance by Mediating Levels of Glucagon-like Peptide 1. Nat. Rev. Endocrinol. 2015, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Hitch, T.C.A.; Bisdorf, K.; Afrizal, A.; Riedel, T.; Overmann, J.; Strowig, T.; Clavel, T. A Taxonomic Note on the Genus Prevotella: Description of Four Novel Genera and Emended Description of the Genera Hallella and Xylanibacter. Syst. Appl. Microbiol. 2022, 45, 126354. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson’s Disease. Neuroscience 2020, 432, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Wang, Z.; Brown, J.M.; Engen, P.A.; Naqib, A.; Goetz, C.G.; Hall, D.A.; Metman, L.V.; Shaikh, M.; Forsyth, C.B.; et al. Gut Microbial Metabolites in Parkinson’s Disease: Association with Lifestyle, Disease Characteristics, and Treatment Status. Neurobiol. Dis. 2022, 170, 105780. [Google Scholar] [CrossRef] [PubMed]
- Gątarek, P.; Sekulska-Nalewajko, J.; Bobrowska-Korczaka, B.; Pawełczyk, M.; Jastrzębski, K.; Głąbiński, A.; Kałużna-Czaplińska, J. Plasma Metabolic Disturbances in Parkinson’s Disease Patients. Biomedicines 2022, 10, 3005. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, D.M.; Lankelma, J.M.; Vachot, L.; Cerrato, E.; Pachot, A.; Wiersinga, W.J.; Textoris, J. Comparison of Host Immune Responses to LPS in Human Using an Immune Profiling Panel, in Vivo Endotoxemia versus Ex Vivo Stimulation. Sci. Rep. 2020, 10, 9918. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S. Biochemistry, Lipopolysaccharide; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Murros, K.E.; Huynh, V.A.; Takala, T.M.; Saris, P.E.J. Desulfovibrio Bacteria Are Associated With Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11, 378. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota Modulation Counteracts Alzheimer’s Disease Progression Influencing Neuronal Proteolysis and Gut Hormones Plasma Levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Syeda, T.; Sanchez-Tapia, M.; Pinedo-Vargas, L.; Granados, O.; Cuervo-Zanatta, D.; Rojas-Santiago, E.; Díaz-Cintra, S.A.; Torres, N.; Perez-Cruz, C. Bioactive Food Abates Metabolic and Synaptic Alterations by Modulation of Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 1657–1682. [Google Scholar] [CrossRef]
- Sanguinetti, E.; Collado, M.C.; Marrachelli, V.G.; Monleon, D.; Selma-Royo, M.; Pardo-Tendero, M.M.; Burchielli, S.; Iozzo, P. Microbiome-Metabolome Signatures in Mice Genetically Prone to Develop Dementia, Fed a Normal or Fatty Diet. Sci. Rep. 2018, 8, 4907. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal Microbiota Transplantation Alleviated Alzheimer’s Disease-like Pathogenesis in APP/PS1 Transgenic Mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.; Ling, Z.; Wang, F.; Ling, Y.; Gong, T.; Fang, N.; Ye, S.; Si, J.; Liu, J. Fructooligosaccharides Ameliorating Cognitive Deficits and Neurodegeneration in APP/PS1 Transgenic Mice through Modulating Gut Microbiota. J. Agric. Food Chem. 2019, 67, 3006–3017. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Saxena, J.; Pesaresi, T.G.; Carrell, C.D.; Ashby, G.B.; Liao, M.-K.; Freeman, L.R. High Fat Diet Alters Gut Microbiota but Not Spatial Working Memory in Early Middle-Aged Sprague Dawley Rats. PLoS ONE 2019, 14, e0217553. [Google Scholar] [CrossRef]
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Gut Dysbiosis Develops before Metabolic Disturbance and Cognitive Decline in High-Fat Diet–Induced Obese Condition. Nutrition 2020, 69, 110576. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased Microglial Activation through Gut-Brain Axis by Prebiotics, Probiotics, or Synbiotics Effectively Restored Cognitive Function in Obese-Insulin Resistant Rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.-R. Obese-Type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kishimoto, T.; Mizuno, S.; Masaoka, T.; Naganuma, M.; Liang, K.-C.; Kitazawa, M.; Nakashima, M.; Shindo, C.; Suda, W.; et al. The Effect of Fecal Microbiota Transplantation on Psychiatric Symptoms among Patients with Irritable Bowel Syndrome, Functional Diarrhea and Functional Constipation: An Open-Label Observational Study. J. Affect. Disord. 2018, 235, 506–512. [Google Scholar] [CrossRef]
- Heym, N.; Heasman, B.C.; Hunter, K.; Blanco, S.R.; Wang, G.Y.; Siegert, R.; Cleare, A.; Gibson, G.R.; Kumari, V.; Sumich, A.L. The Role of Microbiota and Inflammation in Self-Judgement and Empathy: Implications for Understanding the Brain-Gut-Microbiome Axis in Depression. Psychopharmacology 2019, 236, 1459–1470. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef]
- Marcondes Ávila, P.R.; Fiorot, M.; Michels, M.; Dominguini, D.; Abatti, M.; Vieira, A.; de Moura, A.B.; Behenck, J.P.; Borba, L.A.; Botelho, M.E.M.; et al. Effects of Microbiota Transplantation and the Role of the Vagus Nerve in Gut-Brain Axis in Animals Subjected to Chronic Mild Stress. J. Affect. Disord. 2020, 277, 410–416. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. Gut Microbiome and Modulation of CNS Function. Compr. Physiol. 2019, 10, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Hickman, R.A.; Hussein, M.A.; Pei, Z. Consequences of Gut Dysbiosis on the Human Brain. In The Gut Microbiome-Implications for Human Disease; InTechOpen: London, UK, 2016; ISBN 978-953-51-2751-2. [Google Scholar]
- Jena, P.K.; Sheng, L.; di Lucente, J.; Jin, L.-W.; Maezawa, I.; Wan, Y.-J.Y. Dysregulated Bile Acid Synthesis and Dysbiosis Are Implicated in Western Diet-Induced Systemic Inflammation, Microglial Activation, and Reduced Neuroplasticity. FASEB J. 2018, 32, 2866–2877. [Google Scholar] [CrossRef]
- el Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Collyer, R.; Clancy, A.; Borody, T. Faecal Microbiota Transplantation Alleviates Symptoms of Depression in Individuals with Irritable Bowel Syndrome: A Case Series. Med. Microecol. 2020, 6, 100029. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and Probiotics for Depression and Anxiety: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Ikeda, M.; Hamada, K.; Sumitom, N.; Okamoto, H.; Sakakibara, B. Serum Amyloid A, Cytokines, and Corticosterone Responses in Germfree and Conventional Mice after Lipopolysaccharide Injection. Biosci. Biotechnol. Biochem. 1999, 63, 1006–1010. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic Gastrointestinal Inflammation Induces Anxiety-Like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterology 2010, 139, 2102–2112.e1. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Varcoe, J.J.; Bailey, M.T. Anxiogenic Effect of Subclinical Bacterial Infection in Mice in the Absence of Overt Immune Activation. Physiol. Behav. 1998, 65, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.A.; Goehler, L.E. Induction of Anxiety-like Behavior in Mice during the Initial Stages of Infection with the Agent of Murine Colonic Hyperplasia Citrobacter Rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef]
- Goehler, L.E.; Park, S.M.; Opitz, N.; Lyte, M.; Gaykema, R.P.A. Campylobacter Jejuni Infection Increases Anxiety-like Behavior in the Holeboard: Possible Anatomical Substrates for Viscerosensory Modulation of Exploratory Behavior. Brain Behav. Immun. 2008, 22, 354–366. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef]
- Neufeld, K.-A.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of Intestinal Microbiota on Anxiety-like Behavior. Commun. Integr. Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Sherry, C.L.; Kim, S.S.; Dilger, R.N.; Bauer, L.L.; Moon, M.L.; Tapping, R.I.; Fahey, G.C.; Tappenden, K.A.; Freund, G.G. Sickness Behavior Induced by Endotoxin Can Be Mitigated by the Dietary Soluble Fiber, Pectin, through up-Regulation of IL-4 and Th2 Polarization. Brain Behav. Immun. 2010, 24, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Niida, S.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Toba, K.; Sakurai, T. Analysis of the Relationship between the Gut Microbiome and Dementia: A Cross-Sectional Study Conducted in Japan. Sci. Rep. 2019, 9, 1008. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut Microbiota Is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered Microbiomes Distinguish Alzheimer’s Disease from Amnestic Mild Cognitive Impairment and Health in a Chinese Cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Eicher, T.P.; Hasan Mohajeri, M. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef]
- Cheng, S.; Han, B.; Ding, M.; Wen, Y.; Ma, M.; Zhang, L.; Qi, X.; Cheng, B.; Li, P.; Kafle, O.P.; et al. Identifying Psychiatric Disorder-Associated Gut Microbiota Using Microbiota-Related Gene Set Enrichment Analysis. Brief. Bioinform. 2020, 21, 1016–1022. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota−Gut−Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef]
- Łoniewski, I.; Misera, A.; Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Kaźmierczak-Siedlecka, K.; Misiak, B.; Marlicz, W.; Samochowiec, J. Major Depressive Disorder and Gut Microbiota–Association Not Causation. A Scoping Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110111. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural Changes of Gut Microbiota in Parkinson’s Disease and Its Correlation with Clinical Features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal Involvement of Autism Spectrum Disorder: Focus on Gut Microbiota. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Grant, C.V.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Manipulations of the Gut Microbiome Alter Chemotherapy-Induced Inflammation and Behavioral Side Effects in Female Mice. Brain Behav. Immun. 2021, 95, 401–412. [Google Scholar] [CrossRef]
- Tremlett, H.; Zhu, F.; Arnold, D.; Bar-Or, A.; Bernstein, C.N.; Bonner, C.; Forbes, J.D.; Graham, M.; Hart, J.; Knox, N.C.; et al. The Gut Microbiota in Pediatric Multiple Sclerosis and Demyelinating Syndromes. Ann. Clin. Transl. Neurol. 2021, 8, 2252–2269. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, H.; Yang, S.; Zeng, Y.; Li, W.; Wang, L.; Luo, G.; Fang, F.; Zeng, T.; Cheng, W. Salidroside Attenuates Cognitive Dysfunction in Senescence-Accelerated Mouse Prone 8 (SAMP8) Mice and Modulates Inflammation of the Gut-Brain Axis. Front. Pharmacol. 2020, 11, 568423. [Google Scholar] [CrossRef]
- Scassellati, C.; Marizzoni, M.; Cattane, N.; Lopizzo, N.; Mombelli, E.; Riva, M.A.; Cattaneo, A. The Complex Molecular Picture of Gut and Oral Microbiota-Brain-Depression System: What We Know and What We Need to Know. Front. Psychiatry 2021, 12, 722335. [Google Scholar] [CrossRef]
- Fang, P.; Kazmi, S.A.; Jameson, K.G.; Hsiao, E.Y. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe 2020, 28, 201–222. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Chattipakorn, N.; Chattipakorn, S.C. Possible Links between Gut–Microbiota and Attention-Deficit/Hyperactivity Disorders in Children and Adolescents. Eur. J. Nutr. 2020, 59, 3391–3403. [Google Scholar] [CrossRef]
- Bull-Larsen, S.; Mohajeri, M.H. The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 2019, 11, 2805. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.; Zhao, J.; Lai, W.; Wang, M.; Xu, D.; Liu, Y.; Guo, Y.; Xu, S.; Deng, W.; et al. Similarly in Depression, Nuances of Gut Microbiota: Evidences from a Shotgun Metagenomics Sequencing Study on Major Depressive Disorder versus Bipolar Disorder with Current Major Depressive Episode Patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef]
- Knuesel, T.; Mohajeri, M.H. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients 2021, 14, 37. [Google Scholar] [CrossRef]
- Knudsen, J.K.; Bundgaard-Nielsen, C.; Hjerrild, S.; Nielsen, R.E.; Leutscher, P.; Sørensen, S. Gut Microbiota Variations in Patients Diagnosed with Major Depressive Disorder—A Systematic Review. Brain Behav. 2021, 11, e02177. [Google Scholar] [CrossRef]
- Sublette, M.E.; Cheung, S.; Lieberman, E.; Hu, S.; Mann, J.J.; Uhlemann, A.; Miller, J.M. Bipolar Disorder and the Gut Microbiome: A Systematic Review. Bipolar. Disord. 2021, 23, 544–564. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Guo, R.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Metagenome-Wide Association of Gut Microbiome Features for Schizophrenia. Nat. Commun. 2020, 11, 1612. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Kang, Y.; Zhuo, C.; Huang, X.-F.; Song, X. The Gut Microbiota Promotes the Pathogenesis of Schizophrenia via Multiple Pathways. Biochem. Biophys. Res. Commun. 2019, 512, 373–380. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef]
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the Gut Microbiome in Autism Spectrum Disorders. Adv. Exp. Med. Biol. 2019, 1118, 253–269. [Google Scholar]
- Peralta-Marzal, L.N.; Prince, N.; Bajic, D.; Roussin, L.; Naudon, L.; Rabot, S.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci. 2021, 22, 10052. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.K.; Rose, D.; Ashwood, P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Thu Thuy Nguyen, V.; Endres, K. Targeting Gut Microbiota to Alleviate Neuroinflammation in Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2022, 188, 114418. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and Dysbiosis: The Two Sides of the Microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Ahmadi, S.; Nagpal, R.; Wang, S.; Gagliano, J.; Kitzman, D.W.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Read, R.; Yadav, H. Prebiotics from Acorn and Sago Prevent High-Fat-Diet-Induced Insulin Resistance via Microbiome-Gut-Brain Axis Modulation. J. Nutr. Biochem. 2019, 67, 1–13. [Google Scholar] [CrossRef]
- Ahmadi, A.R.; Sadeghian, M.; Alipour, M.; Taheri, S.A.; Rahmani, S.; Abbasnezhad, A. The Effects of Probiotic/Synbiotic on Serum Level of Zonulin as a Biomarker of Intestinal Permeability: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2020, 49, 1222–1231. [Google Scholar]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Playford, R.J.; Choudhry, N.; Kelly, P.; Marchbank, T. Effects of Bovine Colostrum with or without Egg on In Vitro Bacterial-Induced Intestinal Damage with Relevance for SIBO and Infectious Diarrhea. Nutrients 2021, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Chen, C.; Kling, D.E.; Liu, B.; McCoy, J.M.; Merighi, M.; Heidtman, M.; Newburg, D.S. The Principal Fucosylated Oligosaccharides of Human Milk Exhibit Prebiotic Properties on Cultured Infant Microbiota. Glycobiology 2013, 23, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Markowiak, P.; Ślizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Franco-Robles, E.; Ramírez-Emiliano, J.; Sergio López-Briones, J.; Doriany Balcón-Pacheco, C. Prebiotics and the Modulation on the Microbiota-GALT-Brain Axis. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Barile, D.; Marotta, M.; Chu, C.; Mehra, R.; Grimm, R.; Lebrilla, C.B.; German, J.B. Neutral and Acidic Oligosaccharides in Holstein-Friesian Colostrum during the First 3 Days of Lactation Measured by High Performance Liquid Chromatography on a Microfluidic Chip and Time-of-Flight Mass Spectrometry. J. Dairy Sci. 2010, 93, 3940–3949. [Google Scholar] [CrossRef]
- Tao, N.; DePeters, E.J.; German, J.B.; Grimm, R.; Lebrilla, C.B. Variations in Bovine Milk Oligosaccharides during Early and Middle Lactation Stages Analyzed by High-Performance Liquid Chromatography-Chip/Mass Spectrometry. J. Dairy Sci. 2009, 92, 2991–3001. [Google Scholar] [CrossRef]
- Tao, N.; DePeters, E.J.; Freeman, S.; German, J.B.; Grimm, R.; Lebrilla, C.B. Bovine Milk Glycome. J. Dairy Sci. 2008, 91, 3768–3778. [Google Scholar] [CrossRef]
- Divyashri, G.; Sadanandan, B.; Chidambara Murthy, K.N.; Shetty, K.; Mamta, K. Neuroprotective Potential of Non-Digestible Oligosaccharides: An Overview of Experimental Evidence. Front. Pharmacol. 2021, 12, 2035. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; Barile, D. Bovine Milk as a Source of Functional Oligosaccharides for Improving Human Health. Adv. Nutr. 2011, 2, 284–289. [Google Scholar] [CrossRef]
- Gopal, P.K.; Gill, H.S. Oligosaccharides and Glycoconjugates in Bovine Milk and Colostrum. Br. J. Nutr. 2000, 84, 69–74. [Google Scholar] [CrossRef]
- Karav, S.; le Parc, A.; Leite Nobrega de Moura Bell, J.M.; Frese, S.A.; Kirmiz, N.; Block, D.E.; Barile, D.; Mills, D.A. Oligosaccharides Released from Milk Glycoproteins Are Selective Growth Substrates for Infant-Associated Bifidobacteria. Appl. Environ. Microbiol. 2016, 82, 3622–3630. [Google Scholar] [CrossRef]
- Roberts, E.; Frankel, S. γ-aminobutyric acid in brain: Its formation from glutamic acid. J. Biol. Chem. 1950, 187, 55–63. [Google Scholar] [CrossRef]
- Cryan, J.F.; Kaupmann, K. Don’t Worry ‘B’ Happy!: A Role for GABAB Receptors in Anxiety and Depression. Trends Pharmacol. Sci. 2005, 26, 36–43. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-Modulating Bacteria of the Human Gut Microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review. Microorganisms 2022, 10, 2268. [Google Scholar] [CrossRef]
- Noonan, S.; Zaveri, M.; Macaninch, E.; Martyn, K. Food & Mood: A Review of Supplementary Prebiotic and Probiotic Interventions in the Treatment of Anxiety and Depression in Adults. BMJ Nutr. Prev. Health 2020, 3, 351–362. [Google Scholar] [CrossRef]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions. Nutrients 2022, 14, 2512. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancrediid, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot Study of Probiotic/Colostrum Supplementation on Gut Function in Children with Autism and Gastrointestinal Symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).