Therapeutic Approach for Trigeminal Neuralgia: A Systematic Review
Abstract
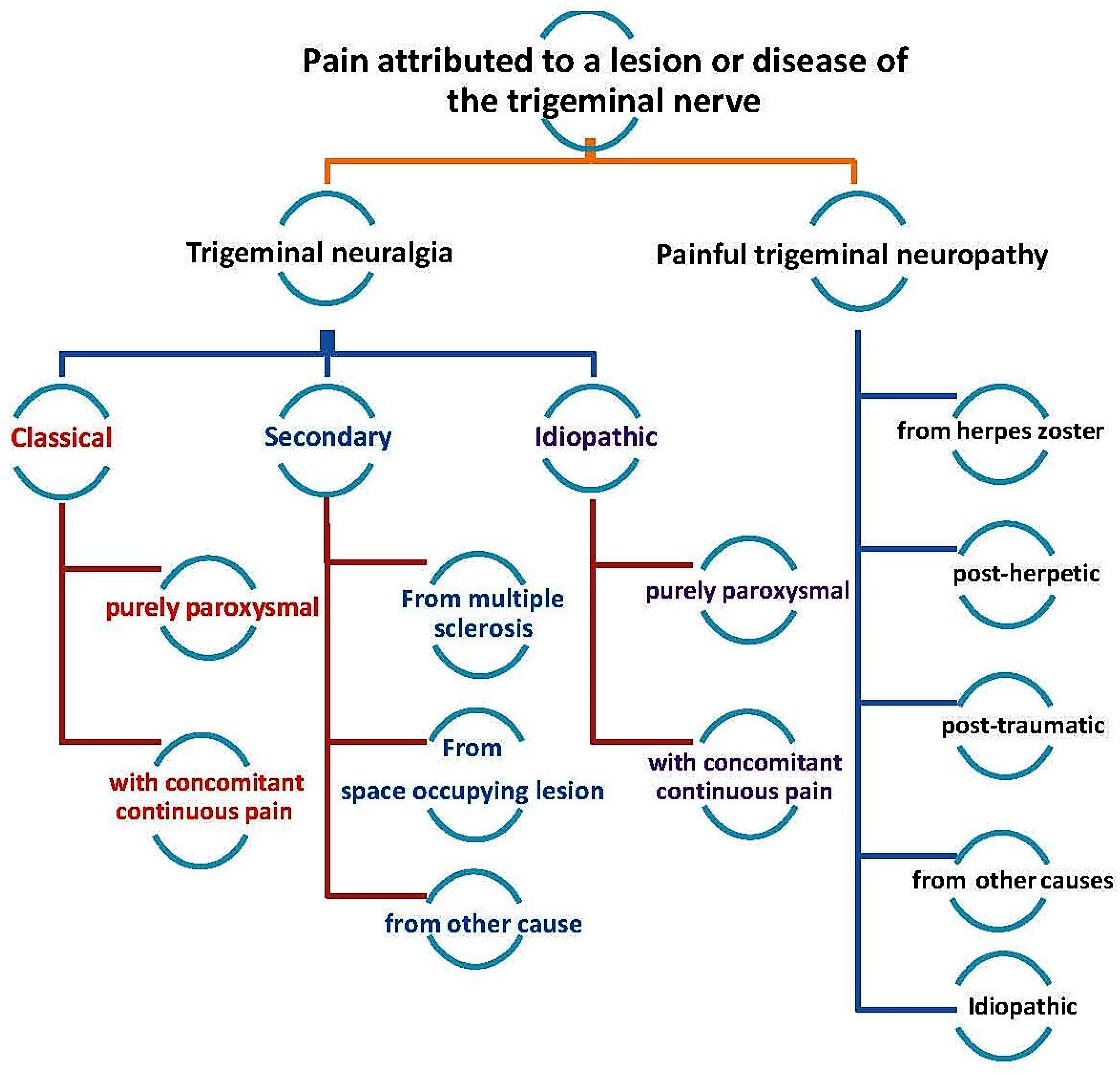
:1. Introduction
2. Methodology
Data Synthesis
3. Results
3.1. Additional Information about the Drugs Included in the Review Articles
3.1.1. Carbamazepine and Oxcarbazepine
3.1.2. Eslicarbazepine
3.1.3. Gabapentin
3.1.4. Ropivacaine and Gabapentin
3.1.5. Ropivacaine and Carbamazepine
3.1.6. Lamotrigine
3.1.7. Levetiracetam
3.1.8. Phenytoin
3.1.9. Pregabalin
3.1.10. Topiramate
3.1.11. Proparacaine
3.1.12. Dextromethorphan
3.1.13. Tizanidine
3.1.14. Pimozide
3.1.15. Tocainide
3.1.16. Lidocaine
3.1.17. Baclofen
3.1.18. Sumatriptan
3.1.19. Botulinum Toxin A
3.1.20. Minocycline
3.1.21. Novel Agents Used to Treat TN
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, R.D. Principles of Neurology; Health Professions Division, McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Bista, P.; Imlach, W.L. Pathological Mechanisms and Therapeutic Targets for Trigeminal Neuropathic Pain. Medicines 2019, 6, 91. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, Q.; Chen, J.; Li, L.; Wang, D.; Zhou, J. International Classification of Headache Disorders 3rd edition beta-based field testing of vestibular migraine in China: Demographic, clinical characteristics, audiometric findings and diagnosis statues. Cephalalgia 2016, 36, 240–248. [Google Scholar] [CrossRef]
- Eide, P.K. Familial occurrence of classical and idiopathic trigeminal neuralgia. J. Neurol. Sci. 2022, 434, 120101. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Chong, M.S.; Shetty, A.; Zakrzewska, J.M. A systematic review of rescue analgesic strategies in acute exacerbations of primary trigeminal neuralgia. Br. J. Anaesth. 2019, 123, e385–e396. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, G.; Truini, A.; Cruccu, G. Current and Innovative Pharmacological Options to Treat Typical and Atypical Trigeminal Neuralgia. Drugs 2018, 78, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Wiffen Philip, J.; Derry, S.; Moore, R.A.; KalsoEija, A. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014, CD005451. [Google Scholar]
- Wang, Q.P.; Bai, M. Topiramate versus carbamazepine for the treatment of classical trigeminal neuralgia: A meta-analysis. CNS Drugs 2011, 25, 847–857. [Google Scholar] [CrossRef]
- Yuan, M.; Zhou, H.Y.; Xiao, Z.L.; Wang, W.; Li, X.L.; Chen, S.J.; Yin, X.-P.; Xu, L.-J. Efficacy and Safety of Gabapentin vs. Carbamazepine in the Treatment of Trigeminal Neuralgia: A Meta-Analysis. Pain Pract. 2016, 16, 1083–1091. [Google Scholar] [CrossRef]
- Steinberg, D.I. Review: In trigeminal neuralgia, carbamazepine, botulinum toxin type A, or lidocaine improve response rate vs placebo. ACP J. Club. 2018, 169, C43-JC. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, R.; Mehta, N.; Gungor, S.; Gulati, A. A Systematic Review of NMDA Receptor Antagonists for Treatment of Neuropathic Pain in Clinical Practice. Clin. J. Pain. 2018, 34, 450–467. [Google Scholar] [CrossRef]
- Corrigan, R.; Derry, S.; Wiffen, P.J.; Moore, R.A. Clonazepam for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, CD009486. [Google Scholar] [CrossRef] [PubMed]
- de León-Casasola, O.A.; Mayoral, V. The topical 5% lidocaine medicated plaster in localized neuropathic pain: A reappraisal of the clinical evidence. J. Pain. Res. 2016, 9, 67–79. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Moore, R.A.; Quinlan, J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014, Cd010958. [Google Scholar]
- Plested, M.; Budhia, S.; Gabriel, Z. Pregabalin, the lidocaine plaster and duloxetine in patients with refractory neuropathic pain: A systematic review. BMC Neurol. 2010, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Masic, D.; Liang, E.; Long, C.; Sterk, E.J.; Barbas, B.; Rech, M.A. Intravenous Lidocaine for Acute Pain: A Systematic Review. Pharmacotherapy 2018, 38, 1250–1259. [Google Scholar] [CrossRef]
- Shaheen, A.; Alam, S.M.; Ahmad, A.; Khan, M. Clinical efficacy and tolerability of Gabapentinoids with current prescription patterns in patients with Neuropathic pain. Pak. J. Med. Sci. 2019, 35, 1505–1510. [Google Scholar] [CrossRef]
- Raj Paudel, K.; Bhattacharya, S.K.; Rauniar, G.P.; Das, B.P. Comparison of antinociceptive effect of the antiepileptic drug gabapentin to that of various dosage combinations of gabapentin with lamotrigine and topiramate in mice and rats. J. Neuro. Rural. Pract. 2011, 2, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Banday, M.; Syed Sameer, A.; Farhat, S.; Aziz, R. Gabapentin: A pharmacotherapeutic panacea. Int. J. Pharm. Pharm. Sci. 2013, 5, 84–94. [Google Scholar]
- Wiffen, P.J.; Derry, S.; Bell, R.F.; Rice, A.S.C.; Tölle, T.R.; Phillips, T.; Moore, R.A. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, CD007938. [Google Scholar] [CrossRef]
- Moore, R.A.; Wiffen, P.J.; Derry, S.; Rice, A.S.C. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014, CD007938. [Google Scholar] [CrossRef]
- Ta, P.C.P.; Dinh, H.Q.; Nguyen, K.; Lin, S.; Ong, Y.L.; Ariyawardana, A. Efficacy of gabapentin in the treatment of trigeminal neuralgia: A systematic review of randomized controlled trials. J. Investig. Clin. Dent. 2019, 10, 4. [Google Scholar] [CrossRef]
- Hearn, L.; Derry, S.; Moore, R.A. Lacosamide for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, CD009318. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, CD008242. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Lamotrigine for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2013, Cd006044. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Lunn, M.P.T. Levetiracetam for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014, CD010943. [Google Scholar] [CrossRef]
- Lunn, M.P.T.; Hughes, R.A.C.; Wiffen, P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev. 2014, CD007115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, N.; He, L.; Yang, M.; Zhu, C.; Wu, F. Oxcarbazepine for neuropathic pain. Cochrane Database Syst. Rev. 2017, Cd007963. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Phillips, T.; Moore, R.A.; Wiffen, P.J. Milnacipran for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, CD011789. [Google Scholar] [CrossRef]
- Birse, F.; Derry, S.; Moore, R.A. Phenytoin for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, Cd009485. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, Cd011209. [Google Scholar] [CrossRef]
- Blanco Tarrio, E.; GálvezMateos, R.; Zamorano Bayarri, E.; López Gómez, V.; Pérez Páramo, M. Effectiveness of Pregabalin as Monotherapy or Combination Therapy for Neuropathic Pain in Patients Unresponsive to Previous Treatments in a Spanish Primary Care Setting. Clin. Drug Investig. 2013, 33, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst. Rev. 2019, CD007076. [Google Scholar] [CrossRef] [PubMed]
- Dauri, M.; Lazzari, M.; Casali, M.; Tufaro, G.; Sabato, E.; Sabato, A. Long-Term Efficacy of OROS Hydromorphone Combined with Pregabalin for Chronic Non-Cancer Neuropathic Pain. Clin. Drug Investig. 2014, 34, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, H.C.; Gallagher, R.M.; Butler, M.; Buggy, D.J.; Henman, M.C. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, Cd011091. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Lunn, M.P.T.; Moore, R.A. Topiramate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2013, CD008314. [Google Scholar] [CrossRef]
- Hearn, L.; Moore, R.A.; Derry, S.; Wiffen, P.J.; Phillips, T. Desipramine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014, CD011003. [Google Scholar] [CrossRef]
- Gill, D.; Derry, S.; Wiffen, P.J.; Moore, R.A. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2011, CD009183. [Google Scholar] [CrossRef]
- Seidel, S.; Aigner, M.; Ossege, M.; Pernicka, E.; Wildner, B.; Sycha, T. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst. Rev. 2013, CD004844. [Google Scholar] [CrossRef]
- Moore, R.A.; Wiffen, P.J.; Derry, S.; Lunn, M.P. Zonisamide for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, CD011241. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, A.; Woron, J.; Dobrogowski, J.; Jakowicka-Wordliczek, J.; Wordliczek, J. Topical clonidine for neuropathic pain. Cochrane Database Syst. Rev. 2015, CD010967. [Google Scholar] [CrossRef]
- Duehmke, R.M.; Derry, S.; Wiffen, P.J.; Bell, R.F.; Aldington, D.; Moore, R.A. Tramadol for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, CD003726. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane Database Syst. Rev. 2019, CD010528. [Google Scholar] [CrossRef]
- Aiyer, R.; Gulati, A.; Gungor, S.; Bhatia, A.; Mehta, N. Treatment of chronic pain with various buprenorphine formulations: A systematic review of clinical studies. Anesth. Analg. 2018, 127, 529–538. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Stannard, C.; Aldington, D.; Cole, P.; Knaggs, R. Buprenorphine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, CD011603. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Chi, C.C.; Wiffen, P.J.; Derry, S.; Rice, A.S. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst. Rev. 2015, CD010902. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Knaggs, R.; Derry, S.; Cole, P.; Phillips, T.; Moore, R.A. Paracetamol [acetaminophen] with or without codeine or dihydrocodeine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2016, CD012227. [Google Scholar] [CrossRef]
- Derry, S.; Stannard, C.; Cole, P.; Wiffen, P.J.; Knaggs, R.; Aldington, D.; Moore, R.A. Fentanyl for neuropathic pain in adults. Cochrane Database Syst. Rev. 2016, CD011605. [Google Scholar] [CrossRef]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, CD012182. [Google Scholar] [CrossRef]
- Stannard, C.; Gaskell, H.; Derry, S.; Aldington, D.; Cole, P.; Cooper, T.E.; Knaggs, R.; Wiffen, P.J.; Moore, R.A. Hydromorphone for neuropathic pain in adults. Cochrane Database Syst. Rev. 2016, CD011604. [Google Scholar] [CrossRef]
- Derry, S.; Moore, R.A. Topical capsaicin [low concentration] for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, CD010111. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Rice, A.S.C.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin [high concentration] for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, CD007393. [Google Scholar] [CrossRef]
- McNicol, E.D.; Ferguson, M.C.; Schumann, R. Methadone for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, CD012499. [Google Scholar] [CrossRef]
- Cooper, T.E.; Chen, J.; Wiffen, P.J.; Derry, S.; Carr, D.B.; Aldington, D.; Cole, P.; Moore, R.A. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, CD011669. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, H.; Derry, S.; Stannard, C.; Moore, R.A. Oxycodone for neuropathic pain in adults. Cochrane Database Syst. Rev. 2016, CD010692. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, H.; Moore, R.A.; Derry, S.; Stannard, C. Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014, CD010692. [Google Scholar] [CrossRef]
- Azevedo Alves, T.C.; Santos Azevedo, G.; Santiago de Carvalho, E. Pharmacological treatment of trigeminal neuralgia: Systematic review and metanalysis. Revista Anest. 2004, 54, 842–849. [Google Scholar]
- Finnerup, N.B.; Sindrup, S.H.; Jensen, T.S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, R.; Oh, U.; Yang, S.; Lapane, K.L. A systematic review of pharmacological pain management in multiple sclerosis. Drugs 2013, 73, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, M.; Zhou, M.; He, L.; Chen, N.; Zakrzewska, J.M. Non-antiepileptic drugs for trigeminal neuralgia. Cochrane Database Syst. Rev. 2013, Cd004029. [Google Scholar] [CrossRef]
- Yang, F.; Lin, Q.; Dong, L.; Gao, X.; Zhang, S. Efficacy of 8 different drug treatments for patients with trigeminal neuralgia: A network meta-analysis. Clin. J. Pain. 2018, 34, 685–690. [Google Scholar] [CrossRef]
- Vadalouca, A.; Siafaka, I.; Argyra, E.; Vrachnou, E.V.I.; Moka, E. Therapeutic Management of Chronic Neuropathic Pain. Ann. N. Y. Acad. Sci. 2006, 1088, 164–186. [Google Scholar] [CrossRef] [PubMed]
- Murnion, B.P. Neuropathic pain: Current definition and review of drug treatment. Aust. Prescr. 2018, 41, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Henze, T.; Rieckmann, P.; Toyka, K.V. Symptomatic treatment of multiple sclerosis: Multiple Sclerosis Therapy Consensus Group [MSTCG] of the German Multiple Sclerosis Society. Euro Neuro 2006, 56, 78–105. [Google Scholar] [CrossRef]
- Chole, R.; Patil, R.; Degwekar, S.S.; Bhowate, R.R. Drug treatment of trigeminal neuralgia: A systematic review of the literature. J. Oral Maxillofac. Surg. 2007, 65, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, L.E.; Wiffen, P.J.; Moore, R.A.; Gilron, I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst. Rev. 2012, CD008943. [Google Scholar] [CrossRef]
- Benoliel, R.; Zini, A.; Khan, J.; Almoznino, G.; Sharav, Y.; Haviv, Y. Trigeminal neuralgia [part II]: Factors affecting early pharmacotherapeutic outcome. Cephalalgia 2016, 36, 747–759. [Google Scholar] [CrossRef]
- Backonja, M.-M.; Serra, J. REVIEW PAPERS Pharmacologic Management Part 1: Better-Studied Neuropathic Pain Diseases. Pain Med. 2004, 5, S28–S47. [Google Scholar] [CrossRef] [PubMed]
- Oomens, M.; Forouzanfar, T. Pharmaceutical Management of Trigeminal Neuralgia in the Elderly. Drugs Aging 2015, 32, 717–726. [Google Scholar] [CrossRef]
- Bendtsen, L.; Zakrzewska, J.M.; Abbott, J.; Braschinsky, M.; Di Stefano, G.; Donnet, A.; Eide, P.K.; Leal, P.R.L.; Maarbjerg, S.; May, A.; et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur. J. Neurol. 2019, 26, 831–849. [Google Scholar] [CrossRef]
- Gagpal, K.; Singh, S.K.; Mishra, D.N. Minocycline encapsulated chitosan nanoparticles for central antinociceptive activity. Int. J. Biol. Macromol. 2015, 72, 131–135. [Google Scholar] [CrossRef]
- Papadakis, M.A.; McPhee, S.J. (Eds.) Trigeminal Neuralgia; Quick Medical Diagnosis & Treatment; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Du, Z.; Zhang, J.; Han, X.; Yu, W.; Gu, X. Potential novel therapeutic strategies for neuropathic pain. Front. Mol. Neurosci. 2023, 16, 1138798. [Google Scholar] [CrossRef]
- Katsuki, M.; Kawamura, S.; Kashiwagi, K.; Tachikawa, S.; Koh, A. Erenumab in the treatment of comorbid trigeminal neuralgia in patients with migraine. Cureus 2023, 15, e35913. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Sivaramakrishnan, G. Interventions for Refractory Trigeminal Neuralgia: A Bayesian Mixed Treatment Comparison Network Meta-Analysis of Randomized Controlled Clinical Trials. Clin. Drug Investig. 2017, 37, 819–831. [Google Scholar] [CrossRef]
- Di Stefano, G.; Truini, A. Pharmacological treatment of trigeminal neuralgia. Expert Rev. Neurother. 2017, 17, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Rubis, A.; Juodzbalys, G. The Use of Botulinum Toxin A in the Management of Trigeminal Neuralgia: A Systematic Literature Review. J. Oral Maxillofac. Res. 2020, 11, e2. [Google Scholar] [PubMed]
- Cruccu, G.; Gronseth, G.; Alksne, J.; Argoff, C.; Brainin, M.; Burchiel, K.; Nurmikko, T.; Zakrzewska, J.M. AAN-EFNS guidelines on trigeminal neuralgia management. Eur. J. Neurol. 2008, 15, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; Conforti, G.; Di Bonaventura, R.; Meglio, M.; Fernandez, E.; Papacci, F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther. Clin. Risk Manag. 2015, 24, 289–299. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, X.; Fan, L.; Li, M.; Liao, Y.; Nie, Z.; Jin, L. Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: A systematic review. J. Headache Pain 2013, 14, 72. [Google Scholar] [CrossRef] [PubMed]

| Category | Drug | Category | Drug |
|---|---|---|---|
| Antiepileptic | Carbamazepine [6,7,8,9] | Anesthetics | Ketamine *, Methadone * [10] |
| Clonazepam [11] | Lidocaine [12,13,14,15] | ||
| Gabapentin [8,16,17,18,19,20,21] | |||
| Lacosamide [22] | Amitriptyline [23] | ||
| Lamotrigine [17,24] | Antidepressants | ||
| Levetiracetam [25] | Duloxetine [14,26] | ||
| Oxcarbazepine [27] | Milnacipran [28] | ||
| Phenytoin [29] | Nortriptyline [30] | ||
| Pregabalin [14,31,32,33] | Venlafaxine [34] | ||
| Topiramate [7,17,35] | Desipramine [36] | ||
| Valproic acid [37] | Antipsychotics [38] | ||
| Zonisamide [39] | Alpha-adrenoceptor stimulants | Clonidine [40] | |
| Opioids | Tramadol [41] | ||
| Herbal [42] | Buprenorphine [43,44] | ||
| NSAIDs [45] | Paracetamol [46] | Fentanyl [47] | |
| Miscellaneous | Cannabis [48] | Hydromorphone [33,49] | |
| Capsaicin [50,51] | Methadone [52] | ||
| Morphine [53] | |||
| Oxycodone [54,55] |
| Participants | Intervention | Control | Primary Outcomes | Secondary Outcomes | Study Design |
|---|---|---|---|---|---|
| Patients who are undergoing/underwent treatment for TN, irrespective of age, gender, and ethnicity. | All drugs used for the treatment of TN, irrespective of the route of administration (Table 1). | Pain relief with orthodox analgesics/opioids/placebo/another active drug. | 1. Intensity of pain quantitatively assessed using the visual analog scale, numerical rating scale. 2. Incidence of painful episodes. | 1. Therapeutic success based on the subjects’ reporting. 2. Quality of life measure. 3. Adverse effects. | Reviews and meta-analysis. |
| Interface | Database | Search Query | Article Output |
|---|---|---|---|
| National Library of Medicine | PubMed | Search: (trigeminal neuralgia) AND (pharmacological) AND (meta-analysis[Filter) OR review (Filter) OR (trigeminal neuralgia) AND (drug) AND (meta-analysis) Filter OR review (Filter) OR (trigeminal neuralgia) AND (medical) AND meta-analysis (Filter) OR review (Filter) OR (tic douloureux) AND (pharmacological) AND meta-analysis (Filter) OR review (Filter) OR (tic douloureux) AND (drug) AND meta-analysis (Filter) OR review (Filter) OR (tic douloureux) AND (medical) AND meta-analysis (Filter) OR review (Filter) Filters: Meta-Analysis, Review | 85 |
| CRD database center for reviews and dissemination (available till 2015) | DARE (Database of Abstracts of Reviews of Effects), NHS EED (National Health Services Economic Evaluation Database), and HTA (Health Technology Assessment) | Trigeminal neuralgia (or) tic douloureux AND pharmacological OR Trigeminal neuralgia (or) tic douloureux AND drug OR Trigeminal neuralgia (or) tic douloureux AND medical | 25 |
| EBSCO | Academic Search Ultimate, CINAH, MEDLINE, and Dentistry & Oral Sciences Source | “Trigeminal neuralgia OR tic douloureux AND systematic review AND pharmacological Scholarly (Peer Reviewed0 Journals AND Apply equivalent subjects Limiters– Scholarly [Peer Reviewed] Journals Expanders– Apply equivalent subjects Narrow by Subject Thesaurus: pregabalin, placebos, phenytoin, nonopioid analgesics nerve block, medical marijuana, local anesthetics, duloxetine, clonidine, baclofen, anticonvulsants, amitriptyline, indomethacin, hyponatremia, gabapentin, drug therapy, combination drug therapy, antidepressants, sodium channel blockers, pain management, lidocaine, lamotrigine, carbamazepine, therapeutics, trigeminal neuralgia. Narrow by Language: English Search modes– Find all my search terms | 83 |
| Web of Science | SCI-Expanded, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI, and CCR-Expanded | TS = Trigeminal neuralgia OR tic douloureux AND AB = pharmacological TS = [Trigeminal neuralgia * OR tic douloureux AND AB = drug TS = Trigeminal neuralgia * OR tic douloureux AND AB = medical LANGUAGE: [English]; DOCUMENT TYPES: [Review] IC Timespan = All years | 235 |
| Scopus | Scopus | ALL “Trigeminal neuralgia” and TITLE-ABS “pharmacological” and d TITLE-ABS [metanalysis] OR [ALL [“Trigeminal neuralgia”] AND TITLE-ABS [“drug”] AND d TITLE-ABS [metanalysis] OR [ALL [“Trigeminal neuralgia”] AND TITLE-ABS [“medical”] AND d TITLE-ABS [metanalysis] OR ALL [“Trigeminal neuralgia”] and TITLE-ABS [“pharmacological”] AND d TITLE-ABS [systematic AND review] OR ALL [“Trigeminal neuralgia”] AND TITLE-ABS [“drug”] and d TITLE-ABS [systematic AND review] OR ALL [“Trigeminal neuralgia”] and TITLE-ABS [“medical”] AND d TITLE-ABS [systematic AND review]]] | 103 |
| Cochrane Library | Cochrane Database of Systematic Reviews | Trigeminal neuralgia [or] tic douloureux AND pharmacological OR Trigeminal neuralgia [or] tic douloureux AND drug OR Trigeminal neuralgia [or] tic douloureux AND medical | 57 |
| TOTAL | 588 | ||
| Drug | Control | Authors—Year—Ref | Reported Level of Evidence | Efficacy of the Drug over Control | Adverse Effects | Study Conclusions | Our Comments |
|---|---|---|---|---|---|---|---|
| Carbamazepine |
| Yang, F. et al., 2018 [60]; Di Stefano, G. et al., 2018 [5] | High | Yes | Headache, reduced neuromuscular coordination, vertigo, sleepiness, nausea and vomiting, nephrosis, arrhythmias, constipation, dermatological reactions [68] | While carbamazepine and oxcarbazepine are successful in most patients, the side effects may discourage the patients from continuing the treatment, especially older adults. | Carbamazepine and oxcarbazepine continue to be the first drug of choice for trigeminal neuralgia. However, the incidence of side effects in the patients needs to be monitored, particularly in elderly patients. |
| Yang, F. et al., 2018 [60] | Based on network meta-analysis (NMA) | Yes | ||||
| Oxcarbazepine |
| Yang, F. et al., 2018 [60] | Based on NMA | Yes | Similar to but milder than that of oxcarbazepine [5] | Oxcarbazepine has lesser drug interactions than carbamazepine. | Carbamazepine and oxcarbazepine continue to be the first drug of choice for trigeminal neuralgia. However, the incidence of side effects in the patients needs to be monitored, particularly in elderly patients. |
| Di Stefano, G. et al., 2018 [5] | Low | Similar | Safer than carbamazepine | Effective in classical TN. However, the efficacy is diminished in continuous concomitant pain. | The dissimilarity in the conclusions is due to differences in the source of the data. The Di Stefano conclusion was based on one randomized controlled trial, whereas the review by Yang F. et al. included two studies, one double-blind RCT and one retrospective study. | |
| Yang, F. et al., 2018 [60] | Based on NMA | No | Safer than carbamazepine | Oxcarbazepine can be chosen for fewer drug interactions and better tolerability. | |||
| Yang, F. et al., 2018 [60] | Based on NMA | Yes | Not mentioned | Not mentioned. | ||
| Eslicarbazepine | None | Di Stefano, G. et al., 2018 [5] | Low: retrospective, open-label, low participant count | Yes | Hyponatremia | Both the American Academy of Neurology Society and the European Federation of Neurological Society (AAN-EFNS) neither endorse nor refute the use of this drug in secondary TN. | It may be helpful in secondary TN and refractory TN. Needs further studies. |
| Gabapentin |
| Di Stefano, G. et al., 2018; Yaun et al., 2016 [5,8] | Low: methodological deficiencies | Similar | Sleepiness, vertigo, diminished neuromuscular control, fatigue diplopia, hand tremor; the side effects are lower than those observed with carbamazepine; negligible drug interactions [21] | The superiority of gabapentin over carbamazepine could not be determined. One of the second-line drugs for secondary TN [5]. | The data were deficient in determining the efficacy. Thus, additional studies are necessary. |
| Ta, P.C.P. et al., 2019 [21] | Low: methodological deficiencies and small participant size, short duration | No | A comparison of side effects with oxcarbazepine is not included in the study | Though gabapentin was effective, it was not superior to oxcarbazepine [21]. Gabapentin could be useful in relieving concomitant continuous pain. Can be used in combination with carbamazepine or oxcarbazepine. | Additional methodologically sound randomized controlled trials are needed. | |
| Ropivacaine + gabapentin | Gabapentin alone | Ta, P.C.P. et al., 2019 [21]; Di Stefano, G. et al., 2018 [5] | Methodologically sound, but low participant count; to date, only one study is available | Yes | Not specified but the side effects were less than gabapentin alone | Effective even after 11 months in many cases. Analgesia, quality of life scores, and oral functions were enhanced. | Though the study was well designed, further studies are needed on a sizable population. |
| Ropivacaine (nerve block) + carbamazepine (oral) | Carbamazepine | Di Stefano, G. et al., 2018 [5] | Methodologically sound but low participant count; to date, only one study is available | Yes | Less than carbamazepine | Substantial reduction in the pain intensity and daily incidences despite reducing the daily dose of carbamazepine. | The combination therapy can reduce the side effects and limitations of carbamazepine. |
| Lamotrigine |
| Yang, F. et al., 2018 [60] | NMA | Yes | Dermatological reactions, vertigo, headache, constipation, nausea, dysgeusia, mental distress [68] | One of the second-line drugs for secondary TN [5]. The EFNS 2010 advocated the use of lamotrigine for intractable trigeminal neuralgia [70]. | The EFNS advocates lamotrigine for intractable cases. |
| Yang, F. et al., 2018 [60]; Di Stefano et al., 2017 [5] | NMA | No | Less than carbamazepine [68] | Not discussed. | ||
| Yang, F. et al., 2018 [60] | NMA | No | Not mentioned | Not discussed. | ||
| Lamotrigine + carbamazepine or phenytoin | Placebo | Di Stefano, G. et al., 2018 [5] | Low | Yes, slightly more effective | Not mentioned in the study | Not discussed. | |
| Levetiracetam | Low: open-label, unspecified control/observational trial. | Di Stefano, G. et al., 2018 [5] | Low | Yes | Not mentioned in the study | Not discussed. | It can be used in classical TN and refractory TN |
| Phenytoin | Low: there were no methodologically sound studies to date. | Di Stefano, G. et al., 2018 [5] | None | Yes? | Not mentioned in the study | Not discussed. | Phenytoin was the foremost drug used for TN, yielding encouraging results. However, additional methodologically sound, randomized controlled trials are needed. |
| Pregabalin |
| Di Stefano, G. et al., 2018 [5] | Low | Yes | Not mentioned in the study | Pregabalin may be effective in relieving concomitant continuous pain. Combination with carbamazepine or oxcarbazepine is recommended. | Additional methodologically sound, randomized controlled trials are needed. |
| Sridharan and Sivaramakrishnan 2016 [70] | Low | Yes; according to the RCT | Side effects of pregabalin are less than lamotrigine | The reviewers could not perform a meta-analysis on this comparison. | Further studies on larger sample sizes are needed. | |
| Pregabalin + carbamazepine | Lamotrigine + carbamazepine | Di Stefano, G. et al., 2018 [5] | Low: open-label, low participant count | Similar | Pregabalin produced fewer adverse effects than lamotrigine | The combination can be used in refractory TN. | Effective in the case of refractory trigeminal neuralgia. |
| Topiramate |
| Alves TCA 2004 [56] | No | Nausea, diarrhea, tiredness, drowsiness, and diminished cognition; however, no patient dropped out [56] | One of the second-line drugs for secondary TN [5]. | The findings in the review by Alves et al. are from one study only. The later studies reported positive results. | |
| Wang et al., 2011 [7] | From NMA. Low: quality deficient in methods and topography of the study | Yes [after 2 months] | Similar | The initial efficacy and sides effects were similar. | Further studies are needed. | |
| Proparacaine |
| Yang, F. et al., 2018 [60]; Zhang 2013 [59]; Alves TCA 2004 [56] | High (low risk of bias) | No | Negligible [68] | Proparacaine might not be producing long-lasting actions. A combination with lidocaine could be investigated [56]. | Based on the evidence, proparacaine could be ruled out for use in TN. |
| Yang, F. et al., 2018 [60] | NMA | No | Not mentioned | Proparacaine produced inferior results to placebo. | ||
| Yang, F. et al., 2018 [60] | NMA | No | ||||
| Dextromethorphan | Lorazepam | Alves TCA 2004 [56] | No | Diminished cognition, dizziness, and ataxia; no patient dropouts [56] | Many patients under dextromethorphan reported a rise in pain. | The drug was proven to be inefficient. | |
| Tizanidine |
| Yang, F. et al., 2018 [60] | NMA | Yes | Vertigo and tiredness [68] | Not discussed. | Extensive sample-sized studies are required. |
| Yang, F. et al., 2018 [60]; Di Stefano, G. et al., 2018 [5]; Zhang et al. 2013 [59] | NMA (Yang et al.); small sample size | No/No significant difference | Side effects of tizanidine are fewer than those of carbamazepine [59] | Extensive sample-sized studies are required. | ||
| Pimozide |
| Yang, F. et al., 2018 [60] | NMA | No | Physical and psychological impedance, hand shivers, diminishing cognition [68] | Not discussed. | The finding was based on NMA. The authors, however, did not discuss the disagreement with other studies. |
| Yang, F. et al., 2018 [60] | NMA | No | According to this NMA, the effect of pimozide was inferior to those of the other drugs compared, including the placebo | Not discussed. | Based on NMA, the findings contradict those of Di Stefano, G. et al., 2018 and Zhang et al., 2013. However, the review did not discuss the conflict. | |
| Alves TCA 2004 [56]; Di Stefano, G. et al., 2018 [5]; Zhang et al., 2013 [59] | Low | Yes | The side effects of pimozide were worse than carbamazepine [68] | One review advocates lamotrigine and pimozide as second-line drugs for refractory cases [56]. | The findings are contradictory to those of Yang F 2018. Lamotrigine and pimozide can be considered as one of the second-line drugs for intractable cases. | ||
| Tocainide | Carbamazepine | Alves TCA 2004 [56]; Di Stefano, G. et al., 2018 [5]; Zhang et al., 2013 [59] | Low | No/Inconclusive | Nausea, paresthesia, and dermatological reactions [68] | Significant side effects limited its use [5,59]. | Based on the evidence, the drug is not beneficial for the treatment of TN. |
| Lidocaine 8% topical |
| Yang, F. et al., 2018 [60]; Di Stefano, G. et al., 2018 [5]; Sridharan et al., 2016 [70] | NMA, low participant count | Yes | Transient local irritation [68] | The analgesia lasted for 3–4 h after application. | The studies have several shortcomings; the results need to be verified with further studies. |
| Yang, F. et al., 2018 [60] | NMA | Yes | Not discussed. | |||
| Yang, F. et al., 2018 [60] | NMA | Yes | ||||
| Yang, F. et al., 2018 [60] | NMA | Yes | ||||
| Lidocaine (i.v. 5 mg/kg over 60 min) | Placebo | Di Stefano, G. et al., 2018 [5]; Sridharan and Sivaramakrishnan 2016 [70] | Low participant count | Yes | The side effects were mild and included sleepiness, xerostomia, vertigo, and headache | The analgesia lasted for 24 h. | This technique can be used in acute exacerbations and refractory cases. |
| Baclofen | Placebo | Di Stefano, G. et al., 2018 [5] | Low: low participant count and treatment duration | Yes | Diminished neuromuscular control, tiredness, and nausea [68] | The studies have several shortcomings; hence, the findings should be carefully inferred. | It may be beneficial in refractory TN. Further studies are needed to ascertain the findings. |
| Baclofen + carbamazepine or phenytoin | Placebo | Di Stefano, G. et al., 2017 [71] | Low | Yes | Remissions may occur | It may be beneficial in refractory TN. | |
| Sumatriptan (subcutaneous inj. 3 mg + oral 50 mg) twice daily |
| Di Stefano, G. et al., 2018 [5]; Sridharan and Sivaramakrishnan 2016 [70] | Low [70] | Yes | Tiredness and nausea [68] It is not suggested for long-standing prescription due to triptan-related side effects [5]. | Sridharan and Sivaramakrishnan advocated this as the best drug for intractable TN [70]. | Further studies are needed to ascertain the benefits and possibility of lowering the dose. |
| Sridharan and Sivaramakrishnan 2016 [70] | Low | Yes | ||||
| Botulinum toxin A Subcutaneous/ Dose: 10 IU to 75 IU | Placebo | Rubies et al., 2020 [72]; Sridharan and Sivaramakrishnan 2016 [70] | Unclear risk of bias | Yes | Induced facial asymmetry, headaches, hematoma, local irritation, and pain [68] | It may be given intramuscularly for the mandibular nerve. The side effects usually disappear within a week. | It is a favorable option in patients with intractable TN. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.H.; Khan, A.A.G.; Khalid, I.; Ishfaq, M.; Javali, M.A.; Baig, F.A.H.; Kota, M.Z.; Khader, M.A.; Hameed, M.S.; Shaik, S.; et al. Therapeutic Approach for Trigeminal Neuralgia: A Systematic Review. Biomedicines 2023, 11, 2606. https://doi.org/10.3390/biomedicines11102606
Rana MH, Khan AAG, Khalid I, Ishfaq M, Javali MA, Baig FAH, Kota MZ, Khader MA, Hameed MS, Shaik S, et al. Therapeutic Approach for Trigeminal Neuralgia: A Systematic Review. Biomedicines. 2023; 11(10):2606. https://doi.org/10.3390/biomedicines11102606
Chicago/Turabian StyleRana, Muhammad Haseeb, Abdul Ahad Ghaffar Khan, Imran Khalid, Muhammad Ishfaq, Mukhatar Ahmed Javali, Fawaz Abdul Hamid Baig, Mohammad Zahir Kota, Mohasin Abdul Khader, Mohammad Shahul Hameed, Sharaz Shaik, and et al. 2023. "Therapeutic Approach for Trigeminal Neuralgia: A Systematic Review" Biomedicines 11, no. 10: 2606. https://doi.org/10.3390/biomedicines11102606
APA StyleRana, M. H., Khan, A. A. G., Khalid, I., Ishfaq, M., Javali, M. A., Baig, F. A. H., Kota, M. Z., Khader, M. A., Hameed, M. S., Shaik, S., & Das, G. (2023). Therapeutic Approach for Trigeminal Neuralgia: A Systematic Review. Biomedicines, 11(10), 2606. https://doi.org/10.3390/biomedicines11102606







