Inflammation-Involved Proteins in Blood Serum of Cataract Patients—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: The right to sight: An analysis for the global burden of disease study. Lancet Glob. Health 2021, 9, e144–e160. [CrossRef]
- Gupta, V.B.; Rajagopala, M.; Ravishankar, B. Etiopathogenesis of cataract: An appraisal. Indian J. Ophthalmol. 2014, 62, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, H.; Theodoratou, E.; Chan, K.Y.; Rudan, I. The national and subnational prevalence of cataract and cataract blindness in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010804. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Öktem, C.; Karabıçak Acer, A.; Kocamış, Ö.; Taşdemir, S. Necessity of periodic ophthalmological examinations in binocular B class driving licence holders over 50 years of age. Turk. J. Ophthalmol. 2016, 46, 73–76. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, C.; Titiyal, J.S. Etiopathogenesis of cataract. Indian J. Ophthalmol. 2009, 57, 165–249. [Google Scholar] [CrossRef]
- Richardson, R.B.; Ainsbury, E.A.; Prescott, C.R.; Lovicu, F.J. Etiology of posterior subcapsular cataracts based on a review of risk factors including aging, diabetes, and ionizing radiation. Int. J. Radiat. Biol. 2020, 96, 1339–1361. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Ridker, P.M.; Glynn, R.J.; Christen, W.G.; Dana, M.R.; Hennekens, C.H. High levels of plasma C-reactive protein and future risk of age-related cataract. Ann. Epidemiol. 1999, 9, 166–171. [Google Scholar] [CrossRef]
- Chen, W.; Lin, H.; Zhong, X.; Liu, Z.; Geng, Y.; Xie, C.; Chen, W. Discrepant expression of cytokines in inflammation- and age-related cataract patients. PLoS ONE 2014, 9, e109647. [Google Scholar] [CrossRef]
- Hilliard, A.; Mendonca, P.; Russell, T.D.; Soliman, K.F.A. The protective effects of flavonoids in cataract formation through the activation of nrf2 and the inhibition of mmp-9. Nutrients 2020, 12, 3651. [Google Scholar] [CrossRef]
- Lesiewska, H.; Woźniak, A.; Reisner, P.; Czosnyka, K.; Stachura, J.; Malukiewicz, G. Is cataract in patients under 60 years associated with oxidative stress? Biomedicines 2023, 11, 1286. [Google Scholar] [CrossRef]
- Krawczyński, J. Enzymatic diagnostics in practical medicine. In Methods of Investigation; PZWL: Warsaw, Poland, 1972. [Google Scholar]
- Błeszyński, W.; Działoszyński, L.M. Purification of soluble arylsulphatases from ox brain. Biochem. J. 1965, 97, 360–364. [Google Scholar] [CrossRef]
- Colowick, S.C.; Kaplan, N.C. Methods in Enzymology, 2nd ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Eriksson, S. Studies in alpha 1-antitrypsin deficiency. Acta Med. Scand. 1965, 432, 1–8. [Google Scholar]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Franceschi, C.; Musarò, A. The hormetic and hermetic role of IL-6. Ageing Res. Rev. 2022, 80, 101697. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.R.; Fasano, R.; Paolisso, G. Adiponectin and cognitive decline. Int. J. Mol. Sci. 2020, 21, 2010. [Google Scholar] [CrossRef] [PubMed]
- Meuleners, L.B.; Feng, Y.R.; Fraser, M.; Brameld, K.; Chow, K. Impact of first and second eye cataract surgery on physical activity: A prospective study. BMJ Open 2019, 9, e024491. [Google Scholar] [CrossRef] [PubMed]
- Paunksnis, A.; Kusleika, S.; Kusleikaite, M. The relationship of the intensity of lens opacity with physical activity. Medicina 2006, 42, 738–743. [Google Scholar] [PubMed]
- Pott, G.B.; Chan, E.D.; Dinarello, C.A.; Shapiro, L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009, 85, 886–895. [Google Scholar] [CrossRef]
- Hamid, S.; Gul, A.; Hamid, Q. Relationship of cytokines and AGE products in diabetic and non-diabetic patients with cataract. Int. J. Health Sci. 2016, 10, 507–515. [Google Scholar] [CrossRef]
- Lewis, A.C. Interleukin-6 in the pathogenesis of posterior capsule opacification and the potential role for interleukin-6 inhibition in the future of cataract surgery. Med. Hypotheses 2013, 80, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Malecaze, F.; Chollet, P.; Cavrois, E.; Vita, N.; Arné, J.L.; Ferrara, P. Role of interleukin 6 in the inflammatory response after cataract surgery. An experimental and clinical study. Arch. Ophthalmol. 1991, 109, 1681–1683. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, C.; Sardinha, L.R.; Rizzo, L.V. Cytokine and chemokine concentration in the tear of patients with age-related cataract. Curr. Eye Res. 2020, 45, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Tsai, L.H.; Chen, C.C.; Lin, C.J.; Lin, S.P.; Cheng, C.Y.; Hsieh, H.P. Risk factor analysis of early-onset cataracts in Taiwan. J. Clin. Med. 2022, 11, 2374. [Google Scholar] [CrossRef]
- Jonas, J.B.; Wei, W.B.; Xu, L.; Wang, Y.X. Systemic inflammation and eye diseases. The Beijing Eye Study. PLoS ONE 2018, 13, e0204263. [Google Scholar] [CrossRef]
- Boey, P.Y.; Tay, W.T.; Lamoureux, E.; Tai, E.S.; Mitchell, P.; Wang, J.J.; Saw, S.M.; Wong, T.Y. C-reactive protein and age-related macular degeneration and cataract: The singapore malay eye study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1880–1885. [Google Scholar] [CrossRef]
- Vasavada, A.R.; Thampi, P.; Yadav, S.; Rawal, U.M. Acid phosphatase and lipid peroxidation in human cataractous lens epithelium. Indian J. Ophthalmol. 1993, 41, 173–175. [Google Scholar]
- Yambire, K.F.; Rostosky, C.; Watanabe, T.; Pacheu-Grau, D.; Torres-Odio, S.; Sanchez-Guerrero, A.; Senderovich, O.; Meyron-Holtz, E.G.; Milosevic, I.; Frahm, J.; et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife 2019, 8, e51031. [Google Scholar] [CrossRef]
- Ge, W.; Li, D.; Gao, Y.; Cao, X. The roles of lysosomes in inflammation and autoimmune diseases. Int. Rev. Immunol. 2015, 34, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, B.; Woźniak, A.; Konca, J.; Górecki, D.; Mila-Kierzenkowska, C.; Szpinda, M.; Sutkowy, P.; Wesołowski, R. Activity of α1-antitrypsin and some lysosomal enzymes in the blood serum of patients with chronic obstructive pulmonary disease after smoking cessation. Biomed. Res. Int. 2015, 2015, 176582. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Abe, A.; Lennikov, A.; Kitaichi, N.; Ishida, S.; Ohguro, H. Increase of lysosomal phospholipase A2 in aqueous humor by uveitis. Exp. Eye Res. 2014, 118, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, A.; Chan, J.L.; Yiannakouris, N.; Kontogianni, M.; Miller, L.C.; Orlova, C.; Mantzoros, C.S. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: Cross-sectional and interventional studies. J. Clin. Endocrinol. Metab. 2003, 88, 4823–4831. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Liang, Z.; Zhong, S.; Liu, X.; Liu, Z.; Poon, W.S.; Song, Y.; Chen, B.; Wang, R. Role of omentin-1 in susceptibility to anxiety and depression like behaviors. Mol. Cell. Endocrinol. 2023, 574, 111990. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 modulates macrophage function via integrin receptors αvβ3 and αvβ5 and reverses plaque vulnerability in animal models of atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef]
- Bednarska-Makaruk, M.; Graban, A.; Wiśniewska, A.; Łojkowska, W.; Bochyńska, A.; Gugała-Iwaniuk, M.; Sławińska, K.; Ługowska, A.; Ryglewicz, D.; Wehr, H. Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology 2017, 18, 561–580. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a novel host defense peptide of innate immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Makiel, K.; Suder, A.; Targosz, A.; Maciejczyk, M.; Haim, A. Exercise-induced alternations of adiponectin, interleukin-8 and indicators of carbohydrate metabolism in males with metabolic syndrome. Biomolecules 2023, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Luo, S.; Li, Z. Multifaceted roles of adiponectin in rheumatoid arthritis. Int. Immunopharmacol. 2015, 28, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Hong, X.; Cao, Q.Q.; So, K.F. Adiponectin, exercise and eye diseases. Int. Rev. Neurobiol. 2019, 147, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Alkady, E.A.; Ahmed, H.M.; Tag, L.; Abdou, M.A. Serum and synovial adiponectin, resistin, and visfatin levels in rheumatoid arthritis patients. Relation to disease activity. Z. Rheumatol. 2011, 70, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Shklyaev, S.S.; Melnichenko, G.A.; Volevodz, N.N.; Falaleeva, N.A.; Ivanov, S.A.; Kaprin, A.D.; Mokrysheva, N.G. Adiponectin: A pleiotropic hormone with multifaceted roles. Probl. Endokrinol. 2021, 67, 98–112. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Mao, D.; Peng, H.; Li, Q.; Wang, J.; Li, P.; Hu, K.; Zhang, X.; Lei, B. Aqueous humor and plasma adiponectin levels in proliferative diabetic retinopathy patients. Curr. Eye Res. 2012, 37, 803–808. [Google Scholar] [CrossRef]
- Luo, Y.; Wan, J.; Luo, C.; Liu, H.; Zhou, Y.; Chen, J.; Duan, Y.; Ning, X.; Zhou, Z.; Wang, K.; et al. Higher aqueous levels of resistin and lipocalin-2 indicated worse visual improvement following anti-vegf therapy in patients with retinal vein occlusion. Curr. Eye Res. 2021, 46, 845–854. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Salvador, J.; Frühbeck, G. Is hyperleptinemia involved in the development of age-related lens opacities? Am. J. Clin. Nutr. 2004, 79, 888–889. [Google Scholar] [CrossRef]
- Bouloumie, A.; Marumo, T.; Lafontan, M.; Busse, R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999, 13, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Wong, T.Y. Obesity and eye diseases. Surv. Ophthalmol. 2007, 52, 180–195. [Google Scholar] [CrossRef] [PubMed]



| Part A | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cataract Patients | Control Group | |||||||||||||
| N | 24 | 33 | ||||||||||||
| Age (years) | 52.7 ± 9.9 | 51.1 ± 8.2 | ||||||||||||
| Sex (F/M) | 15/9 | 22/11 | ||||||||||||
| BMI | 26.5 ± 2.1 | 25.9 ± 1.2 | ||||||||||||
| Part B | ||||||||||||||
| Cataract females | Cataract males | Control females | Control males | |||||||||||
| N | 15 | 9 | 22 | 11 | ||||||||||
| Age (y/o) | 53.0 ± 10.2 | 52.1 ± 9.9 | 50.6 ± 9.1 | 52.2 ± 6.5 | ||||||||||
| BMI | 26.0 ± 2.3 | 27.3 ± 1.8 | 25.9 ± 1.3 | 26.0 ± 1.0 | ||||||||||
| (A) | ||||
|---|---|---|---|---|
| Parameter | Cataract Patients | Controls | ||
| Acid phosphatase [10−4 nmol 4-NP/mg/min] | 11.0 ± 2.9 | 9.6 ± 3.3 | ||
| Cathepsin D [10−2 nmol TYR/mg/min] | 19.4 ± 2.6 | 19.6 ± 2.5 | ||
| Arylsulphatase [10−4 nmol 4-NC/mg/min] | 10.7 ± 4.4 | 9.2 ± 2.1 | ||
| Alpha 1-antitrypsin [10−1 mg TR/mL] | 10.7 ± 1.9 | 11.2 ± 1.4 | ||
| C-reactive protein [10−1 mg/L] | 26.8 ± 10.3 | 27.4 ± 6.6 | ||
| Interleukin 6 [pg/mL] | 5.0 ± 1.9 | 6.5 ± 1.7 × | ||
| Resistin [ng/mL] | 7.4 ± 2.1 | 12.2 ± 3.3 * | ||
| Omentin-1 [10 ng/mL] | 59.1 ± 20.2 | 38.3 ± 9.9 * | ||
| Adiponectin [µg/mL] | 11.2 ± 3.5 | 8.7 ± 3.3 × | ||
| Leptin [10−1 ng/mL] | 56.5 ± 24.6 | 64.2 ± 22.6 | ||
| (B) | ||||
| Parameter | Cataract females (1) | Control females (2) | Cataract males (3) | Control males (4) |
| Acid phosphatase [10−4 nmol 4-NP/mg/min] | 11.4 ± 3.2 a | 9.0 ± 3.6 | 10.2 ± 2.2 | 10.7 ± 2.2 |
| Cathepsin D [10−2 nmol TYR/mg/min] | 20.2 ± 2.8 | 19.5 ± 2.0 | 18.3 ± 1.9 | 19.9 ± 3.4 |
| Arylsulphatase [10−4 nmol 4-NC/mg/min] | 10.3 ± 4.2 | 8.5 ± 1.6 de | 11.4 ± 3.7 | 10.6 ± 2.4 |
| Alpha 1-antitrypsin [10−1 mg TR/mL] | 11.2 ± 1.8 | 11.5 ± 1.4 d | 10.0 ± 1.9 | 10.7 ± 1.3 |
| C-reactive protein [10−1 mg/L] | 28.4 ± 11.9 | 26.1 ± 8.1 | 23.4 ± 5.3 | 29.1 ± 4.6 |
| Interleukin 6 [pg/mL] | 5.4 ± 2.2 c | 6.0 ± 1.7 de | 4.0 ± 0.7 ff | 7.8 ± 0.8 |
| Resistin [ng/mL] | 7.6 ± 2.5 aacc | 12.6 ± 3.8 dd | 7.0 ± 1.3 ff | 11.2 ± 1.7 |
| Omentin-1 [10 ng/mL] | 62.2 ± 20.8 aacc | 38.2 ± 8.0 | 51.3 ± 18.1 | 38.6 ± 13.5 |
| Adiponectin [µg/mL] | 12.1 ± 3.5 bccc | 10.3 ± 3.1 ee | 8.8 ± 2.2 f | 6.0 ± 1.4 |
| Leptin [10−1 ng/mL] | 60.1 ± 24.4 | 80.0 ± 7.1 de | 47.2 ± 19.5 | 54.3 ± 23.6 |
| (A) | |||
|---|---|---|---|
| Cataract Patients | Controls | ||
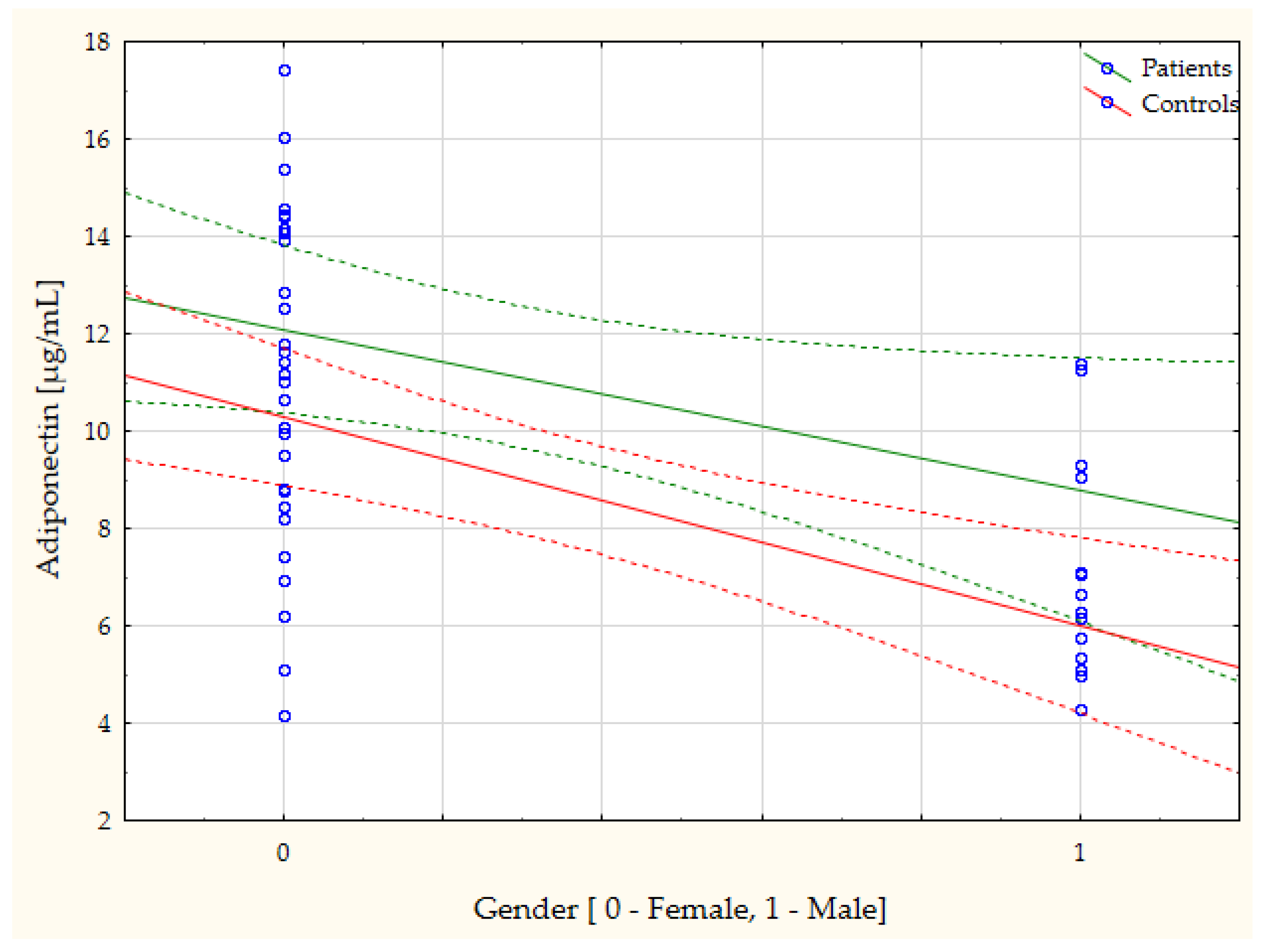
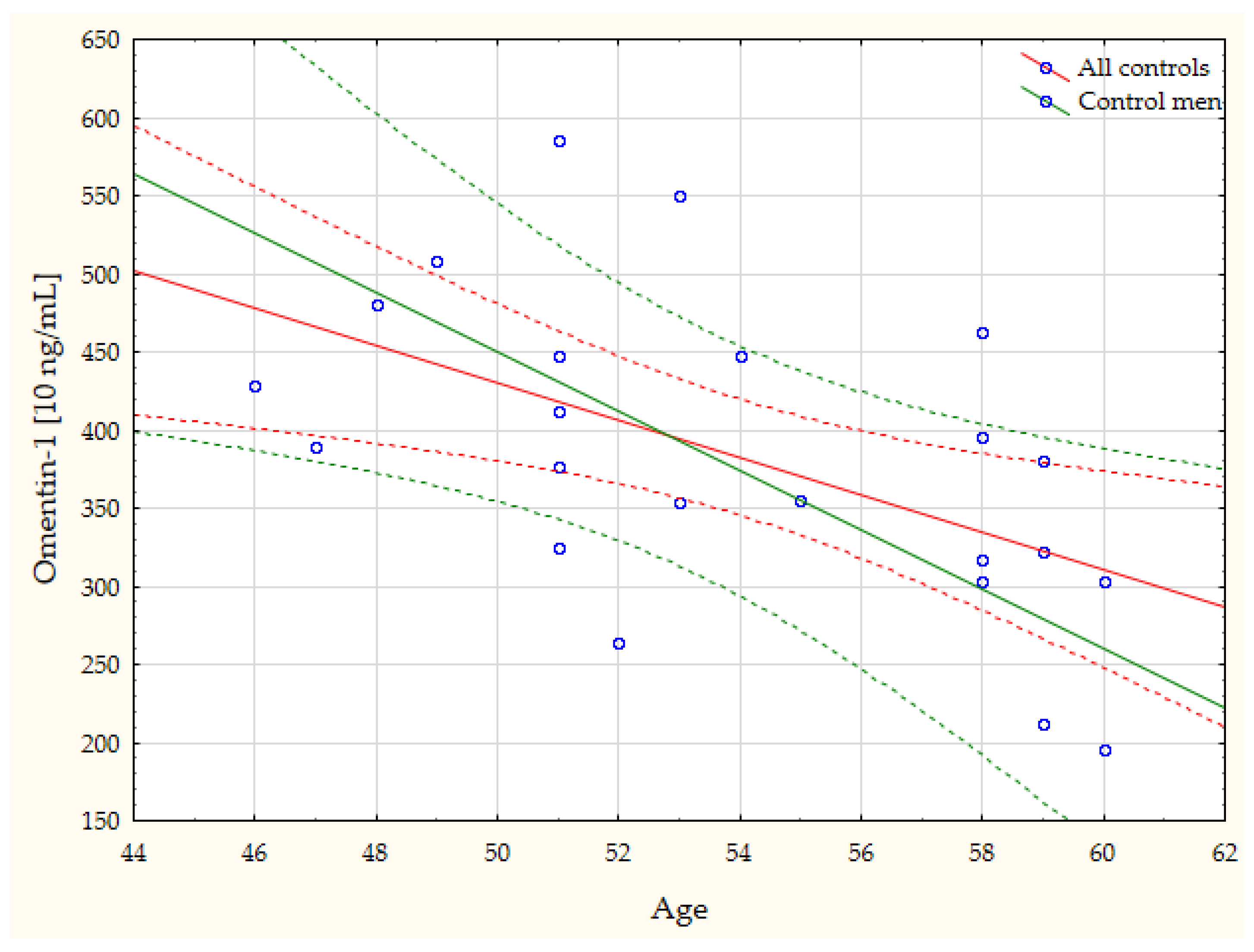
| Gender vs. Adiponectin | r = −0.44, p = 0.046 | Age vs. Omentin-1 | r = −0.54, p = 0.008 |
| AAT vs. Omentin-1 | r = 0.43, p = 0.05 | Gender vs. IL-6 | r = 0.47, p = 0.041 |
| CTS D vs. ASA | r = 0.43, p = 0.039 | Gender vs. ASA | r = 0.48, p = 0.005 |
| CRP vs. Leptin | r = 0.83, p = 0.011 | Gender vs. Adiponectin | r = −0.64, p = 0.001 |
| IL-6 vs. Adiponectin | r = 0.80, p = 0.001 | Gender vs. Leptin | r = −0.58, p = 0.039 |
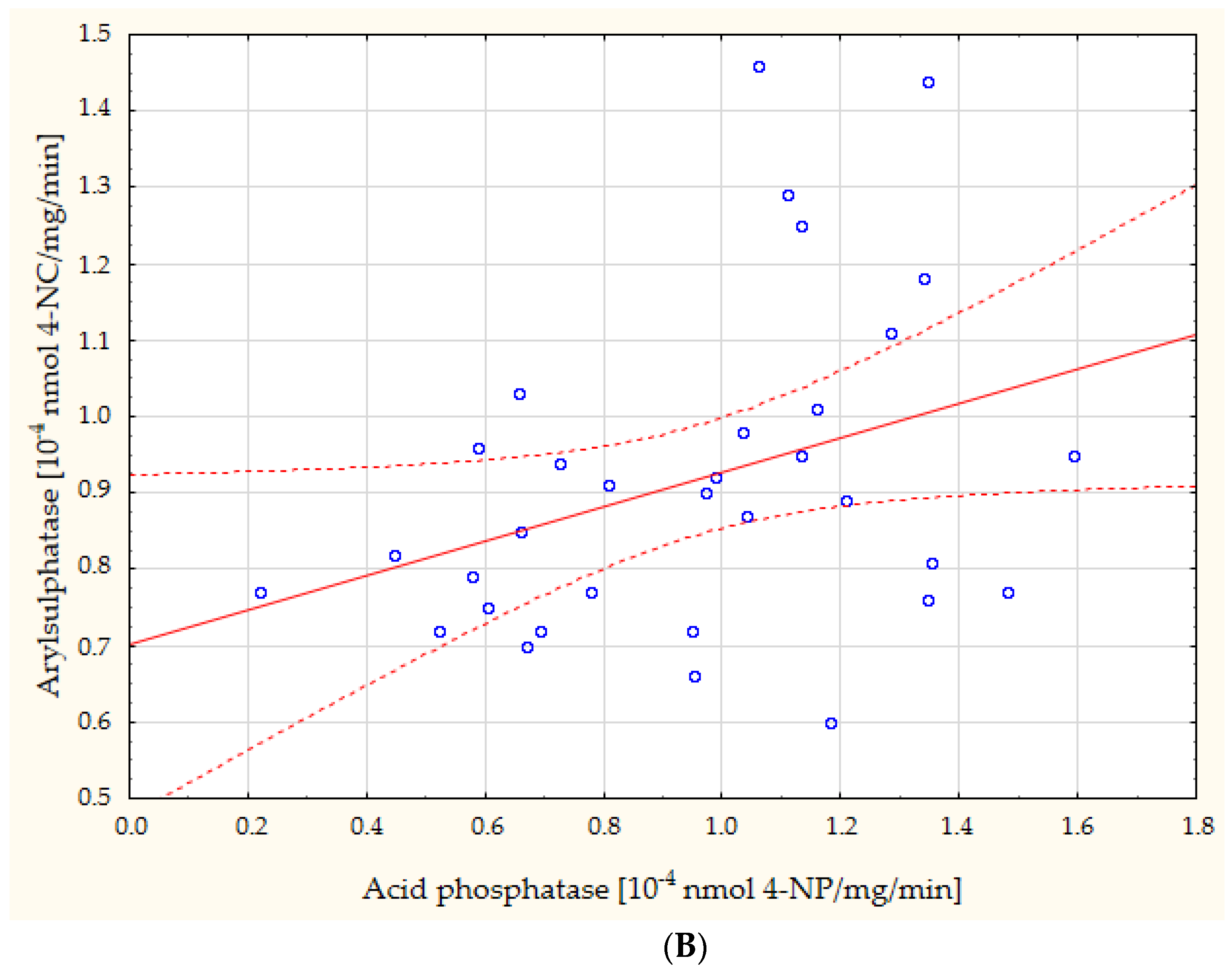
| AcP vs. ASA | r = 0.35, p = 0.046 | ||
| AAT vs. ASA | r = −0.42, p = 0.014 | ||
| ASA vs. Resistin | r = −0.43, p = 0.040 | ||
| (B) | |||
| Females with cataract | Control females | Males with cataract | Control males |
| CRP vs. Leptin: r = 0.95, p = 0.015 | Adiponectin vs. CTS D: r = −0.69, p = 0.005 | BMI vs. Leptin: r = −0.99, p = 0.019 | Age vs. Omentin-1: r = −0.78, p = 0.023 |
| IL-6 vs. Adiponectin: r = 0.89, p = 0.001 | Resistin vs. CTS D: r = 0.51, p = 0.043 | AAT vs. Omentin-1: r = 0.82, p = 0.046 | Age vs. Adiponectin: r = 0.76, p = 0.017 |
| Adiponectin vs. CRP: r = −0.89, p = 0.043 | Adiponectin vs. Leptin: r = −0.99, p = 0.049 | ||
| Resistin vs. CRP: r = 0.85, p = 0.031 | |||
| Resistin vs. Adiponectin: r = −0.62, p = 0.017 | |||
| ASA vs. IL-6: r = −0.58, p = 0.028 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutkowy, P.; Lesiewska, H.; Woźniak, A.; Malukiewicz, G. Inflammation-Involved Proteins in Blood Serum of Cataract Patients—A Preliminary Study. Biomedicines 2023, 11, 2607. https://doi.org/10.3390/biomedicines11102607
Sutkowy P, Lesiewska H, Woźniak A, Malukiewicz G. Inflammation-Involved Proteins in Blood Serum of Cataract Patients—A Preliminary Study. Biomedicines. 2023; 11(10):2607. https://doi.org/10.3390/biomedicines11102607
Chicago/Turabian StyleSutkowy, Paweł, Hanna Lesiewska, Alina Woźniak, and Grażyna Malukiewicz. 2023. "Inflammation-Involved Proteins in Blood Serum of Cataract Patients—A Preliminary Study" Biomedicines 11, no. 10: 2607. https://doi.org/10.3390/biomedicines11102607
APA StyleSutkowy, P., Lesiewska, H., Woźniak, A., & Malukiewicz, G. (2023). Inflammation-Involved Proteins in Blood Serum of Cataract Patients—A Preliminary Study. Biomedicines, 11(10), 2607. https://doi.org/10.3390/biomedicines11102607






