Molecular Processes Involved in the Shared Pathways between Cardiovascular Diseases and Diabetes
Abstract
:1. Introduction
2. What Is Diabetes Mellitus?
2.1. Definition and Types
2.2. Diagnosis
- Random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) with symptoms of hyperglycaemia.
- Oral glucose tolerance test (OGTT) with a 75 g glucose: 2 h plasma glucose (2-hPG) level ≥ 200 mg/dL (11.1 mmol/L).
- Fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L) in two separate tests.
- Haemoglobin A1C level ≥ 6.5% (48 mmol/mol) [12].
- Antibodies against glutamic acid decarboxylase 65 (GAD65);
- Islet cell antigen 2 (IA-2) antibodies;
- Zinc transporter 8 (ZnT8) antibodies [15].
2.3. Molecular Mechanism of Diabetes Mellitus
2.4. Treatment
- Insulin—the main and the most-recognizable treatment of T1D and the last stage of treatment in T2D, when pancreas islets do not produce and secrete enough endogenic insulin. Adequate insulin therapy gives the opportunity to maintain the physiological level of glycaemia. The latest guidelines for treatment suggest using analogues of insulin in preference to human insulin because of their better profile of action [35,36].
- Biguanide derivatives—with metformin as the main representative. The function of this drug is to increase the phosphorylation of the glucose transporter (GLUT), resulting in a boost of insulin sensitivity and uptake and, as consequence, leading to lover levels of glucose and HbA1c. Due to this function, metformin is used in the treatment of T2D. Moreover, metformin could support weight loss [37,38,39].
- Gliptins—work as inhibitors of dipeptidyl peptidase 4 (DPP-4), inhibiting the activation of incretin hormones such as gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). These hormones are responsible for the stimulation of insulin synthesis in response to a meal. To sum up, inhibitors of DPP-4 could help to achieve better glycaemia control in patients with T2D. It is worth mentioning that these medicines have a low hypoglycaemia risk and they are safe for the cardiovascular system (CVS) [43,44,45].
- Glucagon-like peptide 1 (GLP-1) analogues. These medicines are glucose-dependent stimulators of insulin secretion. Moreover, GLP-1 analogues lower glucagon and hepatic glucose production, which could result in better control of glycaemia. Furthermore, they could slow down digestion and decrease food intake due to lower appetite. GLP-1 analogues, similar to DPP-4 inhibitors, could be safe for CVS and stimulate weight loss [46,47,48].
- Gliflozins—inhibit sodium–glucose co-transporter-2 (SGLT-2), resulting in lower glucose reabsorption in renal tubules and higher glucose elimination through the kidneys (by glucosuria). Scientists have described that SGLT-2 inhibitors could have a cardiorenal protective effect coexisting with normoglycaemic function. Nevertheless, these medicines could increase the risk of urinary tract infections [49,50,51].
2.5. Comorbidities
2.5.1. Hypertension
2.5.2. Obesity
2.5.3. Polycystic Ovary Syndrome
2.5.4. Dyslipidaemia
2.5.5. Obstructive Sleep Apnoea
2.5.6. Non-Alcoholic Fatty Liver Disease
2.6. Morbidity of Diabetes Mellitus
3. Cardiovascular Diseases in Patients with Diabetes
3.1. Inflammation and Vascular Remodelling
3.2. AGEs
3.3. Oxidative Stress
3.4. Lipotoxicity and Mitochondrial Dysfunction ER Stress
3.5. Myocardial Cell Death
3.6. Renin–Angiotensin–Aldosterone System
3.7. mRNA
3.8. Epigenetics
4. Drugs Used in Treatment of Diabetes and Cardiovascular Disease
4.1. Sodium–Glucose Co-Transporter-2 Inhibitors
4.2. Glucagon-like Peptide-1 Receptor Agonists
5. Potential Role of Microbiome in Diabetes and Cardiovascular Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleinberger, J.W.; Pollin, T.I. Personalized Medicine in Diabetes Mellitus: Current Opportunities and Future Prospects: Personalized Medicine in Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2015, 1346, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joseph, P.G.; McKee, M.; Anand, S.S.; Teo, K.K.; Schwalm, J.-D.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017, 121, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Kampangkaew, J.; Nambi, V. Prevention of Cardiovascular Disease in Women. Methodist DeBakey Cardiovasc. J. 2017, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-S.; Ko, S.-H. Current Trends in Epidemiology of Cardiovascular Disease and Cardiovascular Risk Management in Type 2 Diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 Inhibitors: Mechanisms of Cardiovascular Benefit beyond Glycaemic Control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Steptoe, A. Effects of Stress on the Development and Progression of Cardiovascular Disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Andrikou, E.; Tsioufis, C.; Andrikou, I.; Leontsinis, I.; Tousoulis, D.; Papanas, N. GLP-1 Receptor Agonists and Cardiovascular Outcome Trials: An Update. Hellenic J. Cardiol. 2019, 60, 347–351. [Google Scholar] [CrossRef]
- Al-awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Kerner, W.; Brückel, J. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Insel, R.A.; Dunne, J.L.; Atkinson, M.A.; Chiang, J.L.; Dabelea, D.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Krischer, J.P.; Lernmark, Å.; et al. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015, 38, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Dayan, C.M.; Korah, M.; Tatovic, D.; Bundy, B.N.; Herold, K.C. Changing the Landscape for Type 1 Diabetes: The First Step to Prevention. Lancet 2019, 394, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Beart, P. Inflammation: Maladies, Models, Mechanisms and Molecules: Inflammation: Maladies, Models, Mechanisms and Molecules. Br. J. Pharmacol. 2016, 173, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Ghorbani, F.; Najafi, S.; Ravaei, N.; Karimian, M.; Kalhor, K.; Movafagh, A.; Mohsen Aghaei Zarch, S. Progress toward Molecular Therapy for Diabetes Mellitus: A Focus on Targeting Inflammatory Factors. Diabetes Res. Clin. Pract. 2022, 189, 109945. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Huang, L.; Liu, X.; Chen, J. Anti-Hyperglycemic, Antioxidant and Anti-Inflammatory Effects of VIP and a VPAC1 Agonist on Streptozotocin-Induced Diabetic Mice. Peptides 2011, 32, 216–222. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Bending, D.; Zaccone, P.; Cooke, A. Inflammation and Type One Diabetes. Int. Immunol. 2012, 24, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes Mellitus and Inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.; McClelland, R.L.; Allison, M.A.; Szklo, M.; Rye, K.-A.; Ong, K.L. The Association of Circulating Fibroblast Growth Factor 21 Levels with Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Metabolism 2023, 143, 155535. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhuang, X.; Luo, M.; Yin, W.; Xiong, L. The Propionic Acid and Butyric Acid in Serum but Not in Feces Are Increased in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. BMC Gastroenterol. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin Resistance: Review of the Underlying Molecular Mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Murabito, A.; Ghigo, A.; Hirsch, E. PI3Ks in Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2017, 70, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.R.; Bogan, J.S. Chapter 7 Intracellular Retention and Insulin-Stimulated Mobilization of GLUT4 Glucose Transporters. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2009; Volume 80, pp. 155–192. ISBN 978-0-12-374408-1. [Google Scholar]
- Achari, A.; Jain, S. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A.; et al. Fibroblast Growth Factor 21 Increases Insulin Sensitivity through Specific Expansion of Subcutaneous Fat. Nat. Commun. 2018, 9, 272. [Google Scholar] [CrossRef]
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B.; Mosiewicz, J. Associations between Fatty Acid-Binding Protein 4–A Proinflammatory Adipokine and Insulin Resistance, Gestational and Type 2 Diabetes Mellitus. Cells 2019, 8, 227. [Google Scholar] [CrossRef]
- Nakamura, R.; Okura, T.; Fujioka, Y.; Sumi, K.; Matsuzawa, K.; Izawa, S.; Ueta, E.; Kato, M.; Taniguchi, S.; Yamamoto, K. Serum Fatty Acid-Binding Protein 4 (FABP4) Concentration Is Associated with Insulin Resistance in Peripheral Tissues, A Clinical Study. PLoS ONE 2017, 12, e0179737. [Google Scholar] [CrossRef]
- Chedid, P.; Boussetta, T.; Dang, P.M.-C.; Belambri, S.A.; Marzaioli, V.; Fasseau, M.; Walker, F.; Couvineau, A.; El-Benna, J.; Marie, J.-C. Vasoactive Intestinal Peptide Dampens Formyl-Peptide-Induced ROS Production and Inflammation by Targeting a MAPK-P47phox Phosphorylation Pathway in Monocytes. Mucosal Immunol. 2017, 10, 332–340. [Google Scholar] [CrossRef]
- Turner, R.C. Glycemic Control With Diet, Sulfonylurea, Metformin, or Insulin in Patients With Type 2 Diabetes MellitusProgressive Requirement for Multiple Therapies (UKPDS 49). JAMA 1999, 281, 2005. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Carr, A.L.J.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 Years of Insulin: Celebrating the Past, Present and Future of Diabetes Therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.M.; Castle, J.R. Recent Advances in Insulin Therapy. Diabetes Technol. Ther. 2020, 22, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef]
- Hostalek, U.; Campbell, I. Metformin for Diabetes Prevention: Update of the Evidence Base. Curr. Med. Res. Opin. 2021, 37, 1705–1717. [Google Scholar] [CrossRef]
- Cho, E.-H. Oldies but Goodies: Thiazolidinedione as an Insulin Sensitizer with Cardioprotection. Diabetes Metab. J. 2022, 46, 827–828. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Sameeh, M.Y.; Khowdiary, M.M.; Nassar, H.S.; Abdelall, M.M.; Amer, H.H.; Hamed, A.; Elhenawy, A.A. Thiazolidinedione Derivatives: In Silico, In Vitro, In Vivo, Antioxidant and Anti-Diabetic Evaluation. Molecules 2022, 27, 830. [Google Scholar] [CrossRef] [PubMed]
- Love, K.M.; Liu, Z. DPP4 Activity, Hyperinsulinemia, and Atherosclerosis. J. Clin. Endocrinol. Metab. 2021, 106, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Kong, X.-Q.; Zhang, K.-F.; Luo, S.; Wang, F.; Zhang, J.-J. DPP4 as a Potential Candidate in Cardiovascular Disease. J. Inflamm. Res. 2022, 15, 5457–5469. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Dipeptidyl Peptidase 4 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 Physiology Informs the Pharmacotherapy of Obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular Mechanisms and Outcomes of a Complex Signaling System. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef]
- Toyama, T.; Neuen, B.L.; Jun, M.; Ohkuma, T.; Neal, B.; Jardine, M.J.; Heerspink, H.L.; Wong, M.G.; Ninomiya, T.; Wada, T.; et al. Effect of SGLT2 Inhibitors on Cardiovascular, Renal and Safety Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2019, 21, 1237–1250. [Google Scholar] [CrossRef]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological Treatment of Hyperglycemia in Type 2 Diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Dong, G.-J.; Guo, M. Targeting Epicardial Adipose Tissue: A Potential Therapeutic Strategy for Heart Failure with Preserved Ejection Fraction with Type 2 Diabetes Mellitus. World J. Diabetes 2023, 14, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Gonzalez-Villalpando, C.; Meigs, J.B.; Ferrannini, E. Hypertension and Diabetes Mellitus: Coprediction and Time Trajectories. Hypertension 2018, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Teck, J. Diabetes-Associated Comorbidities. Prim Care 2022, 49, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, M.-F.; Li, H.-L.; Feng, Q.; Cheung, C.-L.; Cheung, T.T.; Cheung, B.M.Y. Prevalence of Childhood Obesity in the United States in 1999-2018: A 20-Year Analysis. Obes. Facts 2022, 15, 560–569. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S100–S110. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.J.; Cohen, P. How Does Obesity Lead to Insulin Resistance? Elife 2017, 6, e33298. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Mortada, R.; Porter, S. Diagnosis and Treatment of Polycystic Ovary Syndrome. Am. Fam. Physician 2016, 94, 106–113. [Google Scholar]
- Rodgers, R.J.; Avery, J.C.; Moore, V.M.; Davies, M.J.; Azziz, R.; Stener-Victorin, E.; Moran, L.J.; Robertson, S.A.; Stepto, N.K.; Norman, R.J.; et al. Complex Diseases and Co-Morbidities: Polycystic Ovary Syndrome and Type 2 Diabetes Mellitus. Endocr. Connect. 2019, 8, R71–R75. [Google Scholar] [CrossRef]
- Last, A.R.; Ference, J.D.; Menzel, E.R. Hyperlipidemia: Drugs for Cardiovascular Risk Reduction in Adults. Am. Fam. Physician 2017, 95, 78–87. [Google Scholar]
- Screening for Lipid Disorders in Children and Adolescents: Recommendation Statement. Am. Fam. Physician 2016, 94, Online. Available online: https://pubmed.ncbi.nlm.nih.gov/28075093/ (accessed on 30 May 2023).
- Fan, D.; Li, L.; Li, Z.; Zhang, Y.; Ma, X.; Wu, L.; Qin, G. Effect of Hyperlipidemia on the Incidence of Cardio-Cerebrovascular Events in Patients with Type 2 Diabetes. Lipids Health Dis. 2018, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S40–S52. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Adderley, N.J.; Tracy, A.; Taverner, T.; Hanif, W.; Toulis, K.A.; Thomas, G.N.; Tahrani, A.A.; Nirantharakumar, K. Risk of Incident Obstructive Sleep Apnea Among Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Stampfer, M.J.; Tworoger, S.S.; Hu, F.B.; Redline, S. A Population-Based Study of the Bidirectional Association Between Obstructive Sleep Apnea and Type 2 Diabetes in Three Prospective U.S. Cohorts. Diabetes Care 2018, 41, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-Alcoholic Fatty Liver Disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-Alcoholic Fatty Liver Disease and Diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current Guidelines for the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef]
- Miranville, A.; Herling, A.; Biemer-Daub, G.; Voss, M. Differential Adipose Tissue Inflammatory State in Obese Nondiabetic Zucker Fatty Rats Compared to Obese Diabetic Zucker Diabetic Fatty Rats. Horm. Metab. Res. 2012, 44, 273–278. [Google Scholar] [CrossRef]
- Daniels, M.C.; McClain, D.A.; Crook, E.D. Transcriptional Regulation of Transforming Growth Factor Β1 by Glucose: Investigation into the Role of the Hexosamine Biosynthesis Pathway. Am. J. Med. Sci. 2020, 359, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular Mechanisms of Diabetic Cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Prasher, D.; Greenway, S.C.; Singh, R.B. The Impact of Epigenetics on Cardiovascular Disease. Biochem. Cell Biol. 2020, 98, 12–22. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Gillman, M.W.; Kemper, A.R.; Krist, A.H.; et al. Screening for Lipid Disorders in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 316, 625. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Yazdi, F.; Jodari-Karimi, M.; Owen, J.G.; Reisin, E. Obstructive Sleep Apnea and Hypertension: Updates to a Critical Relationship. Curr. Hypertens. Rep. 2022, 24, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated Lipid Metabolism Links NAFLD to Cardiovascular Disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ham, K.; Koster, M.P.H.; Velthuis, B.K.; Budde, R.P.J.; Fauser, B.C.J.M.; Laven, J.S.E.; Louwers, Y.V. Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome. J. Clin. Mol. 2023, 12, 5226. [Google Scholar] [CrossRef]
- Fiordaliso, F.; Leri, A.; Cesselli, D.; Limana, F.; Safai, B.; Nadal-Ginard, B.; Anversa, P.; Kajstura, J. Hyperglycemia Activates P53 and P53-Regulated Genes Leading to Myocyte Cell Death. Diabetes 2001, 50, 2363–2375. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic Cardiomyopathy: A Hyperglycaemia- and Insulin-Resistance-Induced Heart Disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Renin-Angiotensin System in Pathogenesis of Atherosclerosis and Treatment of CVD. Int. J. Mol. Sci. 2021, 22, 6702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, K.; Liu, W.; Cai, Y.; Jin, H. m6A mRNA Methylation Regulates the Development of Gestational Diabetes Mellitus in Han Chinese Women. Genomics 2021, 113, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, L.; Luo, E.; Hou, J.; Yan, G.; Wang, D.; Qiao, Y.; Tang, C. Role of m6A RNA Methylation in Cardiovascular Disease (Review). Int. J. Mol. Med. 2020, 46, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of Diabetes Mellitus and Diabetes Complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Bell, D.S.H. Heart Failure. Diabetes Care 2003, 26, 2433–2441. [Google Scholar] [CrossRef]
- From, A.M.; Leibson, C.L.; Bursi, F.; Redfield, M.M.; Weston, S.A.; Jacobsen, S.J.; Rodeheffer, R.J.; Roger, V.L. Diabetes in Heart Failure: Prevalence and Impact on Outcome in the Population. Am. J. Med. 2006, 119, 591–599. [Google Scholar] [CrossRef]
- Bradley, T.J.; Slorach, C.; Mahmud, F.H.; Dunger, D.B.; Deanfield, J.; Deda, L.; Elia, Y.; Har, R.L.H.; Hui, W.; Moineddin, R.; et al. Early Changes in Cardiovascular Structure and Function in Adolescents with Type 1 Diabetes. Cardiovasc. Diabetol. 2016, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic Cardiomyopathy Revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.A.; Varela-Carver, A.; Mongillo, M.; Kleinert, C.; Khan, M.T.; Leccisotti, L.; Strickland, N.; Matsui, T.; Das, S.; Rosenzweig, A.; et al. Abnormal Myocardial Insulin Signalling in Type 2 Diabetes and Left-Ventricular Dysfunction. Eur. Heart J. 2010, 31, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Khan, M.S.; Patel, K.V.; Butler, J.; Tang, W.H.W.; Vaduganathan, M.; Lam, C.S.P.; Verma, S.; McGuire, D.K.; Pandey, A. Prevalence and Prognostic Implications of Diabetes With Cardiomyopathy in Community-Dwelling Adults. J. Am. Coll. Cardiol. 2021, 78, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Tiwari, S.; Lee, P.; Ndisang, J.F. The Heme Oxygenase System Selectively Enhances the Anti-Inflammatory Macrophage-M2 Phenotype, Reduces Pericardial Adiposity, and Ameliorated Cardiac Injury in Diabetic Cardiomyopathy in Zucker Diabetic Fatty Rats. J. Pharmacol. Exp. Ther. 2013, 345, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Monji, A.; Mitsui, T.; Bando, Y.K.; Aoyama, M.; Shigeta, T.; Murohara, T. Glucagon-like Peptide-1 Receptor Activation Reverses Cardiac Remodeling via Normalizing Cardiac Steatosis and Oxidative Stress in Type 2 Diabetes. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H295–H304. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Rutschow, S.; Jäger, S.; Linderer, A.; Anker, S.; Riad, A.; Unger, T.; Schultheiss, H.-P.; Pauschinger, M.; Tschöpe, C. Contributions of Inflammation and Cardiac Matrix Metalloproteinase Activity to Cardiac Failure in Diabetic Cardiomyopathy. Diabetes 2007, 56, 641–646. [Google Scholar] [CrossRef]
- Alzahrani, S.; Ajjan, R. Review Article: Coagulation and Fibrinolysis in Diabetes. Diabetes Vasc. Dis. Res. 2010, 7, 260–273. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, R.; Bahl, A.; Khullar, M. Molecular Mechanisms and Epigenetic Regulation in Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 725532. [Google Scholar] [CrossRef]
- Hsuan, C.-F.; Teng, S.I.F.; Hsu, C.-N.; Liao, D.; Chang, A.J.-W.; Lee, H.-L.; Hee, S.-W.; Chang, Y.-C.; Chuang, L.-M. Emerging Therapy for Diabetic Cardiomyopathy: From Molecular Mechanism to Clinical Practice. Biomedicines 2023, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Grieve, D.; Cave, A.; Looi, Y.; Shah, A. NADPH Oxidase and Heart Failure. Curr. Opin. Pharmacol. 2006, 6, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kleibert, M.; Zygmunciak, P.; Łakomska, K.; Mila, K.; Zgliczyński, W.; Mrozikiewicz-Rakowska, B. Insight into the Molecular Mechanism of Diabetic Kidney Disease and the Role of Metformin in Its Pathogenesis. Int. J. Mol. Sci. 2023, 24, 13038. [Google Scholar] [CrossRef] [PubMed]
- Son, N.-H.; Yu, S.; Tuinei, J.; Arai, K.; Hamai, H.; Homma, S.; Shulman, G.I.; Abel, E.D.; Goldberg, I.J. PPARγ-Induced Cardiolipotoxicity in Mice Is Ameliorated by PPARα Deficiency despite Increases in Fatty Acid Oxidation. J. Clin. Investig. 2010, 120, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, K.; Bugger, H.; Wende, A.R.; Soto, J.; Jenson, G.A.; Tor, A.R.; McGlauflin, R.; Kenny, H.C.; Zhang, Y.; Souvenir, R.; et al. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induce Post-Translational Modifications of AKAP121, DRP1, and OPA1 That Promote Mitochondrial Fission. Circ. Res. 2018, 122, 58–73. [Google Scholar] [CrossRef]
- Römer, A.; Linn, T.; Petry, S.F. Lipotoxic Impairment of Mitochondrial Function in β-Cells: A Review. Antioxidants 2021, 10, 293. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and Molecular Mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Munasinghe, P.E.; Riu, F.; Dixit, P.; Edamatsu, M.; Saxena, P.; Hamer, N.S.J.; Galvin, I.F.; Bunton, R.W.; Lequeux, S.; Jones, G.; et al. Type-2 Diabetes Increases Autophagy in the Human Heart through Promotion of Beclin-1 Mediated Pathway. Int. J. Cardiol. 2016, 202, 13–20. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the Diabetic Heart: A Potential Pharmacotherapeutic Target in Diabetic Cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef]
- Ghosh, N.; Katare, R. Molecular Mechanism of Diabetic Cardiomyopathy and Modulation of microRNA Function by Synthetic Oligonucleotides. Cardiovasc. Diabetol. 2018, 17, 43. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Jiang, Z.; Gao, Y.; Sun, L.; Li, R.; Chen, M.; Lin, C.; Liu, D. Non-Coding RNAs in Diabetes Mellitus and Diabetic Cardiovascular Disease. Front. Endocrinol. 2022, 13, 961802. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Manning, P.; Katare, R. Cardiovascular microRNAs: As Modulators and Diagnostic Biomarkers of Diabetic Heart Disease. Cardiovasc. Diabetol. 2014, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.; Diao, X.; Wang, X.; Chen, R.; Hu, B. MicroRNAs Involved in the Mitogen-Activated Protein Kinase Cascades Pathway During Glucose-Induced Cardiomyocyte Hypertrophy. Am. J. Pathol. 2011, 179, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Xu, N.; Zhang, H.; Liao, W.; Wang, Y.; Wang, S.; Zhang, S.; Jiang, Y.; Xie, W.; Zhang, Y. Persistent High Glucose Induced EPB41L4A-AS1 Inhibits Glucose Uptake via GCN5 Mediating Crotonylation and Acetylation of Histones and Non-histones. Clin. Transl. Med. 2022, 12, e699. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 Inhibitors and GLP-1 Receptor Agonists: Established and Emerging Indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef] [PubMed]
- van der Aart-van der Beek, A.B.; de Boer, R.A.; Heerspink, H.J.L. Kidney and Heart Failure Outcomes Associated with SGLT2 Inhibitor Use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The Evolving Story of Incretins (GIP and GLP-1) in Metabolic and Cardiovascular Disease: A Pathophysiological Update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Pedrosa, M.R.; Franco, D.R.; Gieremek, H.W.; Vidal, C.M.; Bronzeri, F.; de Cassia Rocha, A.; de Carvalho Cara, L.G.; Fogo, S.L.; Eliaschewitz, F.G. GLP-1 Agonist to Treat Obesity and Prevent Cardiovascular Disease: What Have We Achieved so Far? Curr. Atheroscler. Rep. 2022, 24, 867–884. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, Y.; Meng, C.; Ding, X.; Huang, J.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. The Role of the Microbiome in Diabetes Mellitus. Diabetes Res. Clin. Pract. 2021, 172, 108645. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Joscelyn, J.; Kasper, L.H. Digesting the Emerging Role for the Gut Microbiome in Central Nervous System Demyelination. Mult. Scler. 2014, 20, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Zabell, A.; Tang, W.H.W. Targeting the Microbiome in Heart Failure. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Fuentes, S.; van den Bogert, B.; Honkanen, H.; de Vos, W.M.; Welling, G.W.; Hyöty, H.; Harmsen, H.J.M. Aberrant Gut Microbiota Composition at the Onset of Type 1 Diabetes in Young Children. Diabetologia 2014, 57, 1569–1577. [Google Scholar] [CrossRef]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate Immunity and Intestinal Microbiota in the Development of Type 1 Diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef]
- Menser, M.A.; Forrest, J.M.; Bransby, R.D. Rubella infection and diabetes mellitus. Lancet 1978, 311, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Beyan, H.; Wen, L.; Leslie, R.D. Guts, Germs, and Meals: The Origin of Type 1 Diabetes. Curr. Diabetes Rep. 2012, 12, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.A.; Bittencourt, M.S.; Preston, R.; Singh, A.; Romagnolli, C.; Ghoshhajra, B.; Shah, R.; Abbasi, S.; Abbara, S.; Nasir, K.; et al. Obesity, Metabolic Syndrome and Cardiovascular Prognosis: From the Partners Coronary Computed Tomography Angiography Registry. Cardiovasc. Diabetol. 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Tedjo, D.I.; Blanchet, L.; Bodelier, A.; Pierik, M.J.; Masclee, A.A.M.; Dallinga, J.; Savelkoul, P.H.M.; Jonkers, D.M.A.E.; Penders, J.; et al. Volatile Metabolites in Breath Strongly Correlate with Gut Microbiome in CD Patients. Anal. Chim. Acta 2018, 1025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Dwivedi, G.; O’Gara, F.; Caparros-Martin, J.; Ward, N.C. The Gut Microbiome and Cardiovascular Disease: Current Knowledge and Clinical Potential. Am. J. Physiol.-Heart Circ. Physiol. 2019, 317, H923–H938. [Google Scholar] [CrossRef]
- Emoto, T.; Yamashita, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; Mizoguchi, T.; et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: A Possible Link between Gut Microbiota and Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 908–921. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis Signatures of Gut Microbiota in Coronary Artery Disease. Physiol. Genomics 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Ott, S.J.; El Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kühbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of Diverse Bacterial Signatures in Atherosclerotic Lesions of Patients With Coronary Heart Disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure After Myocardial Infarction in the Rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.-Y. Probiotics and Their Fermented Food Products Are Beneficial for Health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Tokarek, J.; Budny, E.; Saar, M.; Kućmierz, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. Int. J. Mol. Sci. 2023, 24, 1377. [Google Scholar] [CrossRef] [PubMed]
- Thushara, R.M.; Gangadaran, S.; Solati, Z.; Moghadasian, M.H. Cardiovascular Benefits of Probiotics: A Review of Experimental and Clinical Studies. Food Funct. 2016, 7, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.; Shoback, D. Greenspan’s Basic & Clinical Endocrinology, 9th ed.; Gardner, D.G., Greenspan, F.S., Eds.; A Lange Medical Book; McGraw Hill Medical: New York, NY, USA, 2011; ISBN 978-0-07-162243-1. [Google Scholar]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 Receptor Agonists (GLP-1RAs): Cardiovascular Actions and Therapeutic Potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, S.; Gao, L.; Liu, H. Cardiovascular Outcomes Associated with SGLT-2 Inhibitors versus Other Glucose-Lowering Drugs in Patients with Type 2 Diabetes: A Real-World Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0244689. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Simeon, V.; Galiero, R.; Caturano, A.; De Nicola, L.; Chiodini, P.; Rinaldi, L.; Salvatore, T.; Lettieri, M.; Nevola, R.; et al. The Number of Risk Factors Not at Target Is Associated with Cardiovascular Risk in a Type 2 Diabetic Population with Albuminuria in Primary Cardiovascular Prevention. Post-Hoc Analysis of the NID-2 Trial. Cardiovasc. Diabetol. 2022, 21, 235. [Google Scholar] [CrossRef]
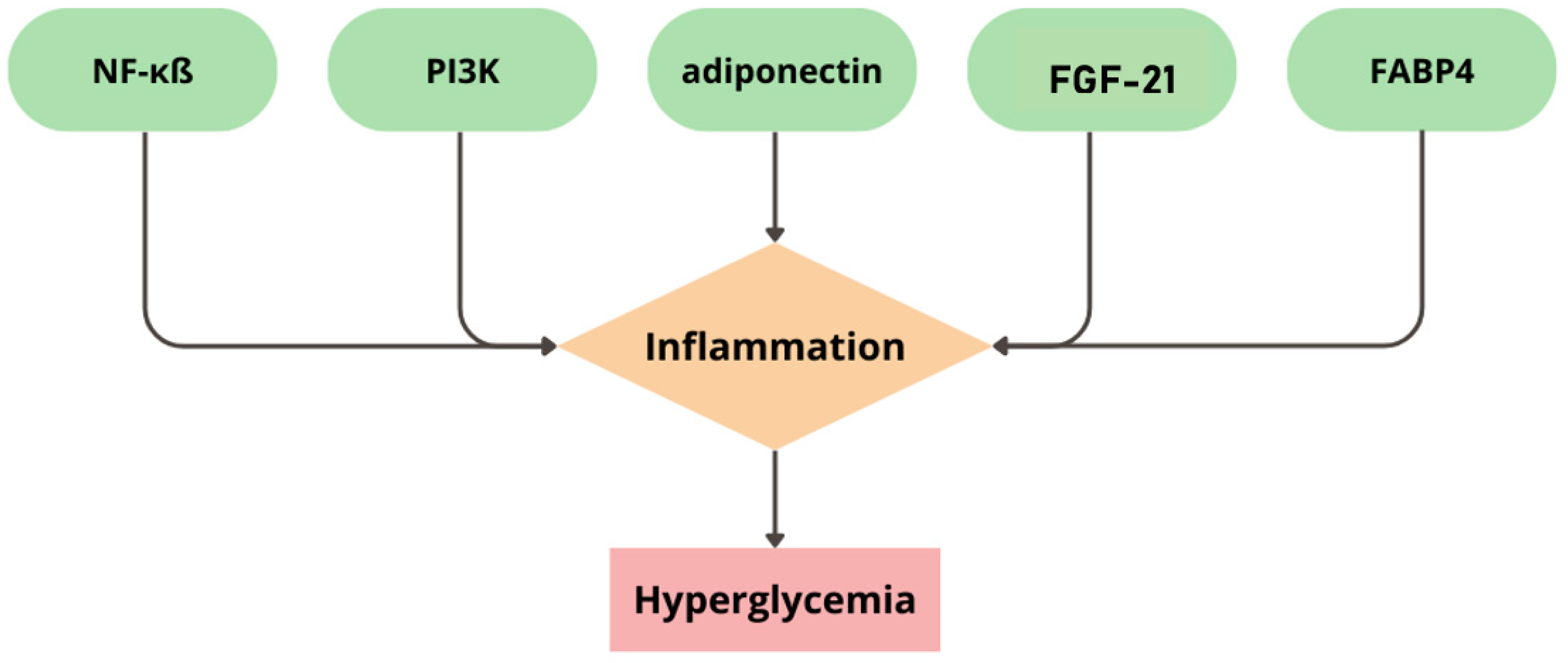

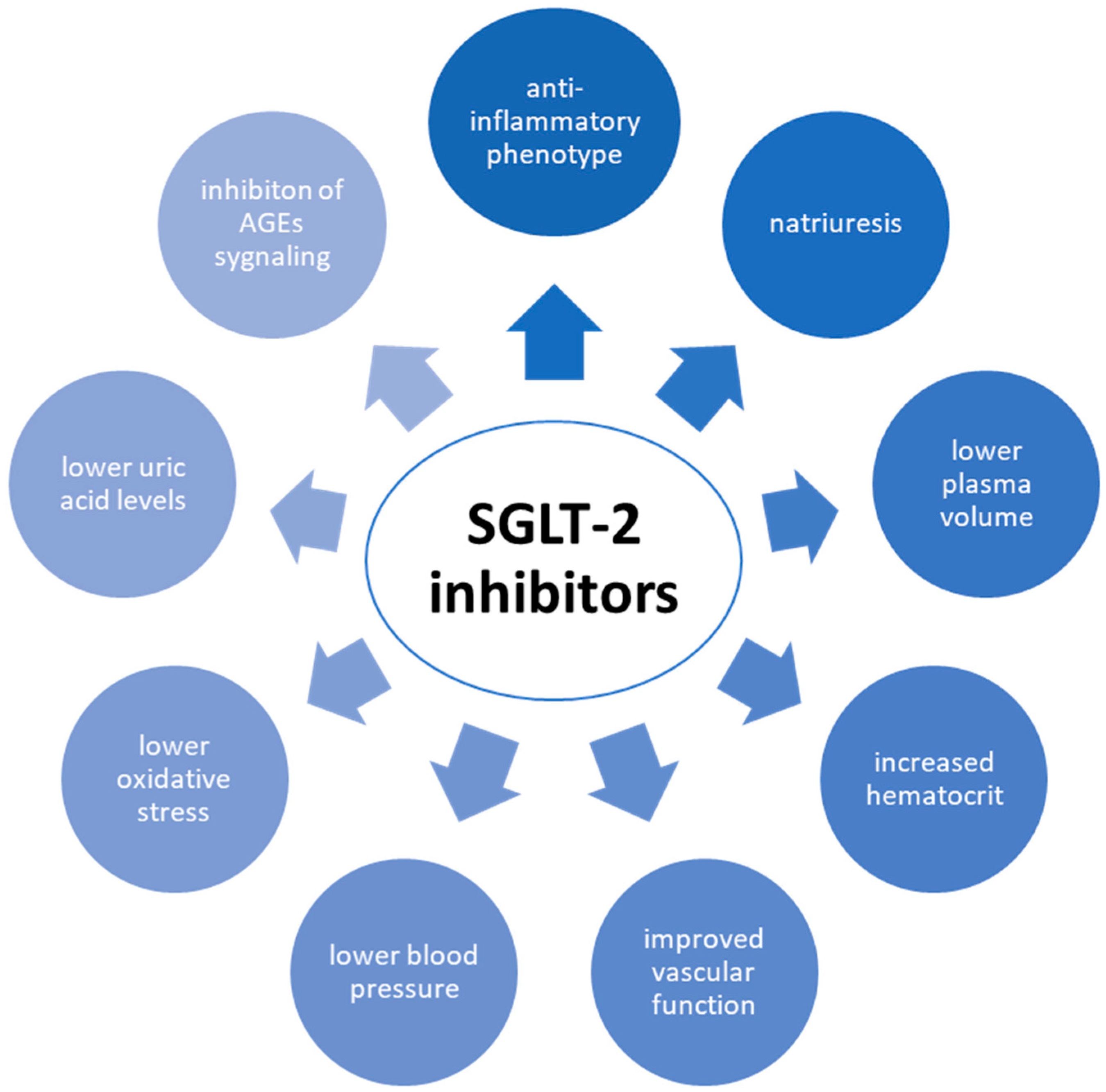
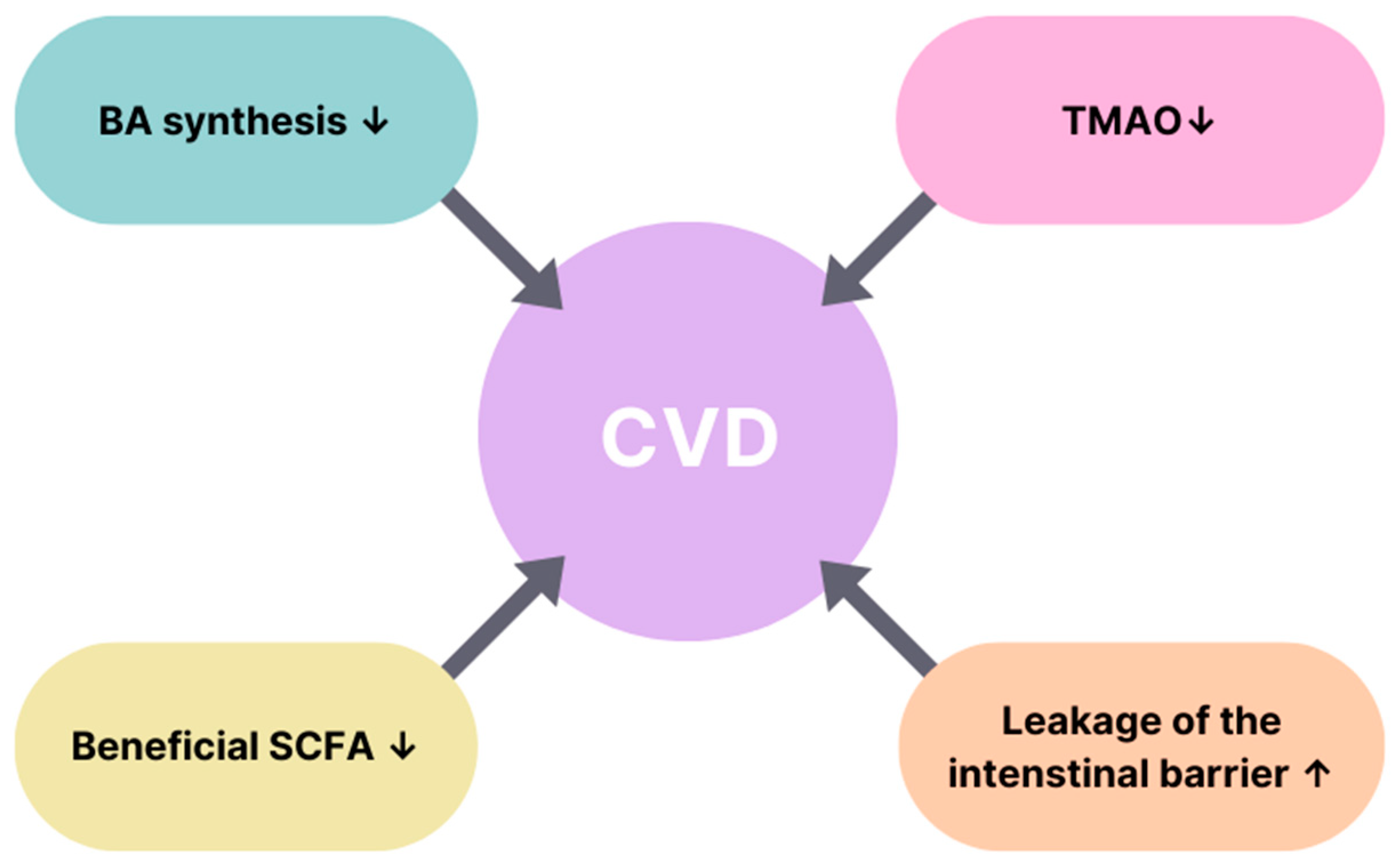
| Risk Factors | Diabetes | CVD |
|---|---|---|
| Inflammatory factors | increased insulin resistance by IRS-1 [70] | endothelial injury, dysregulation of coagulation [71] |
| Oxidative stress | increased hexosamine pathway of glucose oxidation [72] | impaired mitochondrial electron transport, damage of DNA, leading to activation of stress-sensitive pathways [73] |
| Epigenetics | methylation of INS, PDX1, PPARGC1A, and GLP1R [74] | hydroxymethylation of myosin heavy chain 7, inhibition of BET protein [75] |
| Lipid dysregulations | higher amount of VLDL [76] | increased risk of atherosclerosis [62,76] |
| Sleep apnoea | apnoea resulting in lower tissue sensitivity to insulin [63,64,65] | multifactorial pathophysiological processes resulting in HTN [77] |
| NAFLD | leading to bigger insulin resistance [68,69] | increased risk of myocardial infarction and brain stroke [78] |
| PCOS | higher risk of insulin resistance [76,77] | higher risk of hypertension in post-reproductive life [79] |
| RAAS | higher NADPH activity, resulting in cardiac dysfunction [80,81] | leading to local inflammation, which causes arterial thrombosis [82] |
| mRNA | m6A mRNA methylation [83] | m6A mRNA methylation [84] |
| Cardiovascular Disease | Alteration of Bacterial Metabolites |
|---|---|
| Stroke | Faecal butyrate decreased |
| TMAO increased | |
| Carotid artery stenosis | SCFA production by intestinal microbiota decreased |
| Mesenteric ischaemia | SCFA production by intestinal microbiota decreased |
| Carotid artery disease | BA decreased |
| TMAO increased | |
| Peripheral artery disease | Synthesis of BAs decreased |
| TMAO increased |
| Cardiovascular Disease | Changes in the Composition of Microbiota |
|---|---|
| Atherosclerosis | Collinsella, Escherichia coli, Enterobacter aerogenes, Klebsiella sp. increased [134] |
| Carotid artery disease (CAD) | Lactobacillus, Streptococcus, Escherichia/Shigella, Enterococcus increased Bacteroides, Prevotella, Faecalibacterium, Subdoligranulum, Roseburia, Eubacterium rectale decreased [140,141] |
| Atheromatous plaques | Proteus vulgaris, Staphylococcus sp., Klebsiella pneumoniae, Streptococcus sp. increased [142] |
| Comorbidity | Genera of Probiotic Bacteria |
|---|---|
| Diabetes mellitus type 2 | Lactobacillus reuteri Lactobacillus casei Lactobacillus plantarum Bifidobacterium bifidum Lactobacillus rhamnosus Bifidobacterium lactis |
| Hypertension | Lactobacillus bulgaricus Lactobacillus casei Streptococcus thermophilus Lactobacillus helveticus Saccharomyces cerevisia |
| Obesity | Bifidobacterium animalis sp. lactis Lactobacillus rhamnosus Lactobacillus curvatus Lactobacillus plantarum |
| Hypercholesterolaemia | Lactobacillus reuteri Lactobacillus curvatus Streptococcus thermophilus Enterococcus faecalis Lactobacillus casei Saccharomyces boulardii Bifidobacterium longum Lactobacillus acidophilius |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarek, J.; Budny, E.; Saar, M.; Stańczak, K.; Wojtanowska, E.; Młynarska, E.; Rysz, J.; Franczyk, B. Molecular Processes Involved in the Shared Pathways between Cardiovascular Diseases and Diabetes. Biomedicines 2023, 11, 2611. https://doi.org/10.3390/biomedicines11102611
Tokarek J, Budny E, Saar M, Stańczak K, Wojtanowska E, Młynarska E, Rysz J, Franczyk B. Molecular Processes Involved in the Shared Pathways between Cardiovascular Diseases and Diabetes. Biomedicines. 2023; 11(10):2611. https://doi.org/10.3390/biomedicines11102611
Chicago/Turabian StyleTokarek, Julita, Emilian Budny, Maciej Saar, Kamila Stańczak, Ewa Wojtanowska, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2023. "Molecular Processes Involved in the Shared Pathways between Cardiovascular Diseases and Diabetes" Biomedicines 11, no. 10: 2611. https://doi.org/10.3390/biomedicines11102611






