Novel Genetically Engineered Probiotics for Targeted Elimination of Pseudomonas aeruginosa in Intestinal Colonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture
2.2. Plasmid Construction
2.3. Characterization of 3OC12HSL Sensor Module
2.4. Characterization of AMP Secretion Module for the Inducible Production of PA2-GNU7
2.5. 3OC12HSL-Inducible PA2-GNU7 Secretion by Engineered EcN
2.6. Supernatant Activity Test
2.7. P. aeruginosa Growth-Inhibition in Co-Culture with Engineered EcN
2.8. Animal Studies
2.9. Statistical Analysis
3. Results
3.1. Plasmid Design and Construction
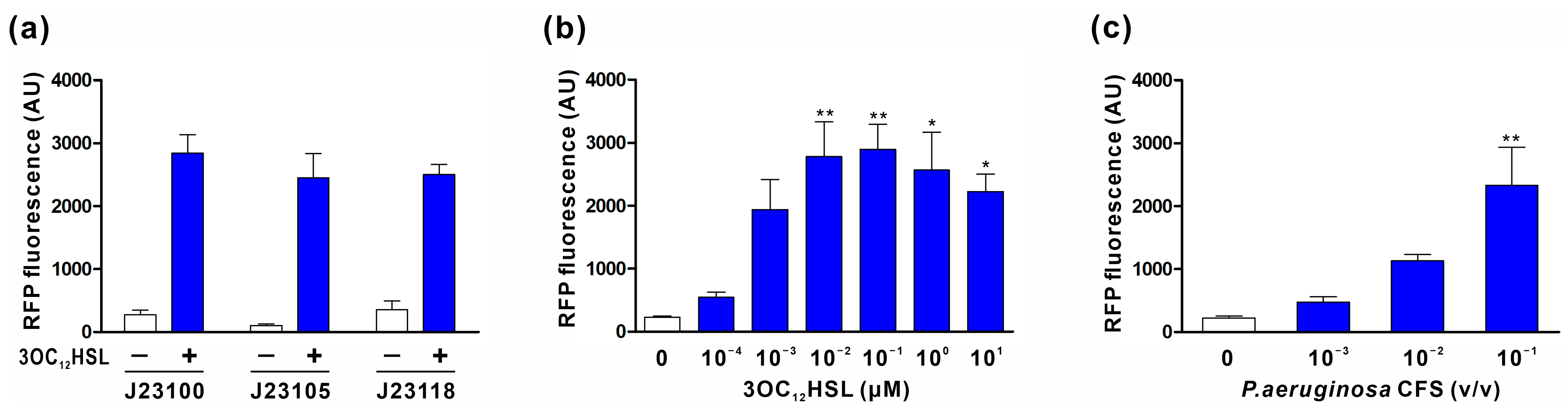
3.2. Characterization of the 3OC12HSL Sensor Module
3.3. Characterization of AMP Secretion Module for the Inducible Production of PA2-GNU7
3.4. 3OC12HSL-Inducible PA2-GNU7 Secretion by Engineered EcN
3.5. Verification of Engineered EcN Activity against P. aeruginosa
3.6. Evaluation of Engineered EcN in a P. aeruginosa-Infected Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neil, J. Trackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/Publications.html (accessed on 22 August 2023).
- Prasad, N.K.; Seiple, I.B.; Cirz, R.T.; Rosenberg, O.S. Leaks in the Pipeline: A Failure Analysis of Gram-Negative Antibiotic Development from 2010 to 2020. Antimicrob. Agents Chemother. 2022, 66, e0005422. [Google Scholar] [CrossRef]
- Kang, C.I.; Kim, S.H.; Kim, H.B.; Park, S.W.; Choe, Y.J.; Oh, M.D.; Kim, E.C.; Choe, K.W. Pseudomonas aeruginosa bacteremia: Risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 2003, 37, 745–751. [Google Scholar] [CrossRef]
- De Bentzmann, S.; Plesiat, P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 2011, 13, 1655–1665. [Google Scholar] [CrossRef]
- Janapatla, R.P.; Dudek, A.; Chen, C.L.; Chuang, C.H.; Chien, K.Y.; Feng, Y.; Yeh, Y.M.; Wang, Y.H.; Chang, H.J.; Lee, Y.C.; et al. Marine prebiotics mediate decolonization of Pseudomonas aeruginosa from gut by inhibiting secreted virulence factor interactions with mucins and enriching Bacteroides population. J. Biomed. Sci. 2023, 30, 9. [Google Scholar] [CrossRef]
- Gomez-Zorrilla, S.; Camoez, M.; Tubau, F.; Canizares, R.; Periche, E.; Dominguez, M.A.; Ariza, J.; Pena, C. Prospective observational study of prior rectal colonization status as a predictor for subsequent development of Pseudomonas aeruginosa clinical infections. Antimicrob. Agents Chemother. 2015, 59, 5213–5219. [Google Scholar] [CrossRef]
- Wheatley, R.M.; Caballero, J.D.; van der Schalk, T.E.; De Winter, F.H.R.; Shaw, L.P.; Kapel, N.; Recanatini, C.; Timbermont, L.; Kluytmans, J.; Esser, M.; et al. Gut to lung translocation and antibiotic mediated selection shape the dynamics of Pseudomonas aeruginosa in an ICU patient. Nat. Commun. 2022, 13, 6523. [Google Scholar] [CrossRef]
- Markou, P.; Apidianakis, Y. Pathogenesis of intestinal Pseudomonas aeruginosa infection in patients with cancer. Front. Cell Infect. Microbiol. 2014, 3, 115. [Google Scholar] [CrossRef]
- Okuda, J.; Hayashi, N.; Okamoto, M.; Sawada, S.; Minagawa, S.; Yano, Y.; Gotoh, N. Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na,K-ATPase regulator, FXYD3. Infect. Immun. 2010, 78, 4511–4522. [Google Scholar] [CrossRef]
- Kim, S.W.; Peck, K.R.; Jung, S.I.; Kim, Y.S.; Kim, S.; Lee, N.Y.; Song, J.H. Pseudomonas aeruginosa as a potential cause of antibiotic-associated diarrhea. J. Korean Med. Sci. 2001, 16, 742–744. [Google Scholar] [CrossRef]
- Hoff, R.T.; Patel, A.; Shapiro, A. Pseudomonas aeruginosa: An Uncommon Cause of Antibiotic-Associated Diarrhea in an Immunocompetent Ambulatory Adult. Case Rep. Gastrointest. Med. 2020, 2020, 6261748. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Janapatla, R.P.; Wang, Y.H.; Chang, H.J.; Huang, Y.C.; Lin, T.Y.; Chiu, C.H. Pseudomonas aeruginosa-associated Diarrheal Diseases in Children. Pediatr. Infect. Dis. J. 2017, 36, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Wang, Y.H.; Chang, H.J.; Chen, H.L.; Huang, Y.C.; Lin, T.Y.; Ozer, E.A.; Allen, J.P.; Hauser, A.R.; Chiu, C.H. Shanghai fever: A distinct Pseudomonas aeruginosa enteric disease. Gut 2014, 63, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Jabbour, J.F.; Kanj, S.S. Current choices of antibiotic treatment for Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2020, 33, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Hancock, R.E. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007, 64, 922–933. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Roca-Pinilla, R.; Lisowski, L.; Aris, A.; Garcia-Fruitos, E. The future of recombinant host defense peptides. Microb. Cell Factories 2022, 21, 267. [Google Scholar] [CrossRef]
- Gardiner, G.E.; Rea, M.C.; O’Riordan, B.; O’Connor, P.; Morgan, S.M.; Lawlor, P.G.; Lynch, P.B.; Cronin, M.; Ross, R.P.; Hill, C. Fate of the two-component lantibiotic lacticin 3147 in the gastrointestinal tract. Appl. Environ. Microbiol. 2007, 73, 7103–7109. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Sheng, H.; Shen, X.; Sun, X.; Yan, Y.; Wang, J.; Yuan, Q. Engineering probiotics as living diagnostics and therapeutics for improving human health. Microb. Cell Factories 2020, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Pitta, A.; Broset, E.; de la Fuente-Nunez, C. Probiotic engineering strategies for the heterologous production of antimicrobial peptides. Adv. Drug Deliv. Rev. 2021, 176, 113863. [Google Scholar] [CrossRef] [PubMed]
- Pedrolli, D.B.; Ribeiro, N.V.; Squizato, P.N.; de Jesus, V.N.; Cozetto, D.A.; Tuma, R.B.; Gracindo, A.; Cesar, M.B.; Freire, P.J.; da Costa, A.F.; et al. Engineering Microbial Living Therapeutics: The Synthetic Biology Toolbox. Trends Biotechnol. 2019, 37, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Geldart, K.; Forkus, B.; McChesney, E.; McCue, M.; Kaznessis, Y.N. pMPES: A Modular Peptide Expression System for the Delivery of Antimicrobial Peptides to the Site of Gastrointestinal Infections Using Probiotics. Pharmaceuticals 2016, 9, 60. [Google Scholar] [CrossRef]
- Volzing, K.; Borrero, J.; Sadowsky, M.J.; Kaznessis, Y.N. Antimicrobial peptides targeting Gram-negative pathogens, produced and delivered by lactic acid bacteria. ACS Synth. Biol. 2013, 2, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Geldart, K.G.; Kommineni, S.; Forbes, M.; Hayward, M.; Dunny, G.M.; Salzman, N.H.; Kaznessis, Y.N. Engineered E. coli Nissle 1917 for the reduction of vancomycin-resistant Enterococcus in the intestinal tract. Bioeng. Transl. Med. 2018, 3, 197–208. [Google Scholar] [CrossRef]
- Zhang, G.; Brokx, S.; Weiner, J.H. Extracellular accumulation of recombinant proteins fused to the carrier protein YebF in Escherichia coli. Nat. Biotechnol. 2006, 24, 100–104. [Google Scholar] [CrossRef]
- Borrero, J.; Chen, Y.; Dunny, G.M.; Kaznessis, Y.N. Modified lactic acid bacteria detect and inhibit multiresistant Enterococci. ACS Synth. Biol. 2015, 4, 299–306. [Google Scholar] [CrossRef]
- Gupta, S.; Bram, E.E.; Weiss, R. Genetically programmable pathogen sense and destroy. ACS Synth. Biol. 2013, 2, 715–723. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Koh, E.; Wong, A.; March, J.C.; Bentley, W.E.; Lee, Y.S.; Chang, M.W. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat. Commun. 2017, 8, 15028. [Google Scholar] [CrossRef]
- Saeidi, N.; Wong, C.K.; Lo, T.M.; Nguyen, H.X.; Ling, H.; Leong, S.S.; Poh, C.L.; Chang, M.W. Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol. Syst. Biol. 2011, 7, 521. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2014, 69, 121–132. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Krupa, R.A.; Zhang, F.; Hajimorad, M.; Holtz, W.J.; Prasad, N.; Lee, S.K.; Keasling, J.D. BglBrick vectors and datasheets: A synthetic biology platform for gene expression. J. Biol. Eng. 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, H.; Jung, I.Y.; Cho, J.H. A20 Inhibits LPS-Induced Inflammation by Regulating TRAF6 Polyubiquitination in Rainbow Trout. Int. J. Mol. Sci. 2021, 22, 9801. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Sondermann, H.; O’Toole, G.A. Type 1 Does the Two-Step: Type 1 Secretion Substrates with a Functional Periplasmic Intermediate. J. Bacteriol. 2018, 200, e00168-18. [Google Scholar] [CrossRef]
- Burdette, L.A.; Leach, S.A.; Wong, H.T.; Tullman-Ercek, D. Developing Gram-negative bacteria for the secretion of heterologous proteins. Microb. Cell Factories 2018, 17, 196. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Luong, H.X.; Ngan, H.D.; Thi Phuong, H.B.; Quoc, T.N.; Tung, T.T. Multiple roles of ribosomal antimicrobial peptides in tackling global antimicrobial resistance. R. Soc. Open Sci. 2022, 9, 211583. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Tan, M.H.; Koh, E.; Ho, C.L.; Poh, C.L.; Chang, M.W. Reprogramming microbes to be pathogen-seeking killers. ACS Synth. Biol. 2014, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Rasouliha, B.H.; Ling, H.; Ho, C.L.; Chang, M.W. A predicted immunity protein confers resistance to pyocin S5 in a sensitive strain of Pseudomonas aeruginosa. ChemBioChem 2013, 14, 2444–2446. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, S.; Starkevic, U.; Misiunas, A.; Vitkauskiene, A.; Gleba, Y.; Razanskiene, A. Plant-expressed pyocins for control of Pseudomonas aeruginosa. PLoS ONE 2017, 12, e0185782. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, S.; Dapkute, V.; Misiunas, A.; Balzaris, M.; Thommes, P.; Sattar, A.; Gleba, Y.; Razanskiene, A. Chimeric bacteriocin S5-PmnH engineered by domain swapping efficiently controls Pseudomonas aeruginosa infection in murine keratitis and lung models. Sci. Rep. 2022, 12, 5865. [Google Scholar] [CrossRef] [PubMed]
- Snopkova, K.; Dufkova, K.; Klimesova, P.; Vanerkova, M.; Ruzicka, F.; Hola, V. Prevalence of bacteriocins and their co-association with virulence factors within Pseudomonas aeruginosa catheter isolates. Int. J. Med. Microbiol. 2020, 310, 151454. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, J.; Pu, L.; Fu, S.; Xing, X.; Zhang, R.; Jin, F. Remotely Controllable Engineered Bacteria for Targeted Therapy of Pseudomonas aeruginosa Infection. ACS Synth. Biol. 2023, 12, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Pons, A.M.; Delalande, F.; Duarte, M.; Benoit, S.; Lanneluc, I.; Sable, S.; Van Dorsselaer, A.; Cottenceau, G. Genetic analysis and complete primary structure of microcin L. Antimicrob. Agents Chemother. 2004, 48, 505–513. [Google Scholar] [CrossRef]
- Scholz, R.L.; Greenberg, E.P. Positive Autoregulation of an Acyl-Homoserine Lactone Quorum-Sensing Circuit Synchronizes the Population Response. mBio 2017, 8, e01079-17. [Google Scholar] [CrossRef]
- Huang, J.J.; Han, J.I.; Zhang, L.H.; Leadbetter, J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003, 69, 5941–5949. [Google Scholar] [CrossRef]
- Kim, S.Y.; Parker, J.K.; Gonzalez-Magaldi, M.; Telford, M.S.; Leahy, D.J.; Davies, B.W. Export of Diverse and Bioactive Small Proteins through a Type I Secretion System. Appl. Environ. Microbiol. 2023, 89, e0033523. [Google Scholar] [CrossRef]
- Parker, J.K.; Davies, B.W. Microcins reveal natural mechanisms of bacterial manipulation to inform therapeutic development. Microbiology 2022, 168, 001175. [Google Scholar] [CrossRef]
- Seo, E.J.; Weibel, S.; Wehkamp, J.; Oelschlaeger, T.A. Construction of recombinant E. coli Nissle 1917 (EcN) strains for the expression and secretion of defensins. Int. J. Med. Microbiol. 2012, 302, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wibowo, D.; Sainsbury, F.; Zhao, C.X. Design and production of a novel antimicrobial fusion protein in Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 8763–8772. [Google Scholar] [CrossRef] [PubMed]
- Mortzfeld, B.M.; Palmer, J.D.; Bhattarai, S.K.; Dupre, H.L.; Mercado-Lubio, R.; Silby, M.W.; Bang, C.; McCormick, B.A.; Bucci, V. Microcin MccI47 selectively inhibits enteric bacteria and reduces carbapenem-resistant Klebsiella pneumoniae colonization in vivo when administered via an engineered live biotherapeutic. Gut Microbes 2022, 14, 2127633. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadis, G.; Destoumieux-Garzon, D.; Lombard, C.; Rebuffat, S.; Peduzzi, J. Isolation and characterization of two members of the siderophore-microcin family, microcins M and H47. Antimicrob. Agents Chemother. 2010, 54, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, M.; Nuccio, S.P.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef]





| Strain/Plasmid | Description | Source |
|---|---|---|
| E. coli TOP10 | Host strain used for cloning and AMP expression | Invitrogen |
| E. coli Nissle 1917 | Nonpathogenic human commensal used in probiotics | Mutaflor |
| P. aeruginosa H103 | PAO1 wild-type prototroph | University of British Columbia |
| P. aeruginosa NCCP 14571 | MeropenemR, ceftazidimeR, tobramycinR, gentamicinR, amikacinR, cefepimeR, cefotaximeR, ciprofloxacinR, imipenemR, piperacillinR, piperacillin-tazobactamR | NCCP |
| pBbE0k-RFP | KanR, ColE1, constitutive mRFP1 | Addgene |
| pBbE1a-RFP | AmpR, ColE1, constitutive LacI (lacIq), Ptrc-controlled mRFP1 | Addgene |
| S100-RFP | pBbE0k, constitutive LasR (J23100), PlasI-controlled mRFP1 | This study |
| S105-RFP | pBbE0k, constitutive LasR (J23105), PlasI-controlled mRFP1 | This study |
| S118-RFP | pBbE0k, constitutive LasR (J23118), PlasI-controlled mRFP1 | This study |
| Y | pBbE1a, Ptrc-controlled YebF with C-terminal 6 × His-tag | This study |
| YP | pBbE1a, Ptrc-controlled YebF-(G4S)2-PA2-GNU7 fusion protein with C-terminal 6 × His-tag | This study |
| P | pBbE1a, Ptrc-controlled PA2-GNU7 with N-terminal microcin V signal peptide (SPmccV) and C-terminal 6 × His-tag | This study |
| PAB | pBbE1a, Ptrc-controlled SPmccV-PA2-GNU7 with C-terminal 6 × His-tag and CvaA/B | This study |
| S100-YP | pBbE0k, constitutive LasR (J23100), PlasI-controlled YebF-(G4S)2-PA2-GNU7 fusion protein with C-terminal 6 × His-tag | This study |
| S100-PAB | pBbE0k, constitutive LasR (J23100), PlasI-controlled SPmccV-PA2-GNU7 with C-terminal 6 × His-tag and CvaA/B | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jang, J.H.; Jung, I.Y.; Kim, H.R.; Cho, J.H. Novel Genetically Engineered Probiotics for Targeted Elimination of Pseudomonas aeruginosa in Intestinal Colonization. Biomedicines 2023, 11, 2645. https://doi.org/10.3390/biomedicines11102645
Kim H, Jang JH, Jung IY, Kim HR, Cho JH. Novel Genetically Engineered Probiotics for Targeted Elimination of Pseudomonas aeruginosa in Intestinal Colonization. Biomedicines. 2023; 11(10):2645. https://doi.org/10.3390/biomedicines11102645
Chicago/Turabian StyleKim, Hyun, Ju Hye Jang, In Young Jung, Ha Rang Kim, and Ju Hyun Cho. 2023. "Novel Genetically Engineered Probiotics for Targeted Elimination of Pseudomonas aeruginosa in Intestinal Colonization" Biomedicines 11, no. 10: 2645. https://doi.org/10.3390/biomedicines11102645




