Targeting FGFR Pathways in Gastrointestinal Cancers: New Frontiers of Treatment
Abstract
:1. Introduction
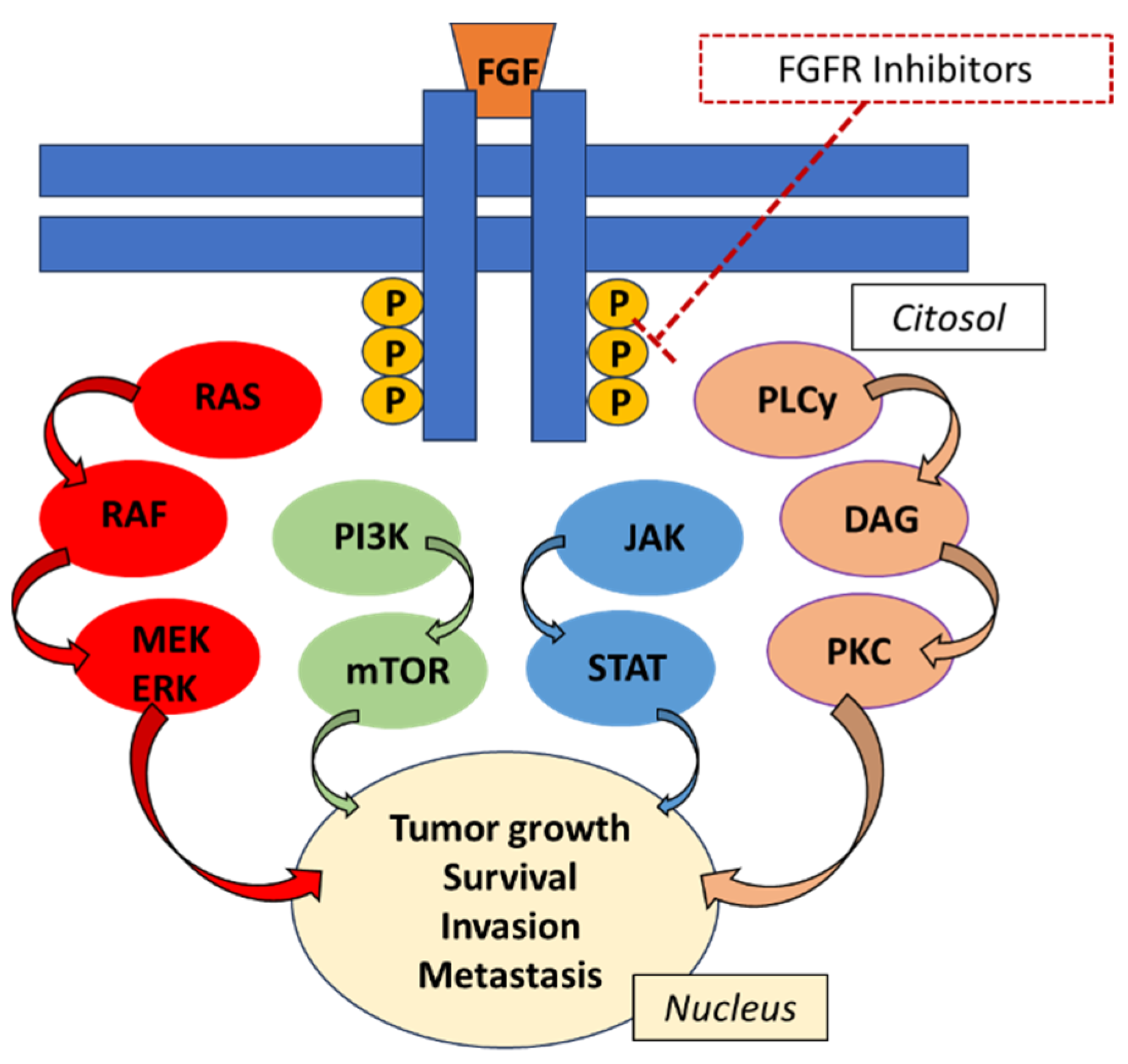
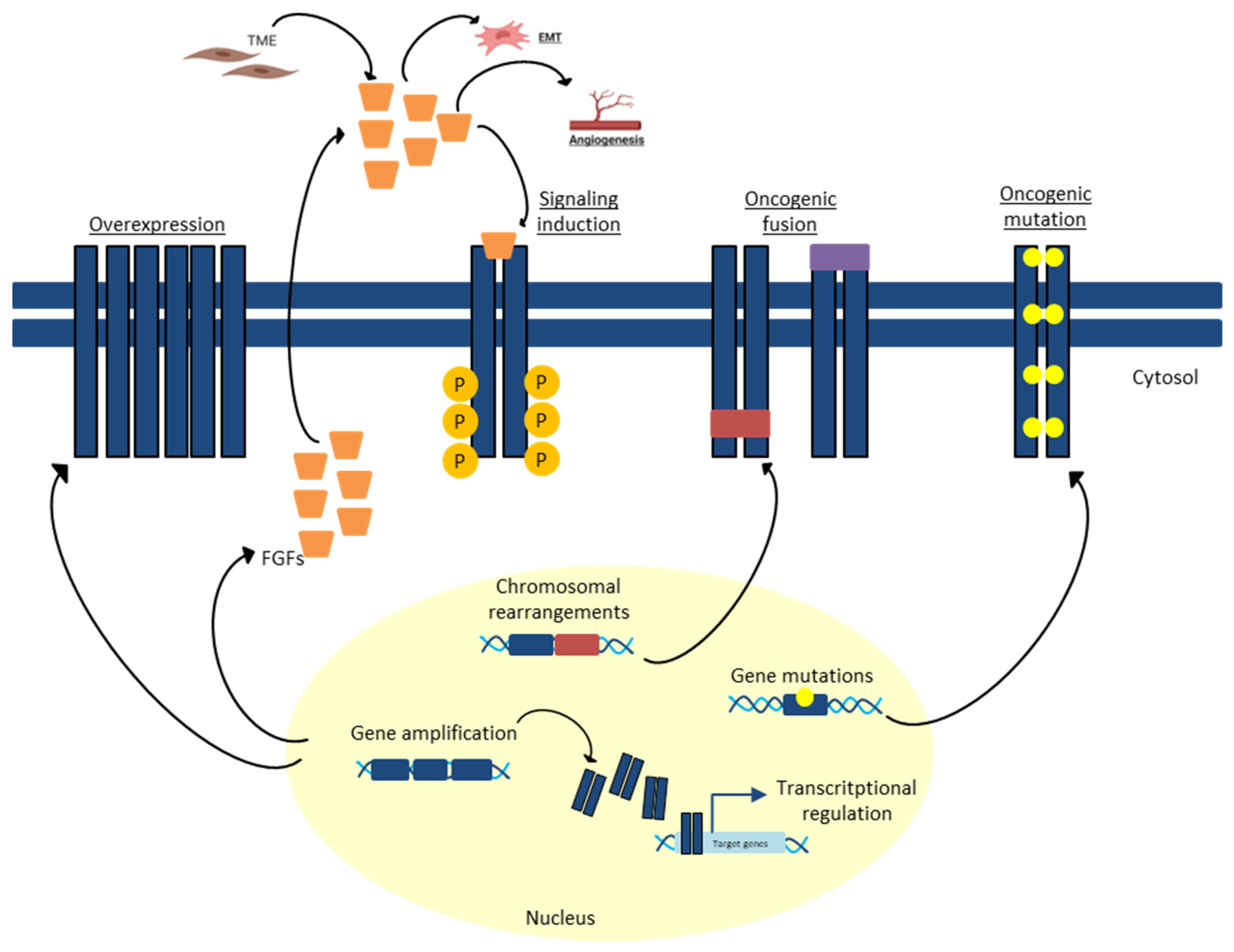
2. FGFR Pathway and Its Alterations in Gastrointestinal Cancers
- -
- FGF overproduction
- -
- FGFR mutations
- -
- Chromosomal translocation
- -
- FGFR gene amplification
- -
- Altered FGFR splicing
- -
- Germline single-nucleotide polymorphisms
3. Targeting FGFR in Cholangiocarcinoma and Pancreatic Cancer
4. Targeting FGFR in Gastric Cancer
5. Targeting FGFR in Colorectal Cancer
6. Discussion
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.T.; Li, N.G.; Zhang, Y.M.; Xie, W.C.; Yang, S.P.; Lu, T.; Shi, Z.H. Recent advance in the development of novel, selective and potent FGFR inhibitors. Eur. J. Med. Chem. 2019, 186, 111884. [Google Scholar] [CrossRef] [PubMed]
- Trueb, B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell Mol. Life Sci. 2011, 68, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Urbini, M.; Indio, V.; Tarantino, G.; Ravegnini, G.; Angelini, S.; Nannini, M.; Saponara, M.; Santini, D.; Ceccarelli, C.; Fiorentino, M.; et al. Gain of FGF4 is a frequent event in KIT/PDGFRA/SDH/RAS-P WT GIST. Genes Chromosomes Cancer 2019, 58, 636–642. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Drier, Y.; Johnstone, S.E.; Hemming, M.L.; Tarjan, D.R.; Hegazi, E.; Shareef, S.J.; Javed, N.M.; Raut, C.P.; Eschle, B.K.; et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature 2019, 575, 229–233. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 1, 259–267. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, K.H.; Park, J.G. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001, 9, 3541–3543. [Google Scholar]
- Ye, Y.; Shi, Y.; Zh, Y.; Du, C.; Wang, C.; Zhan, H.; Zheng, B.; Cao, X.; Sun, M.H.; Fu, H. The fibroblast growth factor receptor-4 Arg388 allele is associated with gastric cancer progression. Ann. Surg. Oncol. 2010, 12, 3354–3361. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, J.Y.; Kim, K.; Kim, K.M.; Kang, S.Y.; Lee, T.; Kim, S.T.; Park, S.H.; Park, Y.S.; Lim, H.Y.; et al. Detection of Fusion Genes Using a Targeted RNA Sequencing Panel in Gastrointestinal and Rare Cancers. J. Oncol. 2020, 2020, 4659062. [Google Scholar] [CrossRef]
- Costa, R.; Carneiro, B.A.; Taxter, T.; Tavora, F.A.; Kalyan, A.; Pai, S.A.; Chae, Y.K.; Giles, F.J. FGFR3-TACC3 fusion in solid tumors: Mini review. Oncotarget 2016, 34, 55924–55938. [Google Scholar] [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 4, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Chmielecki, J.; Tang, C.M.; Wang, K.; Heinrich, M.C.; Kang, G.; Corless, C.L.; Hong, D.; Fero, K.E.; Murphy, J.D.; et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J. Transl. Med. 2016, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ahn, T.; Bang, H.; Ham, J.S.; Kim, J.; Kim, S.T.; Jang, J.; Shim, M.; Kang, S.Y.; Park, S.H.; et al. Acquired resistance to LY2874455 in FGFR2-amplified gastric cancer through an emergence of novel FGFR2-ACSL5 fusion. Oncotarget 2017, 9, 15014–15022. [Google Scholar] [CrossRef]
- Matsumoto, K.; Arao, T.; Hamaguchi, T.; Shimada, Y.; Kato, K.; Oda, I.; Taniguchi, H.; Koizumi, F.; Yanagihara, K.; Sasaki, H.; et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br. J. Cancer 2012, 4, 727–732. [Google Scholar] [CrossRef]
- Gauglhofer, C.; Paur, J.; Schrottmaier, W.C.; Wingelhofer, B.; Huber, D.; Naegelen, I.; Pirker, C.; Mohr, T.; Heinzle, C.; Holzmann, K.; et al. Fibroblast growth factor receptor 4: A putative key driver for the aggressive phenotype of hepatocellular carcinoma. Carcinogenesis 2014, 10, 2331–2338. [Google Scholar] [CrossRef]
- Gordon, A.; Johnston, E.; Lau, D.K.; Starling, N. Targeting FGFR2 Positive Gastroesophageal Cancer: Current and Clinical Developments. Onco Targets Ther. 2022, 15, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.; Dizdar, L.; Behrens, B.; Goering, W.; Knoefel, W.T.; Wruck, W.; Stoecklein, N.H.; Adjaye, J. FGF Signalling in the Self-Renewal of Colon Cancer Organoids. Sci. Rep. 2019, 1, 17365. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Chaar, M.; Kamta, J.; Ait-Oudhia, S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther. 2018, 11, 6227–6237. [Google Scholar] [CrossRef]
- Kalyukina, M.; Yosaatmadja, Y.; Middleditch, M.J.; Patterson, A.V.; Smaill, J.B.; Squire, C.J. Tas-120 cancer target binding: Defining reactivity and revealing the first fibroblast growth factor receptor 1 (FGFR1) irreversible structure. ChemMedChem 2019, 4, 494–500. [Google Scholar] [CrossRef]
- Amadeo, E.; Rossari, F.; Vitiello, F.; Burgio, V.; Persano, M.; Cascinu, S.; Casadei-Gardini, A.; Rimini, M. Past, present, and future of FGFR inhibitors in cholangiocarcinoma: From biological mechanisms to clinical applications. Expert. Rev. Clin. Pharmacol. 2023, 7, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Tesfaye, A.; Tejani, M.; Cheung, E.; Eisenberg, P.; Scott, A.J.; Eng, C.; Hnatyszyn, J.; Marina, N.; Powers, J.; et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol. 2019, 18, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Tiong, K.; Mah, L.; Leong, C. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis Int. J. Program. Cell Death 2013, 12, 1447–1468. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J. Biochem. 2011, 149, 121–130. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 1, 181. [Google Scholar] [CrossRef]
- Xue, W.; Li, M.; Chen, L.; Sun, L.; Li, Y. Recent developments and advances of FGFR as a potential target in cancer. Future Med. Chem. 2018, 17, 2109–2126. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.; Arnedos, M.; Andre, F.; Soria, J. Fibroblast growth factor receptor inhibitors as a cancer treatment: From a biologic rationale to medical perspectives. Cancer Discov. 2013, 3, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.; Van Etten, R. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 2005, 2, 172–187. [Google Scholar] [CrossRef]
- Regeenes, R.; Silva, P.; Chang, H.; Arany, E.; Shukalyuk, A.; Audet, J.; Kilkenny, D.M.; Rocheleau, J.V. Fibroblast growth factor receptor 5 (FGFR5) is a co-receptor for FGFR1 that is up-regulated in beta-cells by cytokine-induced inflammation. J. Biol. Chem. 2018, 44, 17218–17228. [Google Scholar] [CrossRef]
- Roskoski, R. The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder. Pharmacol. Res. 2019, 151, 104567. [Google Scholar] [CrossRef]
- Ferguson, H.R.; Smith, M.P.; Francavilla, C. Fibroblast Growth Factor Receptors (FGFRs) and Noncanonical Partners in Cancer Signaling. Cells 2021, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.N.; Kilgour, E.; Smith, P.D. Molecular pathways: Fibroblast growth factor signaling: A new therapeutic opportunity in cancer. Clin. Cancer Res. 2012, 7, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Perry-Lalley, D.M.; Ohnmacht, G.A.; Bettinotti, M.P.; Yang, J.C. Identification of fibroblast growth factor-5 as an overexpressed antigen in multiple human adenocarcinomas. Cancer Res. 2001, 61, 5511–5516. [Google Scholar]
- Uematsu, S.; Higashi, T.; Nouso, K.; Kariyama, K.; Nakamura, S.; Suzuki, M.; Nakatsukasa, H.; Kobayashi, Y.; Hanafusa, T.; Tsuji, T.; et al. Altered expression of vascular endothelial growth factor, fibroblast growth factor-2 and endostatin in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2005, 20, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Qiu, W.R.; Wang, Y.P.; Hill, D.; Ring, B.D.; Scully, S.; Bolon, B.; DeRose, M.; Luethy, R.; Simonet, W.S.; et al. FGF-18, a novel member of the fibroblast growth factor family, stimulates hepatic and intestinal proliferation. Mol. Cell Biol. 1998, 18, 6063–6074. [Google Scholar] [CrossRef]
- Kin, M.; Sata, M.; Ueno, T.; Torimura, T.; Inuzuka, S.; Tsuji, R.; Sujaku, K.; Sakamoto, M.; Sugawara, H.; Tamaki, S.; et al. Basic fibroblast growth factor regulates proliferation and motility of human hepatoma cells by an autocrine mechanism. J. Hepatol. 1997, 27, 77–87. [Google Scholar] [CrossRef]
- Gauglhofer, C.; Sagmeister, S.; Schrottmaier, W.; Fischer, C.; Rodgarkia-Dara, C.; Mohr, T.; Stättner, S.; Bichler, C.; Kandioler, D.; Wrba, F.; et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology 2011, 53, 854–864. [Google Scholar] [CrossRef]
- Nicholes, K.; Guillet, S.; Tomlinson, E.; Hillan, K.; Wright, B.; Frantz, G.D.; Pham, T.A.; Dillard-Telm, L.; Tsai, S.P.; Stephan, J.P.; et al. A mouse model of hepatocellular carcinoma: Ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 2002, 160, 2295–2307. [Google Scholar] [CrossRef]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef]
- Babina, I.; Turner, N. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 5, 318–332. [Google Scholar] [CrossRef]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; LaQuaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors. JAMA Oncol. 2016, 2, 922. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Nardini, E.; Brents, L.A.; Schröck, E.; Ried, T.; Kuehl, W.M.; Bergsagel, P.L. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3. Nat. Genet. 1997, 16, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Qing, J.; Du, X.; Chen, Y.; Chan, P.; Li, H.; Wu, P.; Marsters, S.; Stawicki, S.; Tien, J.; Totpal, K.; et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J. Clin. Investig. 2009, 119, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017, 3, 252–263. [Google Scholar] [CrossRef]
- Xie, L.; Su, X.; Zhang, D.; Tang, L.; Xu, J.; Wang, M.; Yin, L.; Zhang, J.; Ye, K.; Wang, Z.; et al. Abstract 1643: AZD4547, a potent and selective inhibitor of FGF-receptor tyrosine kinases 1, 2 and 3, inhibits the growth of FGF-receptor 2 driven gastric cancer models in vitro and in vivo. Cancer Res. 2011, 71, 1643. [Google Scholar] [CrossRef]
- Ueda, T.; Sasaki, H.; Kuwahara, Y.; Nezu, M.; Shibuya, T.; Sakamoto, H.; Ishii, H.; Yanagihara, K.; Mafune, K.; Makuuchi, M.; et al. Deletion of the carboxyl-terminal exons of K-sam/FGFR2 by short homology-mediated recombination, generating preferential expression of specific messenger RNAs. Cancer Res. 1999, 59, 6080–6086. [Google Scholar]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef]
- Wesche, J.; Haglund, K.; Haugsten, E.M. Fibroblast growth factors and their receptors in cancer. Biochem. J. 2011, 437, 199–213. [Google Scholar] [CrossRef]
- Easton, D.F.; Pooley, K.A.; Dunning, A.M.; Pharoah, P.D.; Thompson, D.; Ballinger, D.G.; Struewing, J.P.; Morrison, J.; Field, H.; Luben, R.; et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007, 447, 1087–1093. [Google Scholar] [CrossRef]
- Garcia-Closas, M.; Hall, P.; Nevanlinna, H.; Pooley, K.; Morrison, J.; Richesson, D.A.; Bojesen, S.E.; Nordestgaard, B.G.; Axelsson, C.K.; Arias, J.I.; et al. Australian Ovarian Cancer Management Group; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer.Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008, 4, e1000054. [Google Scholar]
- Spinola, M.; Leoni, V.P.; Tanuma, J.; Pettinicchio, A.; Frattini, M.; Signoroni, S.; Agresti, R.; Giovanazzi, R.; Pilotti, S.; Bertario, L.; et al. FGFR4 Gly388Arg polymorphism and prognosis of breast and colorectal cancer. Oncol. Rep. 2005, 14, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 5, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. FOENIX-CCA2 Study Investigators. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 3, 228–239. [Google Scholar] [CrossRef]
- Makawita, S.K.; Abou-Alfa, G.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.Y.; Macarulla, T.T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol. 2020, 30, 2375–2384. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020, 30, 2385–2399. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 2, 165–171. [Google Scholar] [CrossRef]
- Zhang, H.; Hylander, B.L.; LeVea, C.; Repasky, E.A.; Straubinger, R.M.; Adjei, A.A.; Ma, W.W. Enhanced FGFR signalling predisposes pancreatic cancer to the effect of a potent FGFR inhibitor in preclinical models. Br. J. Cancer 2014, 2, 320–329. [Google Scholar] [CrossRef]
- Guan, Z.; Lan, H.; Sun, D.; Wang, X.; Jin, K. A potential novel therapy for FGFR1-amplified pancreatic cancer with bone metastasis, screened by next-generation sequencing and a patient-derived xenograft model. Oncol. Lett. 2019, 2, 2303–2307. [Google Scholar] [CrossRef]
- Subbiah, V.; Iannotti, N.O.; Gutierrez, M.; Smith, D.C.; Féliz, L.; Lihou, C.F.; Tian, C.; Silverman, I.M.; Ji, T.; Saleh, M. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann. Oncol. 2022, 5, 522–533. [Google Scholar] [CrossRef]
- Ng, C.F.; Glaspy, J.; Placencio-Hickok, V.R.; Thomassian, S.; Gong, J.; Osipov, A.; Hendifar, A.E.; Moshayedi, N. Exceptional Response to Erdafitinib in FGFR2-Mutated Metastatic Pancreatic Ductal Adenocarcinoma. J. Natl. Compr. Cancer Netw. 2022, 10, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Poon, D.; Tan, M.H.; Khor, D. Stage 4 pancreatic adenocarcinoma harbouring an FGFR2-TACC2 fusion mutation with complete response to erdafitinib a pan-fibroblastic growth factor receptor inhibitor. BMJ Case Rep. 2021, 9, e244271. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Smyth, E.; Babina, I.S.; Herrera-Abreu, M.T.; Tarazona, N.; Peckitt, C.; Kilgour, E.; Smith, N.R.; Geh, C.; Rooney, C.; et al. High-Level Clonal FGFR Amplification and Response to FGFR Inhibition in a Translational Clinical Trial. Cancer Discov. 2016, 6, 838–851. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Tokunaga, R.; Imamura, Y.; Nakamura, K.; Ishimoto, T.; Nakagawa, S.; Miyake, K.; Nakaji, Y.; Tsuda, Y.; Iwatsuki, M.; Baba, Y.; et al. Fibroblast growth factor receptor 2 expression, but not its genetic amplification, is associated with tumor growth and worse survival in esophagogastric junction adenocarcinoma. Oncotarget 2016, 7, 19748–19761. [Google Scholar] [CrossRef] [PubMed]
- Nagatsuma, A.K.; Aizawa, M.; Kuwata, T.; Doi, T.; Ohtsu, A.; Fujii, H.; Ochiai, A. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric. Cancer 2015, 18, 227–238. [Google Scholar] [CrossRef]
- Pihlak, R.; Fong, C.; Starling, N. Targeted Therapies and Developing Precision Medicine in Gastric Cancer. Cancers (Basel) 2023, 15, 3248. [Google Scholar] [CrossRef] [PubMed]
- Leong, G.B. The expansion of psychiatric participation in social control. Hosp. Commun. Psychiatry 1989, 40, 240–242. [Google Scholar] [CrossRef]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib Is a Novel Irreversible FGFR 1-4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res. 2020, 80, 4986–4997. [Google Scholar] [CrossRef]
- Javle, M.; King, G.; Spencer, K.; Borad, M.J. Futibatinib, an Irreversible FGFR1-4 Inhibitor for the Treatment of FGFR-Aberrant Tumors. Oncologist 2023, oyad149. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1-4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shitara, K.; Kojima, T.; Kuboki, Y.; Matsubara, N.; Bando, H.; Yoh, K.; Naito, Y.; Hirai, H.; Kurokawa, Y.; et al. Phase I study of the irreversible fibroblast growth factor receptor 1-4 inhibitor futibatinib in Japanese patients with advanced solid tumors. Cancer Sci. 2023, 114, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Rasco, D.; Lee, J.; Rha, S.Y.; Lee, K.W.; Bang, Y.J.; Bendell, J.; Enzinger, P.; Marina, N.; Xiang, H.; et al. Phase I Escalation and Expansion Study of Bemarituzumab (FPA144) in Patients With Advanced Solid Tumors and FGFR2b-Selected Gastroesophageal Adenocarcinoma. J. Clin. Oncol. 2020, 38, 2418–2426. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.K.; Qin, S.; Yamaguchi, K.; Kim, I.H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Kang, Y.K.; Saeed, A.; Yamaguchi, K.; Qin, S.; Lee, K.W.; Kim, I.H.; Oh, S.C.; Li, J.; Turk, H.M.; et al. FIGHT: A randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). J. Clin. Oncol. 2021, 39, 4010. [Google Scholar] [CrossRef]
- Porta, R.; Borea, R.; Coelho, A.; Khan, S.; Araújo, A.; Reclusa, P.; Franchina, T.; Van Der Steen, N.; Van Dam, P.; Ferri, J.; et al. FGFR a promising druggable target in cancer: Molecular biology and new drugs. Crit. Rev. Oncol. Hematol. 2017, 113, 256–267. [Google Scholar] [CrossRef]
- Formisano, L.; Lu, Y.; Servetto, A.; Hanker, A.B.; Jansen, V.M.; Bauer, J.A.; Sudhan, D.R.; Guerrero-Zotano, A.L.; Croessmann, S.; Guo, Y.; et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat. Commun. 2019, 10, 1373. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ishiwata, T.; Yamahatsu, K.; Kawahara, K.; Hagio, M.; Peng, W.X.; Yamamoto, T.; Nakazawa, N.; Seya, T.; Ohaki, Y.; et al. Overexpressed fibroblast growth factor receptor 2 in the invasive front of colorectal cancer: A potential therapeutic target in colorectal cancer. Cancer Lett. 2011, 309, 209–219. [Google Scholar] [CrossRef]
- Gavine, P.R.; Mooney, L.; Kilgour, E.; Thomas, A.P.; Al-Kadhimi, K.; Beck, S.; Rooney, C.; Coleman, T.; Baker, D.; Mellor, M.J.; et al. AZD4547: An orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012, 72, 2045–2056. [Google Scholar] [CrossRef]
- Komla-Ebri, D.; Dambroise, E.; Kramer, I.; Benoist-Lasselin, C.; Kaci, N.; Le Gall, C.; Martin, L.; Busca, P.; Barbault, F.; Graus-Porta, D.; et al. Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model. J. Clin. Investig. 2016, 126, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, M.; Min, X.; Dai, S.; Li, M.; Tan, S.; Li, G.; Chen, X.; Ma, Y.; Li, J.; et al. LY2874455 potently inhibits FGFR gatekeeper mutants and overcomes mutation-based resistance. Chem. Commun. 2018, 54, 12089–12092. [Google Scholar] [CrossRef] [PubMed]
- Gozgit, J.M.; Wong, M.J.; Moran, L.; Wardwell, S.; Mohemmad, Q.K.; Narasimhan, N.I.; Shakespeare, W.C.; Wang, F.; Clackson, T.; Rivera, V.M. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol. Cancer Ther. 2012, 11, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Xie, H.; Fetterly, G.; Pitzonka, L.; Whitworth, A.; LeVea, C.; Wilton, J.; Mantione, K.; Schihl, S.; Dy, G.K.; et al. A phase Ib study of the FGFR/VEGFR inhibitor dovitinib with gemcitabine and capecitabine in advanced solid tumor and pancreatic cancer patients. Am. J. Clin. Oncol. 2019, 42, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Guffanti, F.; Chilà, R.; Bello, E.; Zucchetti, M.; Zangarini, M.; Ceriani, L.; Ferrari, M.; Lupi, M.; Jacquet-Bescond, A.; Burbridge, M.F.; et al. In vitro and in vivo activity of lucitanib in FGFR1/2 amplified or mutated cancer models. Neoplasia 2017, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Venetsanakos, E.; Brameld, K.A.; Phan, V.T.; Verner, E.; Owens, T.D.; Xing, Y.; Tam, D.; LaStant, J.; Leung, K.; Karr, D.E.; et al. The irreversible covalent fibroblast growth factor receptor inhibitor PRN1371 exhibits sustained inhibition of FGFR after drug clearance. Mol. Cancer Ther. 2017, 16, 2668–2676. [Google Scholar] [CrossRef]
- Tan, L.; Wang, J.; Tanizaki, J.; Huang, Z.; Aref, A.R.; Rusan, M.; Zhu, S.J.; Zhang, Y.; Ercan, D.; Liao, R.G.; et al. Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors. Proc. Natl. Acad. Sci. USA 2014, 111, E4869–E4877. [Google Scholar] [CrossRef]
- Lu, X.; Chen, H.; Patterson, A.V.; Smaill, J.B.; Ding, K. Fibroblast growth factor receptor 4 (FGFR4) selective inhibitors as hepatocellular carcinoma therapy: Advances and prospects. J. Med. Chem. 2019, 62, 2905–2915. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Chen, X.; Chen, D.; Shi, X.; Song, J.; Wu, J.; Huang, F.; Xia, Q.; Xiang, Y.; et al. The novel FGFR inhibitor F1-7 induces DNA damage and cell death in colon cells. Br. J. Cancer 2022, 6, 1014–1025. [Google Scholar] [CrossRef]
- Freyer, C.W.; Hughes, M.E.; Carulli, A.; Bagg, A.; Hexner, E. Pemigatinib for the treatment of myeloid/lymphoid neoplasms with FGFR1 rearrangement. Expert Rev. Anticancer Ther. 2023, 4, 351–359. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Zemla, T.J.; Hubbard, J.M.; Jia, J.; Gbolahan, O.B.; Sousa, A.; Wilson, L.; Waechter, B.; Ou, F.S.; Nixon, A.B.; et al. A phase II single-arm study of the FGFR inhibitor pemigatinib in patients with metastatic colorectal cancer (mCRC) harboring FGF/FGFR alterations. J. Clin. Oncol. 2023, 41, 139. [Google Scholar] [CrossRef]
- Wu, Q.; Zhen, Y.; Shi, L.; Vu, P.; Greninger, P.; Adil, R.; Merritt, J.; Egan, R.; Wu, M.J.; Yin, X.; et al. EGFR Inhibition Potentiates FGFR Inhibitor Therapy and Overcomes Resistance in FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2022, 5, 1378–1395. [Google Scholar] [CrossRef]
- Goyal, L.; Shi, L.; Liu, L.Y.; Fece de la Cruz, F.; Lennerz, J.K.; Raghavan, S.; Leschiner, I.; Elagina, L.; Siravegna, G.; Ng, R.W.S.; et al. TAS-120 Overcomes Resistance to ATP-Competitive FGFR Inhibitors in Patients with FGFR2 Fusion-Positive Intrahepatic Cholangiocarcinoma. Cancer Discov. 2019, 8, 1064–1079. [Google Scholar] [CrossRef]
- Facchinetti, F.; Hollebecque, A.; Braye, F.; Vasseur, D.; Pradat, Y.; Bahleda, R.; Pobel, C.; Bigot, L.; Deas, O.; Florez Arango, J.D.; et al. Resistance to selective FGFR inhibitors in FGFR-driven urothelial cancer. Cancer Discov. 2023, 13, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Kommalapati, A.; Tella, S.H.; Borad, M.; Javle, M.; Mahipal, A. FGFR Inhibitors in Oncology: Insight on the Management of Toxicities in Clinical Practice. Cancers 2021, 12, 2968. [Google Scholar] [CrossRef] [PubMed]
- FGFR2 Fusions Testing in Intrahepatic Cholangiocarcinoma: ESMO Biomarker Factsheet. Available online: https://oncologypro.esmo.org/education-library/factsheets-on-biomarkers/fgfr2-fusions-testing-in-intrahepatic-cholangiocarcinoma (accessed on 17 July 2023).
- Neumann, O.; Lehmann, U.; Bartels, S.; Pfarr, N.; Albrecht, T.; Ilm, K.; Christmann, J.; Volckmar, A.L.; Goldschmid, H.; Kirchner, M.; et al. First proficiency testing for NGS-based and combined NGS-and FISH-based detection of FGFR2 fusions in intrahepatic cholangiocarcinoma. J. Pathol. Clin. Res. 2023, 2, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lugowska, I.; Stanczak, A.; Roszkowsk, K.; Dziadziuszko, R.; Duchnowska, R.; Kubiatowski, T.; Bodnar, L.; Szczylik, C.; Chorostowska Wynimko, J.; Popiel, D.; et al. 46O—Preliminary results from a phase IA trial of selective FGFR1-3 inhibitor CPL304110 in patients with FGFR-deregulated advanced solid malignancies. Ann. Oncol. 2023, 8, 100896. [Google Scholar] [CrossRef]
- Subbiah, V.; Sahai, V.; Maglic, D.; Bruderek, K.; Toure, B.B.; Zhao, S.; Valverde, R.; O’Hearn, P.J.; Moustakas, D.T.; Schonherr, H.; et al. RLY-4008, the first highly selective FGFR2 inhibitor with activity across FGFR2 alterations and resistance mutations. Cancer Discov. 2023, 9, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
| Alteration | Cancer Type |
|---|---|
| Mutation | 0.6% GC FGFR1 mutation [6] 6% GC FGFR2 mutation [7] 57% GC FGFR4 mutation [8] 5% colorectal cancer FGFR3 mutation [7] 0.9% CCA FGFR1-4 mutation [6] 1.2% PC FGFR1-4 [6] |
| Fusion | 20% GC FGFR3 fusion [9,10] 8% CCA FGFR2 fusion [11] 8% gastrointestinal stromal tumor FGFR1 fusion [12] 0.7% colorectal cancer FGFR2 fusion [11] 1% HCC FGFR2 fusion [11] |
| Copy Number Variation | 1% GC FGFR1 amplification [6] 2–9% GC FGFR2 amplification [13,14] 2–3% HCC FGFR3 amplification [15] 2–3% HCC FGFR4 amplification [15] 2.6% CCA FGFR1-4 amplification [6] 2% colorectal cancer FGFR1 amplification [6] 3.5% PC FGFR1-4 amplification [6] |
| Altered Splicing | 0.7% colon carcinoma FGFR1-4 splice variant [6] 3.5% CCA FGFR1-4 splice variant [6] 1.2% GC FGFR1-4 splice variant [6] 0.6% PC FGFR1-4 splice variant [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.; Orlandi, E.; Hahne, J.C.; Vecchia, S.; Citterio, C.; Anselmi, E.; Toscani, I.; Ghidini, M. Targeting FGFR Pathways in Gastrointestinal Cancers: New Frontiers of Treatment. Biomedicines 2023, 11, 2650. https://doi.org/10.3390/biomedicines11102650
Ratti M, Orlandi E, Hahne JC, Vecchia S, Citterio C, Anselmi E, Toscani I, Ghidini M. Targeting FGFR Pathways in Gastrointestinal Cancers: New Frontiers of Treatment. Biomedicines. 2023; 11(10):2650. https://doi.org/10.3390/biomedicines11102650
Chicago/Turabian StyleRatti, Margherita, Elena Orlandi, Jens Claus Hahne, Stefano Vecchia, Chiara Citterio, Elisa Anselmi, Ilaria Toscani, and Michele Ghidini. 2023. "Targeting FGFR Pathways in Gastrointestinal Cancers: New Frontiers of Treatment" Biomedicines 11, no. 10: 2650. https://doi.org/10.3390/biomedicines11102650
APA StyleRatti, M., Orlandi, E., Hahne, J. C., Vecchia, S., Citterio, C., Anselmi, E., Toscani, I., & Ghidini, M. (2023). Targeting FGFR Pathways in Gastrointestinal Cancers: New Frontiers of Treatment. Biomedicines, 11(10), 2650. https://doi.org/10.3390/biomedicines11102650






