Thoracic Dorsal Root Ganglion Application of Resiniferatoxin Reduces Myocardial Ischemia-Induced Ventricular Arrhythmias
Abstract
1. Introduction
2. Methods
2.1. Surgical Preparation
2.2. Acute Myocardial Ischemia and Reperfusion
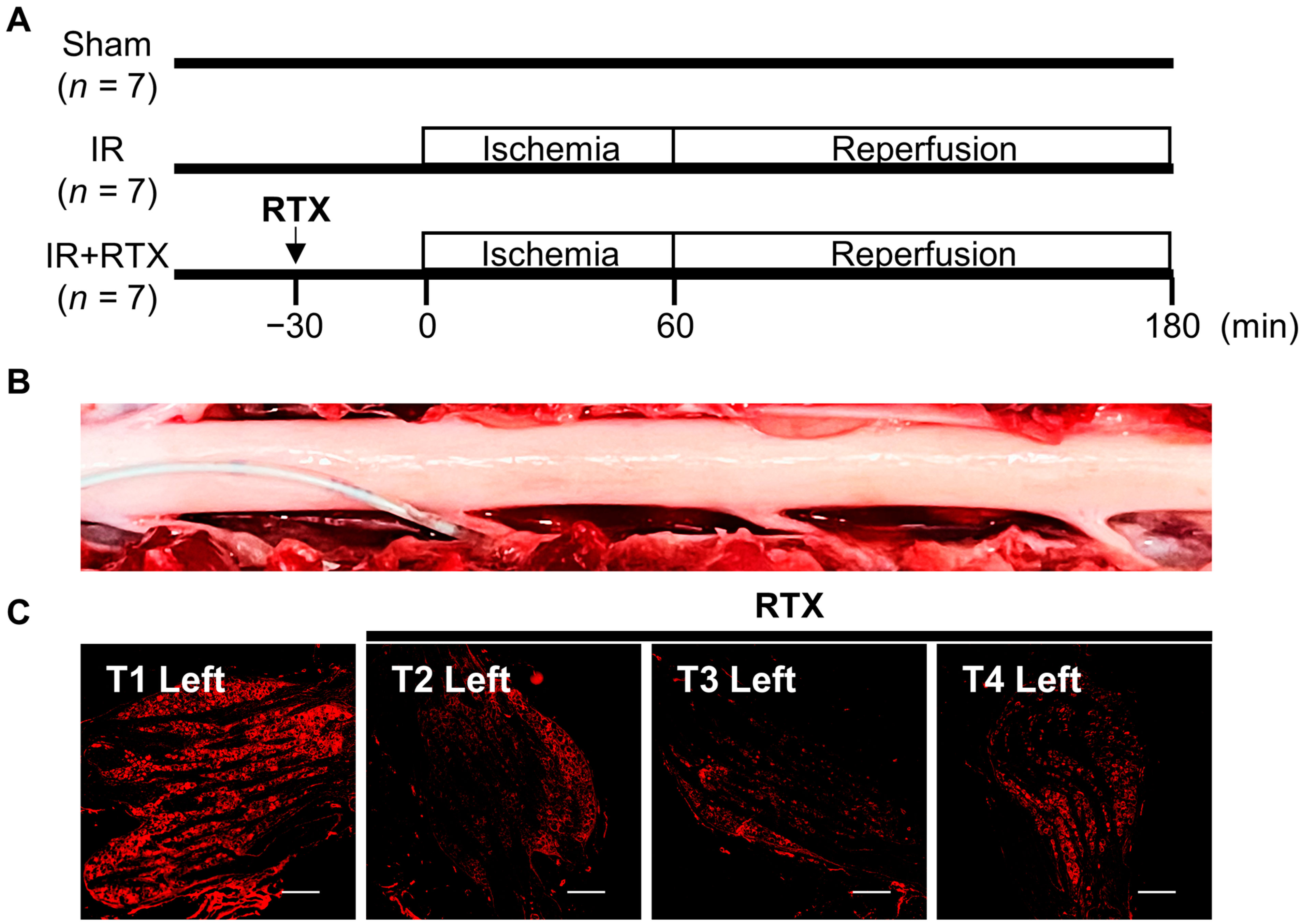
2.3. Epidural Administration of RTX to DRGs
2.4. Experimental Protocols
2.5. Hemodynamic Recording
2.6. Heart Staining
2.7. Cardiac Electrophysiologic Recording
2.8. Arrhythmia Counts
2.9. Immunohistochemistry (IHC)
2.10. Statistical Analysis
3. Results
3.1. Ischemia Area at Risk Assessment
3.2. Hemodynamic Changes
3.3. Cardiac Electrophysiological Changes
3.4. Ventricular Arrhythmia
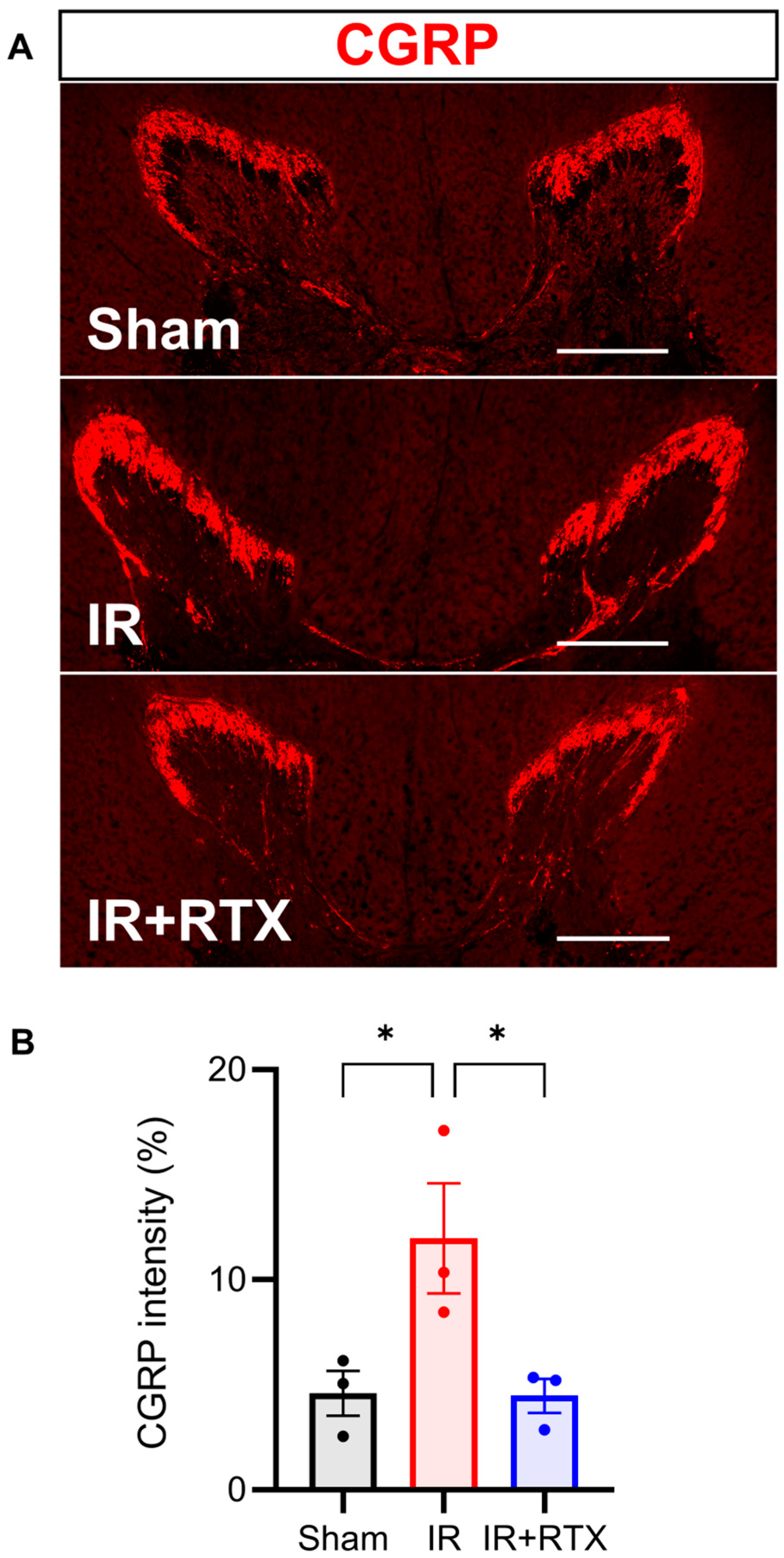
3.5. Immunohistochemistry
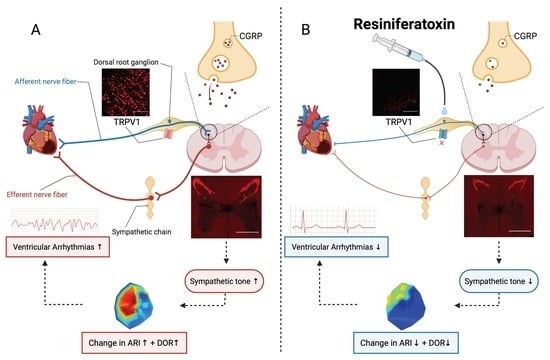
4. Discussion
4.1. Thoracic DRG – RTX Application Reduces Cardiac Sympathoexcitation
4.2. Molecular Mechanism Underlying Action of RTX on Thoracic DRG
4.3. Antiarrhythmic Effect of Thoracic DRG – RTX and Clinical Implications
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DRG | dorsal root ganglion |
| RTX | resiniferatoxin |
| TRPV1 | transient receptor potential vanilloid 1 |
| CGRP | calcitonin gene-related peptide |
| IR | ischemia/reperfusion |
| T1 | thoracic spinal nerve 1 |
| LAD | left anterior descending coronary artery |
| ARI | activation recovery interval |
| APD | action potential duration |
| DOR | dispersion of repolarization |
| PVCs | premature ventricular contractions |
| VT | non-sustained ventricular tachycardias |
| VF | ventricular fibrillation |
References
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac innervation and sudden cardiac death. Circ. Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, O.; Chrispin, J.; Tomaselli, G.F.; Berger, R.D. Clinical management and prevention of sudden cardiac death. Circ. Res. 2015, 116, 2020–2040. [Google Scholar] [CrossRef] [PubMed]
- Zahner, M.R.; Li, D.P.; Chen, S.R.; Pan, H.L. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J. Physiol. 2003, 551, 515–523. [Google Scholar] [CrossRef]
- Yoshie, K.; Rajendran, P.S.; Massoud, L.; Mistry, J.; Swid, M.A.; Wu, X.; Sallam, T.; Zhang, R.; Goldhaber, J.I.; Salavatian, S. Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight 2020, 5, e124477. [Google Scholar] [PubMed]
- Hadaya, J.; Ardell, J.L. Autonomic Modulation for Cardiovascular Disease. Front. Physiol. 2020, 11, 617459. [Google Scholar] [CrossRef]
- Karai, L.; Brown, D.C.; Mannes, A.J.; Connelly, S.T.; Brown, J.; Gandal, M.; Wellisch, O.M.; Neubert, J.K.; Olah, Z.; Iadarola, M.J. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J. Clin. Investig. 2004, 113, 1344–1352. [Google Scholar] [CrossRef]
- Wang, D.; Wu, Y.; Chen, Y.; Wang, A.; Lv, K.; Kong, X.; He, Y.; Hu, N. Focal selective chemo-ablation of spinal cardiac afferent nerve by resiniferatoxin protects the heart from pressure overload-induced hypertrophy. Biomed. Pharmacother. 2019, 109, 377–385. [Google Scholar] [CrossRef]
- Jancso, G.; Kiraly, E.; Jancso-Gabor, A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977, 270, 741–743. [Google Scholar] [CrossRef]
- Szolcsanyi, J.; Szallasi, A.; Szallasi, Z.; Joo, F.; Blumberg, P.M. Resiniferatoxin: An ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 1990, 255, 923–928. [Google Scholar]
- Jeffry, J.A.; Yu, S.-Q.; Sikand, P.; Parihar, A.; Evans, M.S.; Premkumar, L.S. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS ONE 2009, 4, e7021. [Google Scholar]
- Szallasi, A.; Blumberg, P. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid receptor loss in rat sensory ganglia associated with long term desensitization to resiniferatoxin. Neurosci. Lett. 1992, 140, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, Y.; Tang, Y.; Zhou, M.; Xiong, L.; Huang, C. Resiniferatoxin reduces cardiac sympathetic nerve activation to exert a cardioprotective effect during myocardial infarction. Int. J. Clin. Exp. Pathol. 2021, 14, 408. [Google Scholar] [PubMed]
- Xu, S.; Xu, Y.; Cheng, X.; Huang, C.; Pan, Y.; Jin, S.; Xiong, W.; Zhang, L.; He, S.; Zhang, Y. Inhibition of DRG-TRPV1 upregulation in myocardial ischemia contributes to exogenous cardioprotection. J. Mol. Cell. Cardiol. 2020, 138, 175–184. [Google Scholar] [PubMed]
- Wu, Y.; Hu, Z.; Wang, D.; Lv, K.; Hu, N. Resiniferatoxin reduces ventricular arrhythmias in heart failure via selectively blunting cardiac sympathetic afferent projection into spinal cord in rats. Eur. J. Pharmacol. 2020, 867, 172836. [Google Scholar] [PubMed]
- Shanks, J.; De Morais, S.D.; Gao, L.; Zucker, I.H.; Wang, H.-J. TRPV1 (transient receptor potential vanilloid 1) cardiac spinal afferents contribute to hypertension in spontaneous hypertensive rat. Hypertension 2019, 74, 910–920. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Nakamura, K.; Ajijola, O.A.; Vaseghi, M.; Armour, J.A.; Ardell, J.L.; Shivkumar, K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J. Physiol. 2016, 594, 321–341. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Howard-Quijano, K.; Salavatian, S.; Yamaguchi, T.; Saba, S.; Mahajan, A. Thoracic dorsal root ganglion stimulation reduces acute myocardial ischemia induced ventricular arrhythmias. Front. Neurosci. 2023, 17, 1091230. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T. Part 3: Adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020, 142, S366–S468. [Google Scholar]
- Howard-Quijano, K.; Takamiya, T.; Dale, E.A.; Kipke, J.; Kubo, Y.; Grogan, T.; Afyouni, A.; Shivkumar, K.; Mahajan, A. Spinal cord stimulation reduces ventricular arrhythmias during acute ischemia by attenuation of regional myocardial excitability. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H421–H431. [Google Scholar] [CrossRef][Green Version]
- Vaseghi, M.; Yamakawa, K.; Sinha, A.; So, E.L.; Zhou, W.; Ajijola, O.A.; Lux, R.L.; Laks, M.; Shivkumar, K.; Mahajan, A. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1020–H1030. [Google Scholar] [CrossRef] [PubMed]
- Haws, C.W.; Lux, R.L. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 1990, 81, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Camm, A.J.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; Moss, A.J.; Myerburg, R.J. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur. Heart J. 2006, 27, 2099–2140. [Google Scholar] [PubMed]
- Miller, L.E.; Hosick, P.A.; Wrieden, J.; Hoyt, E.; Quindry, J.C. Evaluation of arrhythmia scoring systems and exercise-induced cardioprotection. Med. Sci. Sports Exerc. 2012, 44, 435. [Google Scholar] [CrossRef]
- Brown, J.D.; Saeed, M.; Do, L.; Braz, J.; Basbaum, A.I.; Iadarola, M.J.; Wilson, D.M.; Dillon, W.P. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci. Transl. Med. 2015, 7, 305ra145. [Google Scholar] [CrossRef]
- Salavatian, S.; Ardell, S.M.; Hammer, M.; Gibbons, D.; Armour, J.A.; Ardell, J.L. Thoracic spinal cord neuromodulation obtunds dorsal root ganglion afferent neuronal transduction of the ischemic ventricle. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1134–H1141. [Google Scholar] [CrossRef]
- Dale, E.A.; Kipke, J.; Kubo, Y.; Sunshine, M.D.; Castro, P.A.; Ardell, J.L.; Mahajan, A. Spinal cord neural network interactions: Implications for sympathetic control of the porcine heart. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H830–H839. [Google Scholar] [CrossRef]
- Fu, L.W.; Longhurst, J.C. Regulation of cardiac afferent excitability in ischemia. In Sensory Nerves: Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 185–225. [Google Scholar] [CrossRef]
- Huang, M.H.; Sylven, C.; Horackova, M.; Armour, J.A. Ventricular sensory neurons in canine dorsal root ganglia: Effects of adenosine and substance P. Am. J. Physiol. 1995, 269, R318–R324. [Google Scholar] [CrossRef]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.S.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.D.; et al. Translational neurocardiology: Preclinical models and cardioneural integrative aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef]
- Ardell, J.L.; Armour, J.A. Neurocardiology: Structure-Based Function. Compr. Physiol. 2016, 6, 1635–1653. [Google Scholar] [CrossRef]
- Foreman, R.D.; Blair, R.W.; Weber, R.N. Viscerosomatic convergence onto T2–T4 spinoreticular, spinoreticular-spinothalamic, and spinothalamic tract neurons in the cat. Exp. Neurol. 1984, 85, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Foreman, R.D.; Garrett, K.M.; Blair, R.W. Mechanisms of cardiac pain. Compr. Physiol. 2015, 5, 929–960. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, R.E.; Kovacs, K.J.; Honda, C.N.; Nunez, M.G.; Larson, A.A. Resiniferatoxin (RTX) causes a uniquely protracted musculoskeletal hyperalgesia in mice by activation of TRPV1 receptors. J. Pain 2013, 14, 1629–1641. [Google Scholar] [CrossRef]
- Kee, Z.; Kodji, X.; Brain, S.D. The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front. Physiol. 2018, 9, 1249. [Google Scholar] [CrossRef]
- Kallner, G.; Gonon, A.; Franco-Cereceda, A. Calcitonin gene-related peptide in myocardial ischaemia and reperfusion in the pig. Cardiovasc. Res. 1998, 38, 493–499. [Google Scholar] [CrossRef]
- Devesa, I.; Ferrándiz-Huertas, C.; Mathivanan, S.; Wolf, C.; Luján, R.; Changeux, J.-P.; Ferrer-Montiel, A. αCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc. Natl. Acad. Sci. USA 2014, 111, 18345–18350. [Google Scholar] [CrossRef]
- Assas, B.M.; Pennock, J.I.; Miyan, J.A. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front. Neurosci. 2014, 8, 23. [Google Scholar] [CrossRef]
- Price, T.J.; Flores, C.M. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J. Pain 2007, 8, 263–272. [Google Scholar] [CrossRef]
- Zipes, D.P.; Neuzil, P.; Theres, H.; Caraway, D.; Mann, D.L.; Mannheimer, C.; Van Buren, P.; Linde, C.; Linderoth, B.; Kueffer, F. Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: The DEFEAT-HF study. JACC Heart Fail. 2016, 4, 129–136. [Google Scholar] [CrossRef]
- Hori, Y.; Temma, T.; Wooten, C.; Sobowale, C.; Chan, C.; Swid, M.; Ajijola, O.A. Cardiac afferent signaling partially underlies premature ventricular contraction–induced cardiomyopathy. Heart Rhythm. 2021, 18, 1586–1595. [Google Scholar] [CrossRef] [PubMed]



| Baseline | 5 min after RTX | 30 min after RTX | |
|---|---|---|---|
| HR (beats/min) | 86 ± 4 | 86 ± 3 | 84 ± 3 |
| SBP (mmHg) | 129 ± 5 | 132 ± 5 | 132 ± 5 |
| DBP (mmHg) | 82 ± 4 | 85 ± 3 | 85 ± 4 |
| Sham | IR | IR + RTX | ||||
|---|---|---|---|---|---|---|
| Baseline | Sham30 | Baseline | LAD30 | Baseline | LAD30 | |
| HR (beats/min) | 85 ± 4 | 82 ± 3 | 87 ± 6 | 88 ± 4 | 90 ± 5 | 96 ± 7 |
| MBP (mmHg) | 101 ± 8 | 99 ± 8 | 112 ± 9 | 104 ± 14 | 103 ± 5 | 103 ± 5 |
| LVESP (mmHg) | 110 ± 8 | 102 ± 9 | 117 ± 8 | 111 ± 9 * | 114 ± 8 | 110 ± 7 |
| dP/dtmax (mmHg/s) | 1776 ± 79 | 1778 ± 101 | 2117 ± 186 | 1804 ± 167 * | 1826 ± 127 | 1677 ± 75 |
| dP/dtmin (mmHg/s) | −2751 ± 311 | −2538 ± 297 | −2185 ± 465 | −1744 ± 258 | −3499 ± 794 | −3302 ± 772 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, T.; Salavatian, S.; Kuwabara, Y.; Hellman, A.; Taylor, B.K.; Howard-Quijano, K.; Mahajan, A. Thoracic Dorsal Root Ganglion Application of Resiniferatoxin Reduces Myocardial Ischemia-Induced Ventricular Arrhythmias. Biomedicines 2023, 11, 2720. https://doi.org/10.3390/biomedicines11102720
Yamaguchi T, Salavatian S, Kuwabara Y, Hellman A, Taylor BK, Howard-Quijano K, Mahajan A. Thoracic Dorsal Root Ganglion Application of Resiniferatoxin Reduces Myocardial Ischemia-Induced Ventricular Arrhythmias. Biomedicines. 2023; 11(10):2720. https://doi.org/10.3390/biomedicines11102720
Chicago/Turabian StyleYamaguchi, Tomoki, Siamak Salavatian, Yuki Kuwabara, Abigail Hellman, Bradley K. Taylor, Kimberly Howard-Quijano, and Aman Mahajan. 2023. "Thoracic Dorsal Root Ganglion Application of Resiniferatoxin Reduces Myocardial Ischemia-Induced Ventricular Arrhythmias" Biomedicines 11, no. 10: 2720. https://doi.org/10.3390/biomedicines11102720
APA StyleYamaguchi, T., Salavatian, S., Kuwabara, Y., Hellman, A., Taylor, B. K., Howard-Quijano, K., & Mahajan, A. (2023). Thoracic Dorsal Root Ganglion Application of Resiniferatoxin Reduces Myocardial Ischemia-Induced Ventricular Arrhythmias. Biomedicines, 11(10), 2720. https://doi.org/10.3390/biomedicines11102720