Traumatic Brain Injury and Neuromodulation Techniques in Rehabilitation: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. PICO Evaluation
2.3. Inclusion Criteria
2.4. Exclusion Criteria
3. Results
Neuromodulation Techniques and Rehabilitation in TBI Patients
4. Discussion
Perspective and Neuromodulation
5. Neuromodulations’ Disadvantages and Limitations
6. Study Strengths and Limitations
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.H.; Bandak, F.A.; de Lanerolle, N.C. Neuropathology of Traumatic Brain Injury: Comparison of Penetrating, Nonpenetrating Direct Impact and Explosive Blast Etiologies. Semin. Neurol. 2015, 35, 12–19. [Google Scholar] [CrossRef]
- Takahashi, C.E.; Virmani, D.; Chung, D.Y.; Ong, C.; Cervantes-Arslanian, A.M. Blunt and Penetrating Severe Traumatic Brain Injury. Neurol. Clin. 2021, 39, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Ownbey, M.R.; Pekari, T.B. Acute Mild Traumatic Brain Injury Assessment and Management in the Austere Setting—A Review. Mil. Med. 2022, 187, e47–e51. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, H.S.; Arciniegas, D.B. Treatment of Post-Traumatic Cognitive Impairments. Curr. Treat. Options Neurol. 2012, 14, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Rutland-Brown, W.; Langlois, J.A.; Thomas, K.E.; Xi, Y.L. Incidence of Traumatic Brain Injury in the United States, 2003. J. Head Trauma Rehabil. 2006, 21, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, G.L. The Glasgow Coma Scale. J. Emerg. Med. 2000, 19, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cicerone, K.D.; Dahlberg, C.; Malec, J.F.; Langenbahn, D.M.; Felicetti, T.; Kneipp, S.; Ellmo, W.; Kalmar, K.; Giacino, J.T.; Harley, J.P.; et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature From 1998 Through 2002. Arch. Phys. Med. Rehabil. 2005, 86, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, M.; Polanowska, K.; Seniów, J.; Członkowska, A. Effects of Repeated Anodal tDCS Coupled with Cognitive Training for Patients with Severe Traumatic Brain Injury: A pilot randomized controlled trial. J. Head Trauma Rehabil. 2014, 29, E20–E29. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.P.; Beems, T. Overview of the Clinical Applications of Vagus Nerve Stimulation. J. Clin. Neurophysiol. 2010, 27, 130–138. [Google Scholar] [CrossRef]
- Burke, M.J.; Fried, P.J.; Pascual-Leone, A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. Handb. Clin. Neurol. 2019, 163, 73–92. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar] [CrossRef] [PubMed]
- Galletta, E.E.; Rao, P.R.; Barrett, A.M. Transcranial Magnetic Stimulation (TMS): Potential Progress for Language Improvement in Aphasia. Top. Stroke Rehabil. 2011, 18, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E.M.; Samii, A.; Mercuri, B.; Ikoma, K.; Oddo, D.; Grill, S.E.; Hallett, M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp. Brain Res. 1996, 109, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Dixon, C.E.; Okonkwo, D.O.; Richardson, R.M. Neurostimulation for traumatic brain injury. J. Neurosurg. 2014, 121, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.W.; Boudewyn, M.A.; Carter, C.S.; Phillips, M.L. Transcranial direct current stimulation: A roadmap for research, from mechanism of action to clinical implementation. Mol. Psychiatry 2020, 25, 397–407. [Google Scholar] [CrossRef]
- Dougherty, D.D. Deep Brain Stimulation: Clinical Applications. Psychiatr. Clin. N. Am. 2018, 41, 385–394. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, H.; Nioka, S.; Chance, B. Study of near infrared technology for intracranial hematoma detection. J. Biomed. Opt. 2000, 5, 206–213. [Google Scholar] [CrossRef]
- Fregni, F.; Li, S.; Zaninotto, A.L.; Neville, I.S.; Paiva, W.S.; Nunn, D. Clinical utility of brain stimulation modalities following traumatic brain injury: Current evidence. Neuropsychiatr. Dis. Treat. 2015, 11, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Motes, M.A.; Spence, J.S.; Yeatman, K.; Jones, P.M.; Lutrell, M.; O’Hair, R.; Shakal, S.; DeLaRosa, B.L.; To, W.; Vanneste, S.; et al. High-Definition Transcranial Direct Current Stimulation to Improve Verbal Retrieval Deficits in Chronic Traumatic Brain Injury. J. Neurotrauma 2020, 37, 170–177. [Google Scholar] [CrossRef]
- Choi, G.; Kwak, S.; Lee, H.; Chang, M. Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: A pilot study. J. Rehabil. Med. 2018, 50, 246–252. [Google Scholar] [CrossRef]
- Kricheldorff, J.; Göke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. [Google Scholar] [CrossRef]
- Schönfeldt-Lecuona, C.; Lefaucheur, J.-P.; Cardenas-Morales, L.; Wolf, R.; Kammer, T.; Herwig, U. The value of neuronavigated rTMS for the treatment of depression. Neurophysiol. Clin. 2010, 40, 37–43. [Google Scholar] [CrossRef]
- Giacino, J.; Fins, J.J.; Machado, A.; Schiff, N.D. Central Thalamic Deep Brain Stimulation to Promote Recovery from Chronic Posttraumatic Minimally Conscious State: Challenges and Opportunities. Neuromodulation J. Int. Neuromodulation Soc. 2012, 15, 339–349. [Google Scholar] [CrossRef]
- Chudy, D.; Deletis, V.; Almahariq, F.; Marčinković, P.; Škrlin, J.; Paradžik, V. Deep brain stimulation for the early treatment of the minimally conscious state and vegetative state: Experience in 14 patients. J. Neurosurg. 2018, 128, 1189–1198. [Google Scholar] [CrossRef]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Kreuzer, P.M.; Landgrebe, M.; Frank, E.; Langguth, B. Repetitive Transcranial Magnetic Stimulation for the Treatment of Chronic Tinnitus After Traumatic Brain Injury. J. Head Trauma Rehabil. 2013, 28, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, T.-T.; Song, X.-B.; Zhang, Y.; Li, Z.-H.; Cui, Z.-H.; Hao, Q.; Liu, H.L.; Lei, C.L.; Liu, J. Comparison of different stimulation parameters of repetitive transcranial magnetic stimulation for unilateral spatial neglect in stroke patients. J. Neurol. Sci. 2015, 359, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Neville, I.S.; Hayashi, C.Y.; El Hajj, S.A.; Zaninotto, A.L.C.; Sabino, J.P.; Sousa, L.M.; Nagumo, M.M.; Brunoni, A.R.; Shieh, B.D.F.S.; Amorim, R.L.O.; et al. Repetitive Transcranial Magnetic Stimulation (rTMS) for the cognitive rehabilitation of traumatic brain injury (TBI) victims: Study protocol for a randomized controlled trial. Trials 2015, 16, 440. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.; Fritz, S.L.; Liuzzo, D.M.; Newman-Norlund, R.; Herter, T.M. Using clinical and robotic assessment tools to examine the feasibility of pairing tDCS with upper extremity physical therapy in patients with stroke and TBI: A consideration-of-concept pilot study. NeuroRehabilitation 2014, 35, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.K.; Meek, B.P.; Modirrousta, M.M. Non-Invasive Brain Stimulation for the Treatment of Symptoms Following Traumatic Brain Injury. Front. Psychiatry 2015, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.E.; Krzyzaniak, M.; Costantini, T.W.; De Maio, A.; Baird, A.; Eliceiri, B.P.; Coimbra, R. Vagal Nerve Stimulation Blocks Peritoneal Macrophage Inflammatory Responsiveness After Severe Burn Injury. Shock 2012, 38, 294–300. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, L.; Lin, J.; Lin, J.; Kui, G.; Zhang, J. Neuroprotective effects of vagus nerve stimulation on traumatic brain injury. Neural Regen. Res. 2014, 9, 1585–1591. [Google Scholar] [CrossRef]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive tran-scranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Klomjai, W.; Katz, R.; Lackmy-Vallée, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 2015, 58, 208–213. [Google Scholar] [CrossRef]
- Nasseri, P.; Nitsche, M.A.; Ekhtiari, H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front. Hum. Neurosci. 2015, 9, 54. [Google Scholar] [CrossRef]
- Woods, A.J.; Bryant, V.; Sacchetti, D.; Gervits, F.; Hamilton, R. Effects of Electrode Drift in Transcranial Direct Current Stimulation. Brain Stimul. 2015, 8, 515–519. [Google Scholar] [CrossRef]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. NeuroImage 2015, 109, 140–150. [Google Scholar] [CrossRef]
- Thompson, S.L.; O’leary, G.H.; Austelle, C.W.; Gruber, E.; Kahn, A.T.; Manett, A.J.; Short, B.; Badran, B.W. A Review of Parameter Settings for Invasive and Non-invasive Vagus Nerve Stimulation (VNS) Applied in Neurological and Psychiatric Disorders. Front. Neurosci. 2021, 15, 709436. [Google Scholar] [CrossRef]
- Ghaffarpasand, F.; Razmkon, A.; Khalili, H. Deep Brain Stimulation in Patients with Traumatic Brain Injury; Facts and Figures. Bull. Emerg. Trauma 2014, 2, 101–102. [Google Scholar]
- Haddad, A.R.; Lythe, V.; Green, A.L. Deep Brain Stimulation for Recovery of Consciousness in Minimally Conscious Patients After Traumatic Brain Injury: A Systematic Review. Neuromodulation Technol. Neural Interface 2019, 22, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Katayama, Y.; Obuchi, T.; Kobayashi, K.; Oshima, H.; Fukaya, C. Spinal Cord Stimulation for Treatment of Patients in the Minimally Conscious State. Neurol. Medico Chir. 2012, 52, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yang, Y.; Xu, L.; Chen, X.; Geng, X.; Zhao, J.; He, J. Effects of short-term spinal cord stimulation on patients with prolonged disorder of consciousness: A pilot study. Front. Neurol. 2022, 13, 1026221. [Google Scholar] [CrossRef]
- van Dijk, K.; Scherder, E.; Scheltens, P.; Sergeant, J. Effects of Transcutaneous Electrical Nerve Stimulation (TENS) on Non-Pain Related Cognitive and Behavioural Functioning. Prog. Neurobiol. 2002, 13, 257–270. [Google Scholar] [CrossRef]
- Huang, J.; Yang, C.; Zhao, K.; Zhao, Z.; Chen, Y.; Wang, T.; Qu, Y. Transcutaneous Electrical Nerve Stimulation in Rodent Models of Neuropathic Pain: A Meta-Analysis. Front. Neurosci. 2022, 16, 831413. [Google Scholar] [CrossRef]
- Thunshelle, C.; Hamblin, M.R. Transcranial Low-Level Laser (Light) Therapy for Brain Injury. Photomed. Laser Surg. 2016, 34, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Oron, A.; Oron, U.; Streeter, J.; De Taboada, L.; Alexandrovich, A.; Trembovler, V.; Shohami, E. Near Infrared Transcranial Laser Therapy Applied at Various Modes to Mice following Traumatic Brain Injury Significantly Reduces Long-Term Neurological Deficits. J. Neurotrauma 2012, 29, 401–407. [Google Scholar] [CrossRef]
- Khuman, J.; Zhang, J.; Park, J.; Carroll, J.D.; Donahue, C.; Whalen, M.J. Low-Level Laser Light Therapy Improves Cognitive Deficits and Inhibits Microglial Activation after Controlled Cortical Impact in Mice. J. Neurotrauma 2012, 29, 408–417. [Google Scholar] [CrossRef]
- Poiani, G.D.C.R.; Zaninotto, A.L.; Carneiro, A.M.C.; Zangaro, R.A.; Salgado, A.S.I.; Parreira, R.B.; De Andrade, A.F.; Teixeira, M.J.; Paiva, W.S. Photobiomodulation using low-level laser therapy (LLLT) for patients with chronic traumatic brain injury: A randomized controlled trial study protocol. Trials 2018, 19, 17. [Google Scholar] [CrossRef]
- Kahana, M.J.; Ezzyat, Y.; Wanda, P.A.; Solomon, E.A.; Adamovich-Zeitlin, R.; Lega, B.C.; Jobst, B.C.; Gross, R.E.; Ding, K.; Diaz-Arrastia, R.R. Biomarker-guided neuromodulation aids memory in traumatic brain injury. Brain Stimul. 2023, 16, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.G.F.; Tan, C.O.; Chan, S.-T.; Welt, J.; Avesta, A.; Ratai, E.; Mercaldo, N.D.; Yendiki, A.; Namati, J.; Chico-Calero, I.; et al. Effect of Transcranial Low-Level Light Therapy vs Sham Therapy Among Patients with Moderate Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2017337. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, D.J.; De Carvalho, D.; Paglioni, V.M.; Brunoni, A.R.; Valiengo, L.; Thome-Souza, M.S.; Guirado, V.M.P.; Zaninotto, A.L.; Paiva, W.S. Effects of transcranial direct current stimulation (tDCS) and concurrent cognitive training on episodic memory in patients with traumatic brain injury: A double-blind, randomised, placebo-controlled study. BMJ Open 2021, 11, e045285. [Google Scholar] [CrossRef]
- Sultana, T.; Hasan, M.A.; Kang, X.; Liou-Johnson, V.; Adamson, M.M.; Razi, A. Neural mechanisms of emotional health in traumatic brain injury patients undergoing rTMS treatment. Mol. Psychiatry 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neville, I.S.; Zaninotto, A.L.; Hayashi, C.Y.; Rodrigues, P.A.; Galhardoni, R.; de Andrade, D.C.; Brunoni, A.R.; Amorim, R.L.O.; Teixeira, M.J.; Paiva, W.S. Repetitive TMS does not improve cognition in patients with TBI: A randomized double-blind trial. Neurology 2019, 93, e190–e199. [Google Scholar] [CrossRef]
- Opie, G.M.; Foo, N.; Killington, M.; Ridding, M.C.; Semmler, J.G. Transcranial Magnetic Stimulation-Electroencephalography Measures of Cortical Neuroplasticity Are Altered after Mild Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2774–2784. [Google Scholar] [CrossRef]
- Hou, J.; Mohanty, R.; Chu, D.; Nair, V.A.; Danilov, Y.; Kaczmarek, K.A.; Meyerand, B.; Tyler, M.; Prabhakaran, V. Translingual neural stimulation affects resting-state functional connectivity in mild-moderate traumatic brain injury. J. Neuroimaging 2022, 32, 1193–1200. [Google Scholar] [CrossRef]
- Tyler, M.; Skinner, K.; Prabhakaran, V.; Kaczmarek, K.; Danilov, Y. Translingual Neurostimulation for the Treatment of Chronic Symptoms Due to Mild-to-Moderate Traumatic Brain Injury. Arch. Rehabil. Res. Clin. Transl. 2019, 1, 100026. [Google Scholar] [CrossRef]
- Sacco, K.; Galetto, V.; Dimitri, D.; Geda, E.; Perotti, F.; Zettin, M.; Geminiani, G.C. Concomitant use of transcranial direct current stimulation and computer-assisted training for the rehabilitation of attention in traumatic brain injured patients: Behavioral and neuroimaging results. Front. Behav. Neurosci. 2016, 10, 57. [Google Scholar] [CrossRef]
- Boissonnault, E.; Higgins, J.; LaGarde, G.; Barthélemy, D.; Lamarre, C.; Dagher, J.H. Brain stimulation in attention deficits after traumatic brain injury: A literature review and feasibility study. Pilot Feasibility Stud. 2021, 7, 115. [Google Scholar] [CrossRef]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Sánchez-Kuhn, A.; Pérez-Fernández, C.; Cánovas, R.; Flores, P.; Sánchez-Santed, F. Transcranial direct current stimulation as a motor neurorehabilitation tool: An empirical review. Biomed. Eng. Online 2017, 16, 76. [Google Scholar] [CrossRef]
- Kartje, G.L.; Schwab, M.E. Axonal growth in the adult mammalian nervous system: Regeneration and compensatory plasticity. In Basic Neurochemistry; Siegel, G., Ed.; Lippincott: Philadelphia, PA, USA, 2006. [Google Scholar]
- Pape, T.L.-B.; Rosenow, J.; Lewis, G. Transcranial Magnetic Stimulation: A possible treatment for TBI. J. Head Trauma Rehabil. 2006, 21, 437–451. [Google Scholar] [CrossRef]
- Faught, E.; Tatum, W. Trigeminal stimulation: A superhighway to the brain? Neurology 2013, 80, 780–781. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Shewmon, D.A.; Whitehurst, T. Trigeminal nerve stimulation for epilepsy. Neurology 2003, 61, 421–422. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Soss, J.; Cook, I.A.; Markovic, D.; Gornbein, J.; Murray, D.; Oviedo, S.; Gordon, S.; Corralle-Leyva, G.; Kealey, C.P.; et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 2013, 80, 786–791. [Google Scholar] [CrossRef]
- Cook, I.A.; Abrams, M.; Leuchter, A.F. Trigeminal Nerve Stimulation for Comorbid Posttraumatic Stress Disorder and Major Depressive Disorder. Neuromodulation Technol. Neural Interface 2016, 19, 299–305. [Google Scholar] [CrossRef]
- Shiozawa, P.; Duailibi, M.S.; da Silva, M.E.; Cordeiro, Q. Trigeminal nerve stimulation (TNS) protocol for treating major depression: An open-label proof-of-concept trial. Epilepsy Behav. 2014, 39, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Salgari, G.C.; Ellis, A.; Cowen, J.; Dillon, A.; McGough, J.J. Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder: Cognitive and Electroencephalographic Predictors of Treatment Response. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 856–864.e1. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wu, X.; Xie, M.; Li, X.; Liu, C.; Su, Y.; Chen, Y.; Wu, S.; Ma, C. Trigeminal nerve stimulation successfully awakened an unconscious patient. Brain Stimul. 2019, 12, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Berryhill, M.E.; Dielenberg, V. Can brain stimulation enhance cognition in clinical populations? A critical review. Restor. Neurol. Neurosci. 2022, 40, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, M.E.; Martin, D. Cognitive Effects of Transcranial Direct Current Stimulation in Healthy and Clinical Populations: An Overview. J. ECT 2018, 34, e25–e35. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Fayzullina, R.; Bullard, B.M.; Levina, V.; Reinhart, R.M.G. A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci. Transl. Med. 2023, 15, eabo2044. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Schwertfeger, J.L.; Beyer, C.; Hung, P.; Ung, N.; Madigan, C.; Cortes, A.R.; Swaminathan, B.; Madhavan, S. A map of evidence using transcranial direct current stimulation (tDCS) to improve cognition in adults with traumatic brain injury (TBI). Front. Neuroergonomics 2023, 4, 1170473. [Google Scholar] [CrossRef]
- Pergher, V.; Au, J.; Shalchy, M.A.; Santarnecchi, E.; Seitz, A.; Jaeggi, S.M.; Battelli, L. The benefits of simultaneous tDCS and working memory training on transfer outcomes: A systematic review and meta-analysis. Brain Stimul. 2022, 15, 1541–1551. [Google Scholar] [CrossRef]
- Jones, K.T.; Stephens, J.A.; Alam, M.; Bikson, M.; Berryhill, M.E. Longitudinal Neurostimulation in Older Adults Improves Working Memory. PLoS ONE 2015, 10, e0121904. [Google Scholar] [CrossRef]
- Johnson, E.L.; Arciniega, H.; Jones, K.T.; Kilgore-Gomez, A.; Berryhill, M.E. Individual predictors and electrophysiological signatures of working memory enhancement in aging. NeuroImage 2022, 250, 118939. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Peterson, D.J.; Blacker, K.J.; Berryhill, M.E. Frontoparietal neurostimulation modulates working memory training benefits and oscillatory synchronization. Brain Res. 2017, 1667, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Nguyen, J.A.; Reinhart, R.M. Synchronizing Brain Rhythms to Improve Cognition. Annu. Rev. Med. 2021, 72, 29–43. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Nguyen, J.A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 2019, 22, 820–827. [Google Scholar] [CrossRef]
- Ulam, F.; Shelton, C.; Richards, L.; Davis, L.; Hunter, B.; Fregni, F.; Higgins, K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin. Neurophysiol. 2015, 126, 486–496. [Google Scholar] [CrossRef]
- Johnson, M.D.; Lim, H.H.; Netoff, T.I.; Connolly, A.T.; Johnson, N.; Roy, A.; Holt, A.; Lim, K.O.; Carey, J.R.; Vitek, J.L.; et al. Neuromodulation for brain disorders: Challenges and opportunities. IEEE Trans. Biomed. Eng. 2013, 60, 610–624. [Google Scholar] [CrossRef]
- Silva, S.; Basser, P.; Miranda, P. Elucidating the mechanisms and loci of neuronal excitation by transcranial magnetic stimulation using a finite element model of a cortical sulcus. Clin. Neurophysiol. 2008, 119, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E.M.; Zimmermann, T. Transcranial magnetic brain stimulation: Therapeutic promises and scientific gaps. Pharmacol. Ther. 2012, 133, 98–107. [Google Scholar] [CrossRef]
- Datta, A.; Bikson, M.; Fregni, F. Transcranial direct current stimulation in patients with skull defects and skull plates: High-resolution computational FEM study of factors altering cortical current flow. NeuroImage 2010, 52, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S. The distribution of induced currents in magnetic stimulation of the nervous system. Phys. Med. Biol. 1990, 35, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Roth, Y.; Zangen, A.; Hallett, M. A Coil Design for Transcranial Magnetic Stimulation of Deep Brain Regions. J. Clin. Neurophysiol. 2002, 19, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.J.; Lamberty, G.J.; Gatewood, L.C.; Johnson, L.M. Traumatic brain injury rehabilitation: Analysis of common data elements. In Proceedings of the MEDINFO 2013, Copenhagen, Denmark, 13–20 August 2013; IOS Press: Amsterdam, The Netherlands, 2013; p. 1186. [Google Scholar]
- Fregni, F.; Nitsche, M.A.; Loo, C.K.; Brunoni, A.R.; Marangolo, P.; Leite, J.; Carvalho, S.; Bolognini, N.; Caumo, W.; Paik, N.J.; et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): Review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2015, 32, 22–35. [Google Scholar] [CrossRef]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Vyvere, T.V.; de la Rosa, E.; Wilms, G.; Nieboer, D.; Steyerberg, E.W.; Maas, A.; Verheyden, J.; Hauwe, L.v.D.; Parizel, P.M. Prognostic Validation of the NINDS Common Data Elements for the Radiologic Reporting of Acute Traumatic Brain Injuries: A CENTER-TBI Study. J. Neurotrauma 2020, 37, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- LaPlaca, M.C.; Huie, J.R.; Alam, H.B.; Bachstetter, A.D.; Bayir, H.; Bellgowan, P.F.; Cummings, D.; Dixon, C.E.; Ferguson, A.R.; Ferland-Beckham, C.; et al. pre-clinical common data el-ements for traumatic brain injury research: Progress and use cases. J. Neurotrauma 2021, 38, 1399–1410. [Google Scholar] [CrossRef]

| Neuromodulation Techniques | Description and Characteristics | Picture |
|---|---|---|
| Transcranial Magnetic Stimulation (TMS) | A technique known as transcutaneous magnetic stimulation (TMS) is a noninvasive way to stimulate the brain, producing alternating magnetic fields that change rapidly over time. The extensive capabilities of TMS make it a perfect neurophysiological tool for studying the function of brain regions and their associated networks, as well as studying brain–behavior relationships to identify possible neurobiological substrates of diseases [10]. For single-pulse experiments, monophasic magnetic pulses are commonly used, whereas rTMS experiments usually require biphasic stimulation waveforms due to their lower energy requirements [36]. Low-frequency rTMS studies typically employ a 1 Hz stimulation frequency, with differences in both the intensity and number of pulses during each study, which can suppress the effect. Conversely, high-frequency rTMS (5–250 Hz) is believed to enhance cortical excitability [37]. |   MagstimRapid |
| Transcranial Direct Current Stimulation (tDCS) | A brain stimulation technique called tDCS delivers low electrical current (2–1 mA) to the cerebral cortex as a means of stimulating cognition and regulating symptoms of neurological disorders and psychiatric. Although common, the side effects include mild itching, burning, and headache, but no lasting effects. A range of approaches can be utilized to pinpoint the location of electrodes. Typically, the 10:20 EEG system is utilized. The measurements can then be used in conjunction with a 10:20 EEG system to localize the region of interest. Alternatively, neuronavigation software, which is more accurate than 10:20 EEG systems, can be used [38]. The scalp can be equipped with electrodes through rubber bands, elastic mesh tubing, or neoprene caps. Keeping the electrodes in place during stimulation is crucial. One study found that as little as 5% movement can change the accuracy and intensity of electrical current to a desired cortical area [39]. The target area (prefrontal cortex, motor cortex, etc.) is stimulated using target electrodes, the location of which depends on the hypothesis and task. Alternatively, hemispheric montages (also known as “dual” stimulation) can be used. In this case, the positioning of both target electrodes is fundamental for downregulation in one region (cathode current) and upregulation in a parallel region (anodic current), opposite hemisphere [40]. | 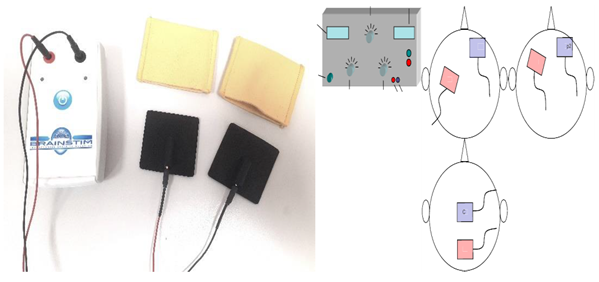 Transcranial electrical stimulator (tDCS) |
| Vague Nerve Stimulation (VNS) | The VNS is a device that can be implanted, which includes an electrode surrounding the left vagus nerve and an attached unit with batteries and corresponding pulse generators placed under the collarbone. The treatment of drug-resistant depression and epilepsy is often achieved through it, resulting in significant antidepressant and antiepileptic effects. It typically denotes the parametric elements that impact on the administration and delivery of electrical stimulation. It includes: (i) Pulse width is the length of time of a square current pulse. This time parameter is specified in microseconds (μs); (ii) current strength is a measure of the amplitude or strength of an electrical impulse. The unit is milliampere (mA); (iii) frequency is a measure of the total periodic cycles (from the beginning of one pulse to the beginning of the next) in one second. In contrast to the pulse width, the time during which no current is applied is taken into account. This is in Hertz (Hz); (iv) on–off time is the amount of time that pacing and nonstimulation periods are delivered during a specified period. The “on” period is the time during which stimulation with an intensity greater than 0 mA is delivered; (v) during VNS treatment, the duration of time is considered the cumulative timing [41]. | 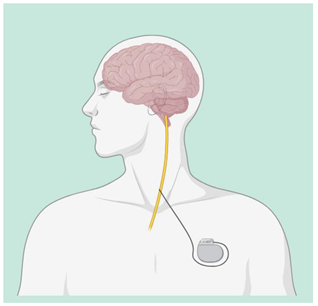 Vagus Nerve Stimulation (VNS) |
| Deep Brain Stimulation (DBS) | DBS is used through electrodes implanted stereotactically at specific targets in the brain. The electrodes are connected to an implantable pulse generator, which is a pacemaker-like device that is implanted under the skin in the chest wall and typically located beneath the collarbone. A computer, which communicates with the implanted pulse generator via a transcutaneous connection, is used by the clinician to establish stimulation parameters after DBS implantation. Stimulation parameters include electrode contacts that give stimulus amplitude, frequency, and pulse width. In the last years, DBS of various targets has been used to promote recovery in patients with disorders of consciousness with varying results, though evidence supporting the use of DBS in MCS patients following TBI is lacking [42,43]. | 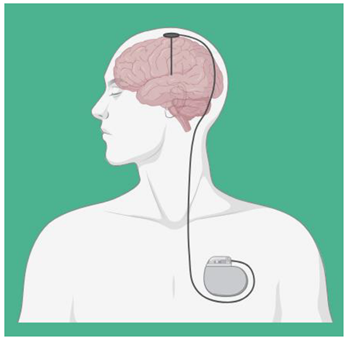 |
| Spinal Cord Stimulation (SCS) | SCS is a form of electrotherapy in which electrodes are implanted into the epidural space of the spinal cord to stimulate the posterior column and modulate nerve function. It is common for the outpatient procedure to last around 1–2 h before a transplant. The surgeon inflates the generator by making an incision, usually on the lower abdomen or buttocks, and then inserts permanent electrodes through a second inlet along one side of the spine after giving local anesthesia. The majority of times, the wound is closed for 2 to 4 weeks after the operation. Advanced leads, advanced remote pulse generators, and traditional SCS are used to treat chronic pain using a variety of stimulation parameters/programs, including high-frequency stimulation, high-frequency burst stimulation, and dorsal root ganglion stimulation [44,45]. |  |
| Transcutaneous electrical nerve stimulation (TENS) | The noninvasive TENS method involves the placement of adhesive electrodes on the skin, which deliver pulsed electrical stimulation with a variable frequency, intensity, and duration. The use of it for pain management is widespread in both acute and chronic pain conditions. General battery-powered TENS machines can adjust pulse width, frequency, and intensity. In general, TENS uses high frequencies (>50 Hz) and intensities below motor contractions (sensory intensity) or low frequencies [46,47]. |  |
| Low Level Laser Therapy (LLLT) | LLLT is a novel noninvasive neurostimulation method that can safely penetrate the brain at specific wavelengths. It is thought to promote cell survival when energy substrates are depleted by interacting with cytochrome c oxidase and promoting oxidative phosphorylation [48,49]. Both animal models and human stroke and TBI patients have reported significant positive effects from LLLT. Kuman et al. showed that LLLT could improve cognitive function in controlled cortical impact (CCI) mice [50]. Poiani et al. [51] used an optical device consisting of an LED emitting 632 nm radiation at full power of 830 mW in patients with TBI. A skull area of 400 cm2 was irradiated for 30 min, corresponding to a total dose of 3.74 J/cm2 per session. |  |
| Author | Aim | Treatment Period | Sample Size | Outcomes Measures | Main Findings |
|---|---|---|---|---|---|
| Kahana et al. 2023 [52] | To assess whether closed-loop tDCS of the temporal lobe cortex can reliably improve memory in a TBI cohort. | 1 year. | 8 patients with TBI. | ENS, EEG. | The stimulus-induced recall of lists was 19% more effective than the non stimulated ones. This discovery provides evidence for closed-loop brain stimulation as a potential therapy for memory impairment caused by TBI. |
| Longo et al. 2020 [53] | To evaluate the feasibility and safety of LLLT in the acute phase after moderate TBI and neural response to LLLT using MRI and cognitive measures | 27 November 2015–11 July 2019. | 68 men and women with TBI. | LLLT, RPQ. | LLLT was successfully administered to all patients in this randomized clinical trial without any adverse effects observed. During the late subacute phase, light therapy caused significant changes in several diffusion tensor parameters. |
| De Freitas et al. 2020 [54] | To see if episodic memory is improved more than just simulated tDCS but enhanced by active tDCS and computer-based cognitive training. | A 20 min. tDCS for 10 days. | 36 participants with TBI. | BDI-II, WAIS, RAVLT, AEQ. | The results proved that delta activity decreased and alpha frequencies increased near active electrodes and found a better performance correlation in neuropsychological tests. |
| Sultana et al. 2023 [55] | To explore the relationship between changes in connectivity and emotional health following rTMS in TBI patients. | 20 sessions in 2 weeks. | 32 patients with TBI. | VR-36, fMRI. | The results showed an overall decrease in the strength of excitatory connectivity and an increase in the strength of inhibitory connectivity among extrinsic connections after neuromodulation. The central area of analysis was the dorsal anterior cingulate cortex (dACC), which is thought to be most affected during emotional health disorders. |
| Neville et al. 2019 [56] | To investigate the potential of high-frequency repetitive rTMS to enhance cognitive abilities in individuals who have suffered from severe TBI. | 90 days. | Individuals between 18 and 60 years. | TMT-B, rTMS. | Cognitive function in chronic DAI patients does not improve with high-frequency rTMS for the left DLPFC. |
| Opie et al. 2018 [57] | In this study, TMS and EEG were used further to investigate the impact of mTBI on these processes. | Not Specificated. | 32 participants. | GCS, LICI, TMS. | TEP measurements showed that GABA-a and GABA-b activation was not affected by injury; TEP measurements also showed that the response to cTBS was increased in patients, suggesting that cortical plasticity is enhanced due to injury. |
| Hou et al. 2022 [58] | It investigated the efficacy of TLNS and associated brain connectivity using the RSFC approach in mmTBI patients. | 2 weeks. | 9 participants with mmTBI. | SOT, DGI. | TLNS in combination with physiotherapy can induce brain plasticity in TBI patients with balance and movement disorders. |
| Tyler et al. 2019 [59] | The effectiveness of noninvasive TLNS and PT in treating chronic balance/foot gait disorders caused by mmTBI is evaluated through comparison. | 26 weeks. | 44 Participants | TLNS, PT, SOT. | Balance scores were significantly improved in both the HFP + PT and LFP + PT groups, and the results were maintained for 12 weeks after TLNS treatment discontinuation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderone, A.; Cardile, D.; Gangemi, A.; De Luca, R.; Quartarone, A.; Corallo, F.; Calabrò, R.S. Traumatic Brain Injury and Neuromodulation Techniques in Rehabilitation: A Scoping Review. Biomedicines 2024, 12, 438. https://doi.org/10.3390/biomedicines12020438
Calderone A, Cardile D, Gangemi A, De Luca R, Quartarone A, Corallo F, Calabrò RS. Traumatic Brain Injury and Neuromodulation Techniques in Rehabilitation: A Scoping Review. Biomedicines. 2024; 12(2):438. https://doi.org/10.3390/biomedicines12020438
Chicago/Turabian StyleCalderone, Andrea, Davide Cardile, Antonio Gangemi, Rosaria De Luca, Angelo Quartarone, Francesco Corallo, and Rocco Salvatore Calabrò. 2024. "Traumatic Brain Injury and Neuromodulation Techniques in Rehabilitation: A Scoping Review" Biomedicines 12, no. 2: 438. https://doi.org/10.3390/biomedicines12020438
APA StyleCalderone, A., Cardile, D., Gangemi, A., De Luca, R., Quartarone, A., Corallo, F., & Calabrò, R. S. (2024). Traumatic Brain Injury and Neuromodulation Techniques in Rehabilitation: A Scoping Review. Biomedicines, 12(2), 438. https://doi.org/10.3390/biomedicines12020438







