Ablative and Immunostimulatory Effects of Histotripsy Ablation in a Murine Osteosarcoma Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Murine OS Model
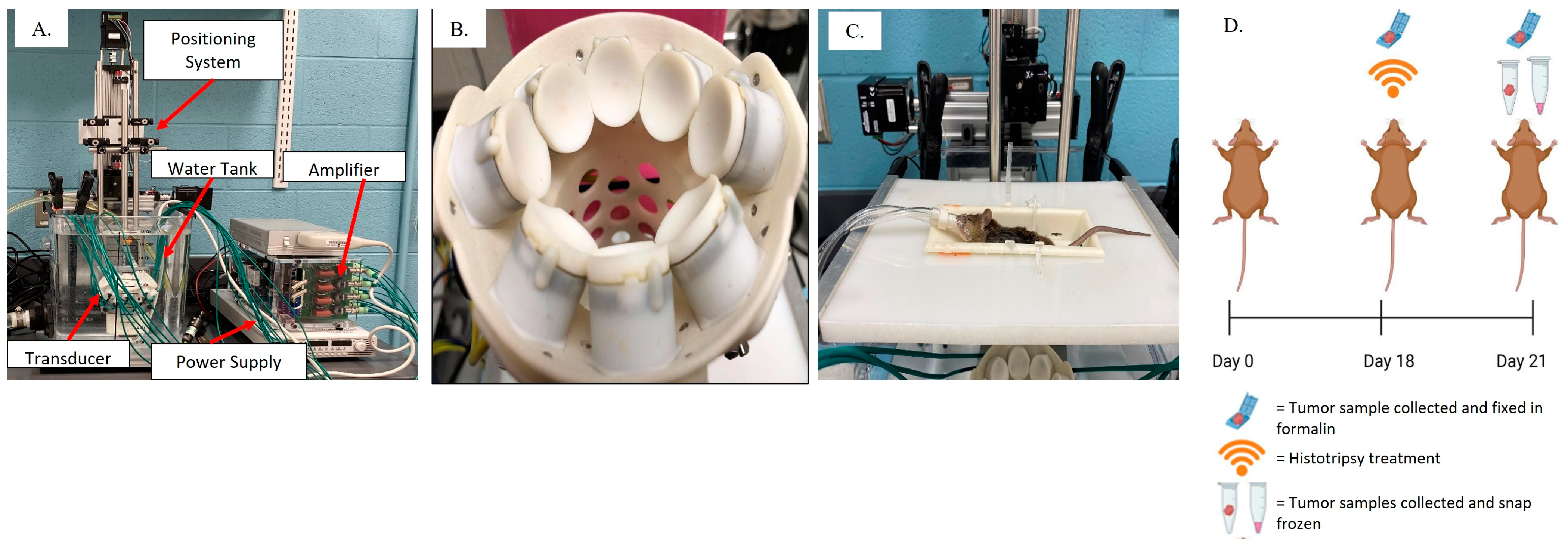
2.2. Histotripsy System and Ablation Parameters
2.3. Tissue Collection and Processing
2.4. Histopathology
2.5. Gene Expression Analysis
2.6. Flow Cytometry Analysis
2.7. Data and Statistical Analysis
3. Results
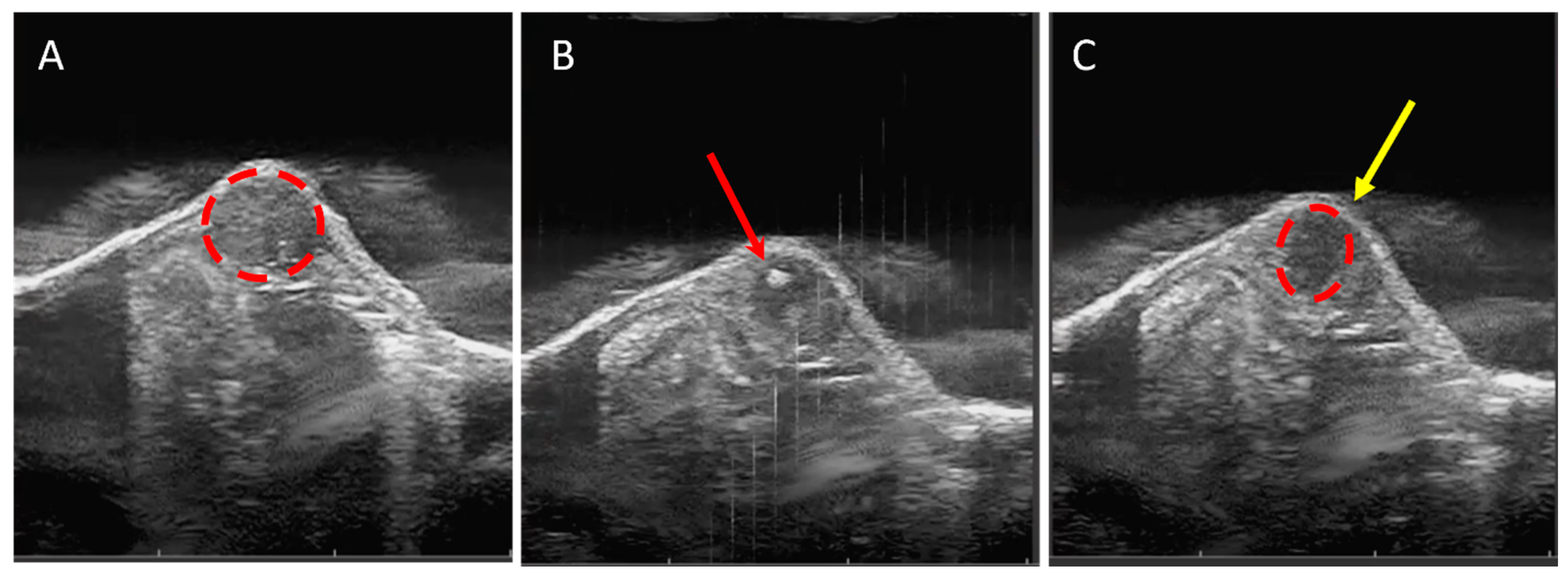
3.1. Tumor Tissue Can Be Effectively and Precisely Targeted and Ablated with Histotripsy
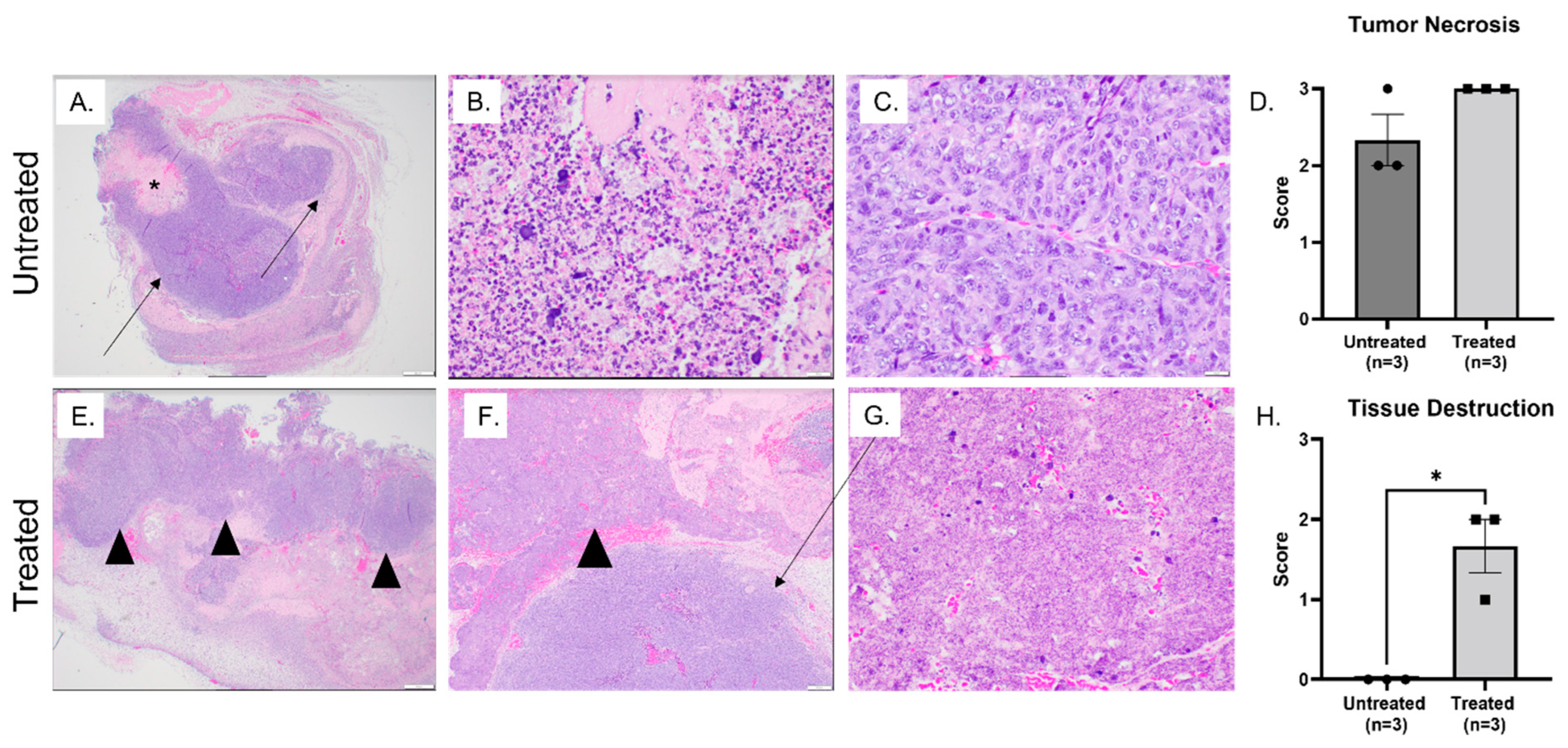
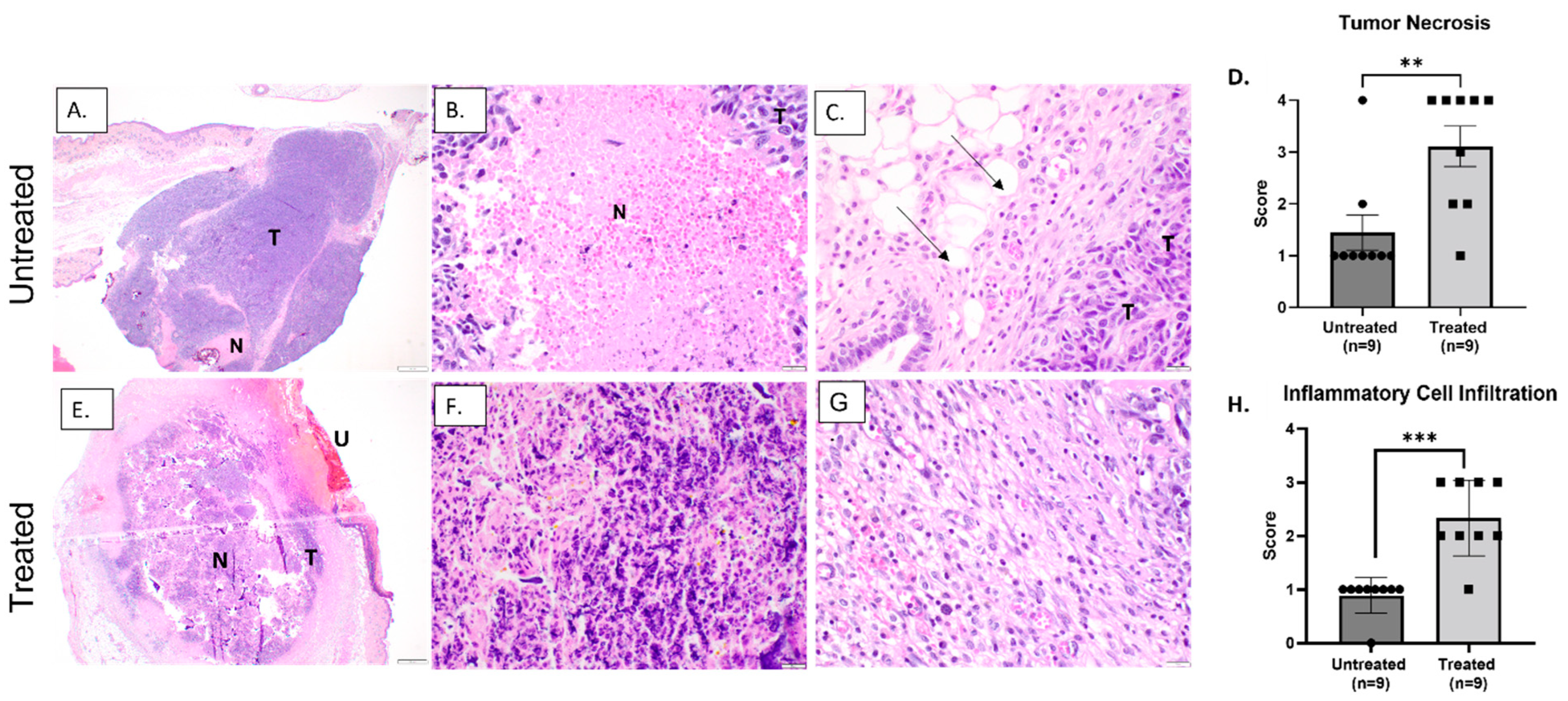
3.2. Histotripsy Ablation Resulted in Effective Tumor Tissue Destruction, Necrosis, and Immune Cell Infiltration
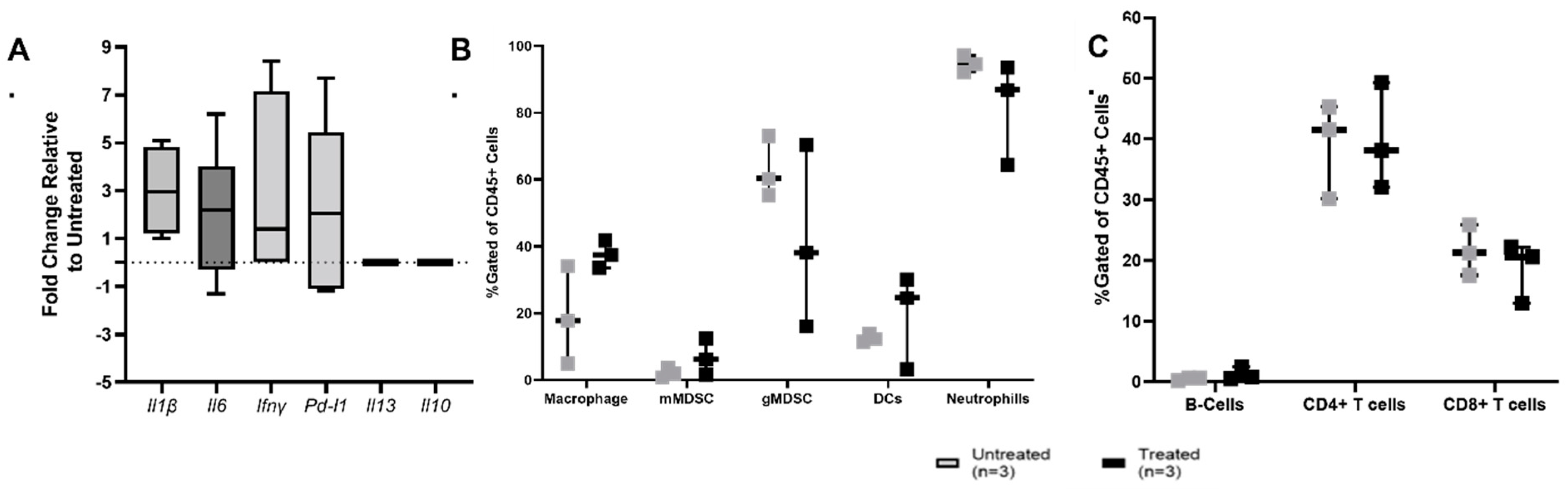
3.3. Histotripsy Ablation Induces Immune Activation within the TME
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. SICOT J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Friebele, J.C.; Peck, J.; Pan, X.; Abdel-Rasoul, M.; Mayerson, J.L. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am. J. Orthop. 2015, 44, 547–553. [Google Scholar] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Peng, H. Osteosarcoma in patients below 25 years of age: An observational study of incidence, metastasis, treatment and outcomes. Oncol. Lett. 2018, 16, 6502–6514. [Google Scholar] [CrossRef]
- Moukengue, B.; Lallier, M.; Marchandet, L.; Baud’huin, M.; Verrecchia, F.; Ory, B.; Lamoureux, F. Origin and Therapies of Osteosarcoma. Cancers 2022, 14, 3503. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.A.; Mirabello, L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 2011, 2011, 548151. [Google Scholar] [CrossRef]
- Meazza, C.; Scanagatta, P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Expert Rev. Anticancer Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, S.Z.; Posta, A.; Weber, K.L.; Wilson, R.J. Limb Salvage and Reconstruction Options in Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1257, 13–29. [Google Scholar] [CrossRef]
- Yang, Y.; Han, L.; He, Z.; Li, X.; Yang, S.; Yang, J.; Zhang, Y.; Li, D.; Yang, Y.; Yang, Z. Advances in limb salvage treatment of osteosarcoma. J. Bone Oncol. 2018, 10, 36–40. [Google Scholar] [CrossRef]
- El Ghoneimy, A.M.; Shehab, A.M.; Farid, N. What is the Cumulative Incidence of Revision Surgery and What Are the Complications Associated with Stemmed Cementless Nonextendable Endoprostheses in Patients 18 Years or Younger with Primary Bone Sarcomas About the Knee. Clin. Orthop. Relat. Res. 2022, 480, 1329–1338. [Google Scholar] [CrossRef]
- Kaneuchi, Y.; Yoshida, S.; Fujiwara, T.; Evans, S.; Abudu, A. Limb salvage surgery has a higher complication rate than amputation but is still beneficial for patients younger than 10 years old with osteosarcoma of an extremity. J. Pediatr. Surg. 2022, 57, 702–709. [Google Scholar] [CrossRef]
- Tóth, L.; Krieg, A.H.; Nowakowski, A.M. How much is a leg worth following radical tumor resection in bone sarcomas? Literature review. Surg. Oncol. 2022, 46, 101900. [Google Scholar] [CrossRef]
- Luo, Z.W.; Liu, P.P.; Wang, Z.X.; Chen, C.Y.; Xie, H. Macrophages in Osteosarcoma Immune Microenvironment: Implications for Immunotherapy. Front. Oncol. 2020, 10, 586580. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex but Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeRenzo, C.; Gottschalk, S. Genetically Modified T-Cell Therapy for Osteosarcoma: Into the Roaring 2020s. Adv. Exp. Med. Biol. 2020, 1257, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.F.; Lézot, F.; Heymann, D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell. Immunol. 2019, 343, 103711. [Google Scholar] [CrossRef]
- Pratt, H.G.; Justin, E.M.; Lindsey, B.A. Applying Osteosarcoma Immunology to Understand Disease Progression and Assess Immunotherapeutic Response. Adv. Exp. Med. Biol. 2020, 1258, 91–109. [Google Scholar] [CrossRef]
- Dubinsky, T.J.; Khokhlova, T.D.; Khokhlova, V.; Schade, G.R. Histotripsy: The Next Generation of High-Intensity Focused Ultrasound for Focal Prostate Cancer Therapy. J. Ultrasound Med. 2020, 39, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Wenger, A.; Hutchison, R.; Vlaisavljevich, E.; Allen, I.C. Immunological Effects of Histotripsy for Cancer Therapy. Front. Oncol. 2021, 11, 681629. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Wenger, A.; Sereno, J.; Gannon, J.; Zeher, A.; Brock, R.M.; Beitel-White, N.; Simon, A.; Davalos, R.V.; Coutermarsh-Ott, S.; Vlaisavljevich, E.; et al. Histotripsy Ablation Alters the Tumor Microenvironment and Promotes Immune System Activation in a Subcutaneous Model of Pancreatic Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Latifi, M.; Hay, A.; Carroll, J.; Dervisis, N.; Arnold, L.; Coutermarsh-Ott, S.L.; Kierski, K.R.; Klahn, S.; Allen, I.C.; Vlaisavljevich, E.; et al. Focused ultrasound tumour ablation in small animal oncology. Vet. Comp. Oncol. 2021, 19, 411–419. [Google Scholar] [CrossRef]
- Pahk, K.J.; Shin, C.H.; Bae, I.Y.; Yang, Y.; Kim, S.H.; Pahk, K.; Kim, H.; Oh, S.J. Boiling Histotripsy-induced Partial Mechanical Ablation Modulates Tumour Microenvironment by Promoting Immunogenic Cell Death of Cancers. Sci. Rep. 2019, 9, 9050. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef]
- Schade, G.R.; Wang, Y.N.; D'Andrea, S.; Hwang, J.H.; Liles, W.C.; Khokhlova, T.D. Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response. Ultrasound Med. Biol. 2019, 45, 137–147. [Google Scholar] [CrossRef]
- Singh, M.P.; Sethuraman, S.N.; Miller, C.; Malayer, J.; Ranjan, A. Boiling histotripsy and in-situ CD40 stimulation improve the checkpoint blockade therapy of poorly immunogenic tumors. Theranostics 2021, 11, 540–554. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres-Creixams, X.; Ziemlewicz, T.J.; Cannata, J.M. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3001–3005. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Maxwell, A.; Mancia, L.; Johnsen, E.; Cain, C.; Xu, Z. Visualizing the Histotripsy Process: Bubble Cloud-Cancer Cell Interactions in a Tissue-Mimicking Environment. Ultrasound Med. Biol. 2016, 42, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.B.; Vlaisavljevich, E.; Maxwell, A.D. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med. Biol. 2019, 45, 1056–1080. [Google Scholar] [CrossRef]
- Allam, C.L.; Wilkinson, J.E.; Cheng, X.; Ives, K.A.; Hall, T.L.; Roberts, W.W. Histotripsy effects on the bladder trigone: Functional and histologic consequences in the canine model. J. Endourol. 2013, 27, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, V.A.; Fowlkes, J.B.; Roberts, W.W.; Schade, G.R.; Xu, Z.; Khokhlova, T.D.; Hall, T.L.; Maxwell, A.D.; Wang, Y.N.; Cain, C.A. Histotripsy methods in mechanical disintegration of tissue: Towards clinical applications. Int. J. Hyperth. 2015, 31, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Kim, Y.; Owens, G.; Roberts, W.; Cain, C.; Xu, Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys. Med. Biol. 2014, 59, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Vidal, J.J.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Lee, F.; Ziemlewicz, T.J. Phase I Study of Safety and Efficacy of Hepatic Histotripsy: Preliminary Results of First in MAn Experience with Robotically-Assisted Sonic Therapy; International Society of Therapeutic Ultrasound: Barcelona, Spain, 2019. [Google Scholar]
- Schuster, T.G.; Wei, J.T.; Hendlin, K.; Jahnke, R.; Roberts, W.W. Histotripsy Treatment of Benign Prostatic Enlargement Using the Vortx R(x) System: Initial Human Safety and Efficacy Outcomes. Urology 2018, 114, 184–187. [Google Scholar] [CrossRef]
- Messas, E.; Jsselmuiden, A.I.; Goudot, G.; Vlieger, S.; Zarka, S.; Puymirat, E.; Cholley, B.; Spaulding, C.; Hagège, A.A.; Marijon, E.; et al. Feasibility and Performance of Noninvasive Ultrasound Therapy in Patients with Severe Symptomatic Aortic Valve Stenosis: A First-in-Human Study. Circulation 2021, 143, 968–970. [Google Scholar] [CrossRef]
- Schade, G.R.; Keller, J.; Ives, K.; Cheng, X.; Rosol, T.J.; Keller, E.; Roberts, W.W. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J. Urol. 2012, 188, 1957–1964. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Arnold, L.; Gannon, J.; Simon, A.; Singh, N.; Sheppard, H.; Nagai-Singer, M.A.; Imran, K.M.; Lee, K.; Clark-Deener, S.; et al. Histotripsy Ablation in Preclinical Animal Models of Cancer and Spontaneous Tumors in Veterinary Patients: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Hendricks-Wenger, A.; Coutermarsh-Ott, S.; Gannon, J.; Hay, A.N.; Dervisis, N.; Klahn, S.; Allen, I.C.; Tuohy, J.; Vlaisavljevich, E. Histotripsy Ablation of Bone Tumors: Feasibility Study in Excised Canine Osteosarcoma Tumors. Ultrasound Med. Biol. 2021, 47, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.N.; Hay, A.N.; Gannon, J.M.; Sheppard, H.O.; Coutermarsh-Ott, S.L.; Daniel, G.B.; Kierski, K.R.; Ciepluch, B.J.; Vlaisavljevich, E.; Tuohy, J.L. Histotripsy Ablation of Spontaneously Occurring Canine Bone Tumors In Vivo. IEEE Trans. Biomed. Eng. 2023, 70, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.; Yang, E.; Gannon, J.; Sheppard, H.; Coutermarsh-Ott, S.; Ziemlewicz, T.J.; Dervisis, N.; Allen, I.C.; Daniel, G.B.; Tuohy, J.; et al. Mechanical High-Intensity Focused Ultrasound (Histotripsy) in Dogs with Spontaneously Occurring Soft Tissue Sarcomas. IEEE Trans. Biomed. Eng. 2023, 70, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Worlikar, T.; Vlaisavljevich, E.; Gerhardson, T.; Greve, J.; Wan, S.; Kuruvilla, S.; Lundt, J.; Ives, K.; Hall, T.; Welling, T.H.; et al. Histotripsy for Non-Invasive Ablation of Hepatocellular Carcinoma (HCC) Tumor in a Subcutaneous Xenograft Murine Model. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 6064–6067. [Google Scholar] [CrossRef]
- Worlikar, T.; Zhang, M.; Ganguly, A.; Hall, T.L.; Shi, J.; Zhao, L.; Lee, F.T.; Mendiratta-Lala, M.; Cho, C.S.; Xu, Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers 2022, 14, 1612. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-man histotripsy of hepatic tumors: The THERESA trial, a feasibility study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Weber, P.; Simon, A.; Saunier, S.; Coutermarsh-Ott, S.; Grider, D.; Vidal-Jove, J.; Allen, I.C.; Luyimbazi, D.; Vlaisavljevich, E. Histotripsy for the Treatment of Cholangiocarcinoma Liver Tumors: In Vivo Feasibility and Ex Vivo Dosimetry Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2953–2964. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.; Fan, W.; Huang, J.; Zhang, F.; Wu, P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010, 116, 3934–3942. [Google Scholar] [CrossRef]
- Scipione, R.; Anzidei, M.; Bazzocchi, A.; Gagliardo, C.; Catalano, C.; Napoli, A. HIFU for Bone Metastases and other Musculoskeletal Applications. Semin. Intervent. Radiol. 2018, 35, 261–267. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Tang, J. Primary malignant tumours of the bony pelvis: US-guided high intensity focused ultrasound ablation. Int. J. Hyperth. 2013, 29, 683–687. [Google Scholar] [CrossRef]
- Yu, W.; Tang, L.; Lin, F.; Yao, Y.; Shen, Z.; Zhou, X. High-intensity focused ultrasound: Noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg. Oncol. 2015, 24, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.H.; Martin, R.C.G. Optimal Dosing and Patient Selection for Electrochemotherapy in Solid Abdominal Organ and Bone Tumors. Bioengineering 2023, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Locquet, M.A.; Brahmi, M.; Blay, J.Y.; Dutour, A. Radiotherapy in bone sarcoma: The quest for better treatment option. BMC Cancer 2023, 23, 742. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.Y.; Davies, D.M.; Maher, J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem. Soc. Trans. 2018, 46, 1449–1462. [Google Scholar] [CrossRef]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2022, 13, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Eranki, A.; Srinivasan, P.; Ries, M.; Kim, A.; Lazarski, C.A.; Rossi, C.T.; Khokhlova, T.D.; Wilson, E.; Knoblach, S.M.; Sharma, K.V.; et al. High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2020, 26, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Dumars, C.; Ngyuen, J.M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology 2017, 6, e1331193. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- DeVito, N.C.; Plebanek, M.P.; Theivanthiran, B.; Hanks, B.A. Role of Tumor-Mediated Dendritic Cell Tolerization in Immune Evasion. Front. Immunol. 2019, 10, 2876. [Google Scholar] [CrossRef]
- Garbi, N.; Kreutzberg, T. Dendritic cells enhance the antigen sensitivity of T cells. Front. Immunol. 2012, 3, 389. [Google Scholar] [CrossRef]
- Kramer, E.D.; Abrams, S.I. Granulocytic Myeloid-Derived Suppressor Cells as Negative Regulators of Anticancer Immunity. Front. Immunol. 2020, 11, 1963. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Clone | Concentration |
|---|---|---|
| CD45 | 30-F11 | 0.25 µg/mL |
| CD11b | M1/70 | 0.156 µg/mL |
| Ly6C | HK1.4 | 0.078 µg/mL |
| Ly6G | 1A8 | 0.3 µg/mL |
| CD11c | N418 | 1.25 µg/mL |
| MHCII | M5/114.15.2 | 0.2 µg/mL |
| F4/80 | BM8 | 2.5 µg/mL |
| CD3 | 17A2 | 1.25 µg/mL |
| CD4 | GK1.5 | 0.156 µg/mL |
| CD8a | 53-6.7 | 0.625 µg/mL |
| CD19 | 6D5 | 0.25 µg/mL |
| FoxP3 | 1054C | 0.25 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hay, A.N.; Imran, K.M.; Hendricks-Wenger, A.; Gannon, J.M.; Sereno, J.; Simon, A.; Lopez, V.A.; Coutermarsh-Ott, S.; Vlaisavljevich, E.; Allen, I.C.; et al. Ablative and Immunostimulatory Effects of Histotripsy Ablation in a Murine Osteosarcoma Model. Biomedicines 2023, 11, 2737. https://doi.org/10.3390/biomedicines11102737
Hay AN, Imran KM, Hendricks-Wenger A, Gannon JM, Sereno J, Simon A, Lopez VA, Coutermarsh-Ott S, Vlaisavljevich E, Allen IC, et al. Ablative and Immunostimulatory Effects of Histotripsy Ablation in a Murine Osteosarcoma Model. Biomedicines. 2023; 11(10):2737. https://doi.org/10.3390/biomedicines11102737
Chicago/Turabian StyleHay, Alayna N., Khan Mohammad Imran, Alissa Hendricks-Wenger, Jessica M. Gannon, Jacqueline Sereno, Alex Simon, Victor A. Lopez, Sheryl Coutermarsh-Ott, Eli Vlaisavljevich, Irving C. Allen, and et al. 2023. "Ablative and Immunostimulatory Effects of Histotripsy Ablation in a Murine Osteosarcoma Model" Biomedicines 11, no. 10: 2737. https://doi.org/10.3390/biomedicines11102737
APA StyleHay, A. N., Imran, K. M., Hendricks-Wenger, A., Gannon, J. M., Sereno, J., Simon, A., Lopez, V. A., Coutermarsh-Ott, S., Vlaisavljevich, E., Allen, I. C., & Tuohy, J. L. (2023). Ablative and Immunostimulatory Effects of Histotripsy Ablation in a Murine Osteosarcoma Model. Biomedicines, 11(10), 2737. https://doi.org/10.3390/biomedicines11102737






