Iron Bioavailability in the Extracellular Environment Is More Relevant Than the Intracellular One in Viability and Gene Expression: A Lesson from Oligodendroglioma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
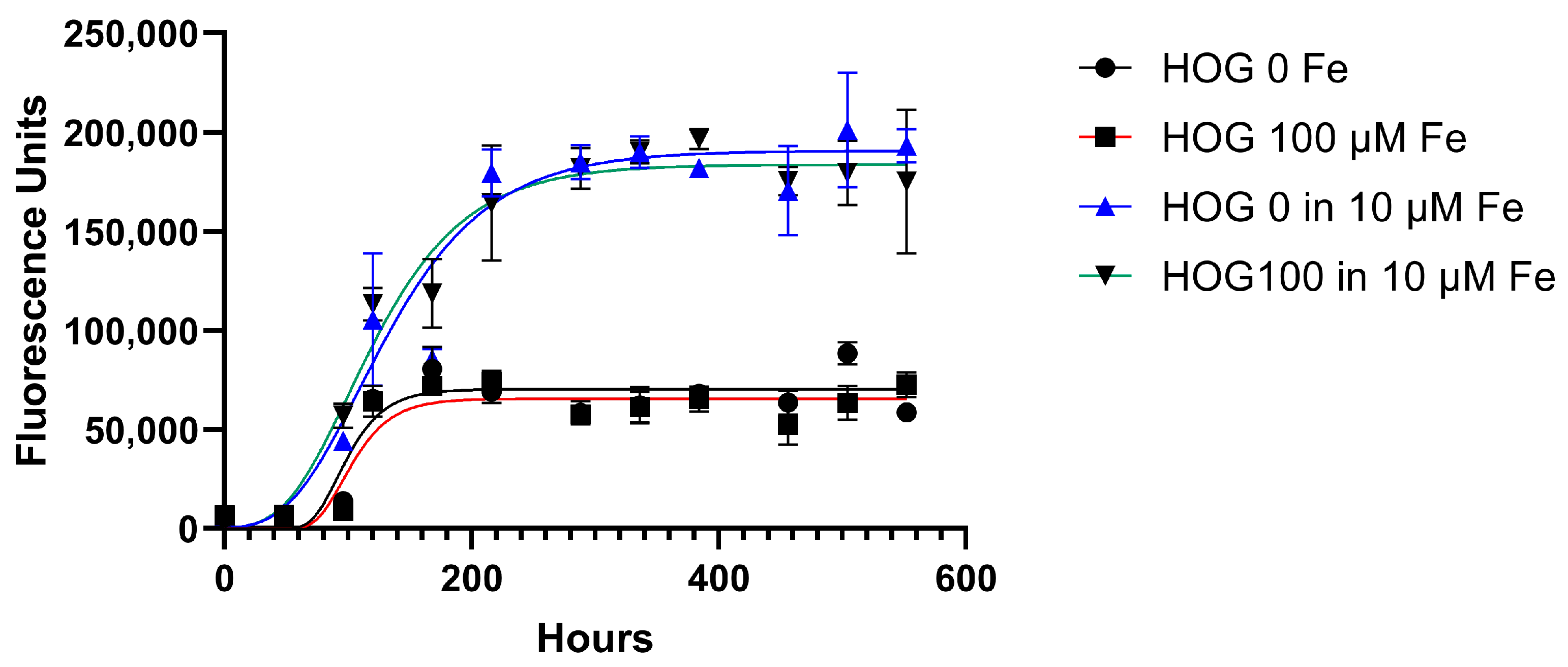
2.2. Viability Measurements (Alamar Blue Assay)
2.3. Sample Preparation for ICP-MS Analysis in Cell Lines
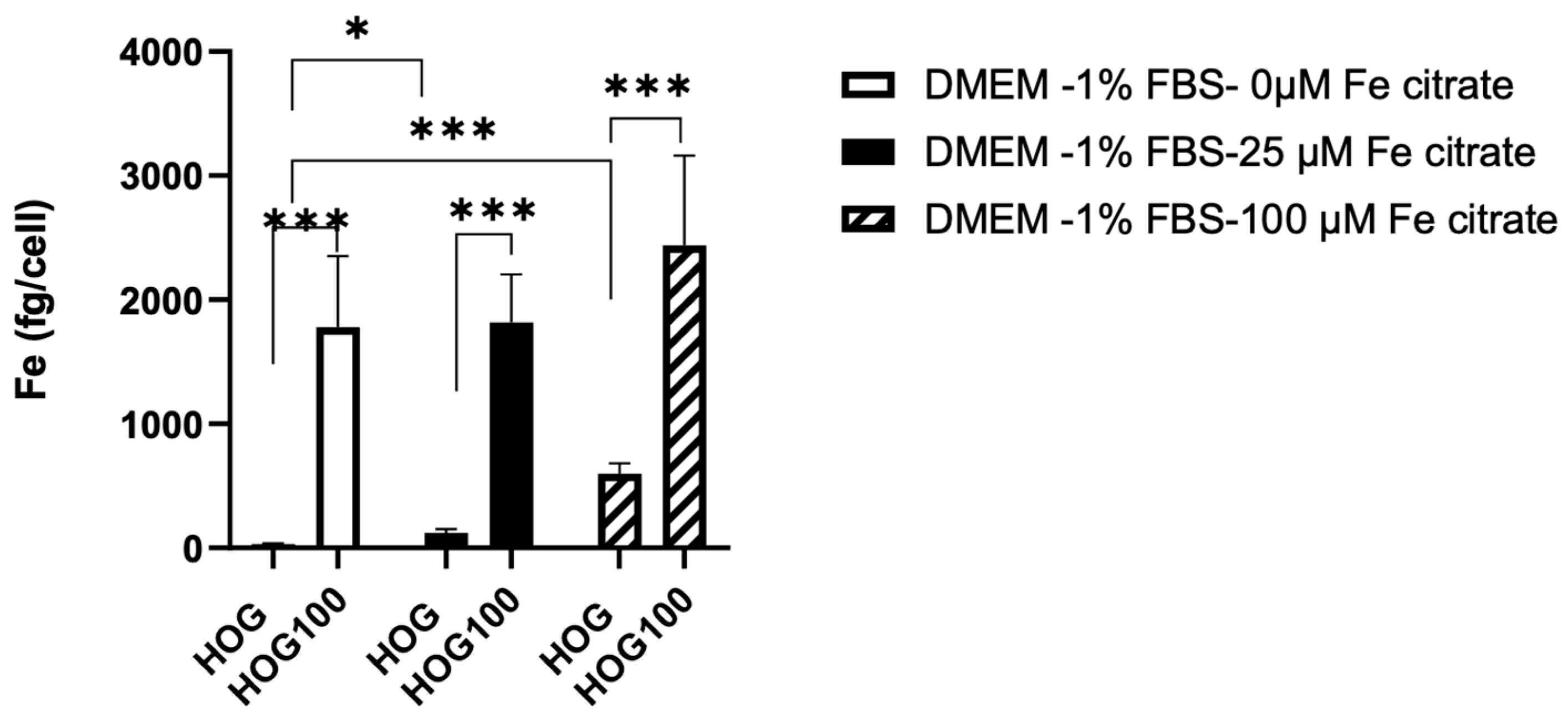
2.4. Quantification of Iron (Fe) in Cell Lysates
2.5. Confocal Laser Scanning Microscope Analysis
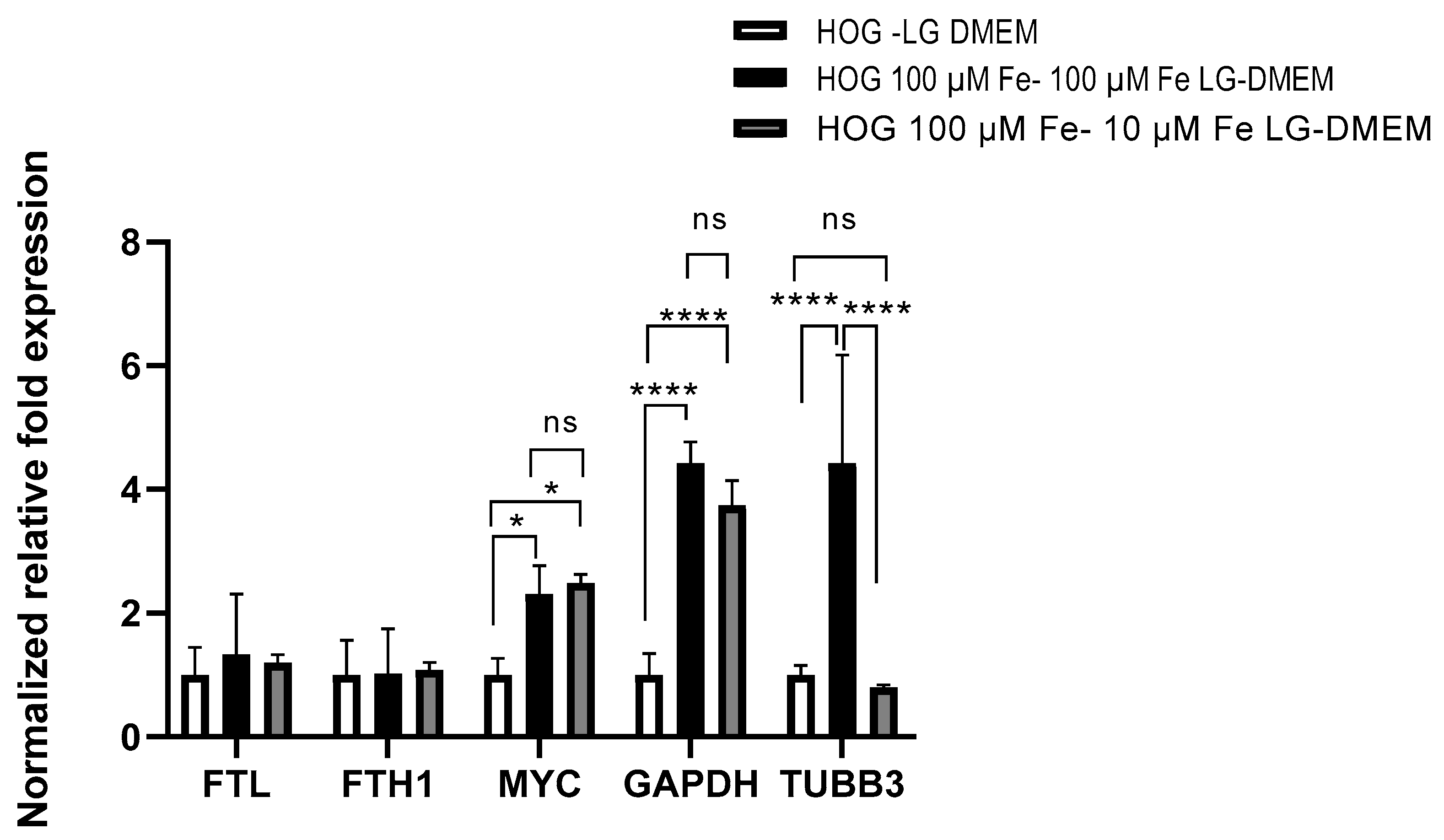
2.6. Gene Expression by Real-Time PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Iron Conditioning on Cell Morphology
3.2. Effect of Medium Composition on Cell Models
3.3. Cell Line Viability and Proliferation after Conditioned Iron Medium
3.4. Confocal Microscopy Confirms the Iron Uptake
3.5. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singla, A.K.; Madan, R.; Gupta, K.; Goyal, S.; Kumar, N.; Sahoo, S.K.; Uppal, D.K.; Ahuja, C.K. Clinical behaviour and outcome in pediatric glioblastoma: Current scenario. Radiat. Oncol. J. 2021, 39, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Adel Fahmideh, M.; Cote, D.J.; Muskens, I.S.; Schraw, J.M.; Scheurer, M.E.; Bondy, M.L. Risk factors for childhood and adult primary brain tumors. Neuro-oncology 2019, 21, 1357–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Chen, J.; Lan, Y.; Zhang, W.; Kang, Z.; Zheng, Y.; Zhang, R.; Yu, J.; Li, W. Signaling pathways in brain tumors and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 8. [Google Scholar] [CrossRef]
- Lau, C.S.; Mahendraraj, K.; Chamberlain, R.S. Oligodendrogliomas in pediatric and adult patients: An outcome-based study from the Surveillance, Epidemiology, and End Result database. Cancer Manag. Res. 2017, 9, 159–166. [Google Scholar] [CrossRef]
- Byer, L.; Kline-Nunnally, C.; Tihan, T.; Mueller, S. Chapter 33—Pediatric oligodendroglioma. In Oligodendroglioma; Paleologos, N.A., Newton, H.B., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 379–386. [Google Scholar]
- Goel, N.J.; Abdullah, K.G.; Lang, S.-S. Outcomes and Prognostic Factors in Pediatric Oligodendroglioma: A Population-Based Study. Pediatr. Neurosurg. 2017, 53, 24–35. [Google Scholar] [CrossRef]
- Fonseca-Nunes, A.; Jakszyn, P.; Agudo, A. Iron and Cancer Risk—A Systematic Review and Meta-analysis of the Epidemiological Evidence. Cancer Epidemiol. Biomark. Prev. 2014, 23, 12–31. [Google Scholar] [CrossRef]
- Hänninen, M.M.; Haapasalo, J.; Haapasalo, H.; Fleming, R.E.; Britton, R.S.; Bacon, B.R.; Parkkila, S. Expression of iron-related genes in human brain and brain tumors. BMC Neurosci. 2009, 10, 36. [Google Scholar] [CrossRef]
- Liu, H.-D.; Li, W.; Chen, Z.-R.; Zhou, M.-L.; Zhuang, Z.; Zhang, D.-D.; Zhu, L.; Hang, C.-H. Increased expression of ferritin in cerebral cortex after human traumatic brain injury. Neurol. Sci. 2013, 34, 1173–1180. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Tao, L.; Chen, L. Iron Transporters and Ferroptosis in Malignant Brain Tumors. Front. Oncol. 2022, 12, 861834. [Google Scholar] [CrossRef]
- Steegmann-Olmedillas, J.L. The role of iron in tumour cell proliferation. Clin. Transl. Oncol. 2011, 13, 71–76. [Google Scholar] [CrossRef]
- Le, N.T.V.; Richardson, D.R. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2002, 1603, 31–46. [Google Scholar] [CrossRef]
- Bishop, G.M.; Dang, T.N.; Dringen, R.; Robinson, S.R. Accumulation of Non-Transferrin-Bound Iron by Neurons, Astrocytes, and Microglia. Neurotox. Res. 2011, 19, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Legendre, C.; Garcion, E. Iron metabolism: A double-edged sword in the resistance of glioblastoma to therapies. Trends Endocrinol. Metab. 2015, 26, 322–331. [Google Scholar] [CrossRef]
- Reith, T.P.; Prah, M.A.; Choi, E.-J.; Lee, J.; Wujek, R.; Al-Gizawiy, M.; Chitambar, C.R.; Connelly, J.M.; Schmainda, K.M. Basal Ganglia Iron Content Increases with Glioma Severity Using Quantitative Susceptibility Mapping: A Potential Biomarker of Tumor Severity. Tomography 2022, 8, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Schonberg, D.L.; Miller, T.E.; Wu, Q.; Flavahan, W.A.; Das, N.K.; Hale, J.S.; Hubert, C.G.; Mack, S.C.; Jarrar, A.M.; Karl, R.T.; et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell 2015, 28, 441–455. [Google Scholar] [CrossRef]
- Gao, J.; Richardson, D.R. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents, IV: The mechanisms involved in inhibiting cell-cycle progression. Blood 2001, 98, 842–850. [Google Scholar] [CrossRef]
- Richardson, D.R.; Tran, E.H.; Ponka, P. The Potential of Iron Chelators of the Pyridoxal Isonicotinoyl Hydrazone Class as Effective Antiproliferative Agents. Blood 1995, 86, 4295–4306. [Google Scholar] [CrossRef]
- Jaksch-Bogensperger, H.; Spiegl-Kreinecker, S.; Arosio, P.; Eckl, P.; Golaszewski, S.; Ebner, Y.; Al-Schameri, R.; Strasser, P.; Weis, S.; Bresgen, N. Ferritin in glioblastoma. Br. J. Cancer 2020, 122, 1441–1444. [Google Scholar] [CrossRef]
- Walter, A.W.; Hancock, M.L.; Pui, C.H.; Hudson, M.M.; Ochs, J.S.; Rivera, G.K.; Pratt, C.B.; Boyett, J.M.; Kun, L.E. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J. Clin. Oncol. 1998, 16, 3761–3767. [Google Scholar] [CrossRef]
- Maule, M.; Scélo, G.; Pastore, G.; Brennan, P.; Hemminki, K.; Tracey, E.; Sankila, R.; Weiderpass, E.; Olsen, J.H.; McBride, M.L.; et al. Risk of Second Malignant Neoplasms After Childhood Leukemia and Lymphoma: An International Study. JNCI J. Natl. Cancer Inst. 2007, 99, 790–800. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Moschovi, M.; Georgoulis, G.; Neroutsou, R.; Stefanaki, K.; Sfakianos, G.; Prodromou, N. Anaplastic oligodendrogliomas after treatment of acute lymphoblastic leukemia in children: Report of 2 cases. J. Neurosurg. Pediatr. PED 2010, 5, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Post, G.R.; Dawson, G. Characterization of a cell line derived from a human oligodendroglioma. Mol. Chem. Neuropathol. 1992, 16, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Buntinx, M.; Vanderlocht, J.; Hellings, N.; Vandenabeele, F.; Lambrichts, I.; Raus, J.; Ameloot, M.; Stinissen, P.; Steels, P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: Morphology and oligodendrocyte-specific gene expression. J. Neurocytol. 2003, 32, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Halonen, P.i.; Mattila, J.; Suominen, P.; Ruuska, T.; Salo, M.K.; Mäkipernaa, A. Iron Overload in Children Who Are Treated for Acute Lymphoblastic Leukemia Estimated by Liver Siderosis and Serum Iron Parameters. Pediatrics 2003, 111, 91–96. [Google Scholar] [CrossRef]
- Bolte, S.; CordeliÈRes, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Kashima, T.; Tiu, S.N.; Merrill, J.E.; Vinters, H.V.; Dawson, G.; Campagnoni, A.T. Expression of Oligodendrocyte-associated Genes in Cell Lines Derived from Human Gliomas and Neuroblastomas. Cancer Res. 1993, 53, 170–175. [Google Scholar]
- De Kleijn, K.M.A.; Zuure, W.A.; Peijnenborg, J.; Heuvelmans, J.M.; Martens, G.J.M. Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning. Cells 2019, 8, 1096. [Google Scholar] [CrossRef]
- Connor, J.R.; Menzies, S.L. Relationship of iron to oligondendrocytes and myelination. Glia 1996, 17, 83–93. [Google Scholar] [CrossRef]
- Brown, R.A.M.; Richardson, K.L.; Kabir, T.D.; Trinder, D.; Ganss, R.; Leedman, P.J. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front. Oncol. 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Merlot, A.M.; Huang, M.L.H.; Bae, D.H.; Jansson, P.J.; Sahni, S.; Kalinowski, D.S.; Richardson, D.R. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 1130–1144. [Google Scholar] [CrossRef]
- Koorts, A.M.; Viljoen, M. Ferritin and ferritin isoforms I: Structure–function relationships, synthesis, degradation and secretion. Arch. Physiol. Biochem. 2007, 113, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, D.; Arosio, P. Biology of ferritin in mammals: An update on iron storage, oxidative damage and neurodegeneration. Arch. Toxicol. 2014, 88, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol. Ther. 2012, 133, 177–188. [Google Scholar] [CrossRef]
- Qian, Z.-M.; Ke, Y. Brain iron transport. Biol. Rev. 2019, 94, 1672–1684. [Google Scholar] [CrossRef]
- Garrick, M.D.; Dolan, K.G.; Horbinski, C.; Ghio, A.J.; Higgins, D.; Porubcin, M.; Moore, E.G.; Hainsworth, L.N.; Umbreit, J.N.; Conrad, M.E.; et al. DMT1: A mammalian transporter for multiple metals. Biometals 2003, 16, 41–54. [Google Scholar] [CrossRef]
- Alarcón-Veleiro, C.; Mato-Basalo, R.; Lucio-Gallego, S.; Vidal-Pampín, A.; Quindós-Varela, M.; Al-Qatarneh, T.; Berrecoso, G.; Vizoso-Vázquez, Á.; Arufe, M.C.; Fafián-Labora, J. Study of Ferroptosis Transmission by Small Extracellular Vesicles in Epithelial Ovarian Cancer Cells. Antioxidants 2023, 12, 183. [Google Scholar] [CrossRef]
- Ali, A.; Shafarin, J.; Abu Jabal, R.; Aljabi, N.; Hamad, M.; Sualeh Muhammad, J.; Unnikannan, H.; Hamad, M. Ferritin heavy chain (FTH1) exerts significant antigrowth effects in breast cancer cells by inhibiting the expression of c-MYC. FEBS Open Bio 2021, 11, 3101–3114. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Liu, B.; Zhang, S.; Wu, L.; Zhu, X.; Chen, Q. Expression of Ferritin Light Chain (FTL) Is Elevated in Glioblastoma, and FTL Silencing Inhibits Glioblastoma Cell Proliferation via the GADD45/JNK Pathway. PLoS ONE 2016, 11, e0149361. [Google Scholar] [CrossRef]
- Cornett, K.; Puderbaugh, A.; Back, O.; Craven, R. GAPDH in neuroblastoma: Functions in metabolism and survival. Front. Oncol. 2022, 12, 979683. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Yoshihara, K.; Umemoto, M.; Igarashi, K.; Hirose, S.; Ohgi, K.; Irie, M. Studies on Salivary Gland Ribonucleases. III. Purification and Properties of Three Ribonucleases from Bovine Parotid Gland. J. Biochem. 1983, 93, 865–874. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Gene Bank Number | Primer Sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) | Primer Efficiency |
|---|---|---|---|---|---|
| GAPDH | NM_002046 | Forward TCTCTGCTCCTCCTGTTC Reverse GCCCAATACGACCAAATCC | 120 | 60 | 1.08 |
| FTH1 | NM_002032 | Forward ACCTGTCCATGTCTTACTACTTTG Reverse GCCACCTCGTTGGTTCTG | 134 | 60 | 1.03 |
| FTL | NM_000146 | Forward GCCTCCTACACCTACCTCTC Reverse GCTGGCTTCTTGATGTCCTGG | 179 | 60 | 1.16 |
| MYC | NM_002467.4 | Forward GCGACTCTGAGGAGGAACA Reverse TGCGTAGTTGTGCTGATGTG | 182 | 59 | 0.96 |
| RPL15 | NM_002948 | Forward GGAGAAAGAAGCAGTCTGATGTC Reverse CCACGGCGAACACGAATC | 175 | 60 | 1.06 |
| TUBB3 | NM_006086 | Forward GCAAGGTGCGTGAGGAGTAT Reverse GCGGAAGCAGATGTCGTAGA | 182 | 60 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braidotti, S.; Curci, D.; Zampieri, D.; Covino, C.; Zanon, D.; Maximova, N.; Sala, R. Iron Bioavailability in the Extracellular Environment Is More Relevant Than the Intracellular One in Viability and Gene Expression: A Lesson from Oligodendroglioma Cells. Biomedicines 2023, 11, 2940. https://doi.org/10.3390/biomedicines11112940
Braidotti S, Curci D, Zampieri D, Covino C, Zanon D, Maximova N, Sala R. Iron Bioavailability in the Extracellular Environment Is More Relevant Than the Intracellular One in Viability and Gene Expression: A Lesson from Oligodendroglioma Cells. Biomedicines. 2023; 11(11):2940. https://doi.org/10.3390/biomedicines11112940
Chicago/Turabian StyleBraidotti, Stefania, Debora Curci, Daniele Zampieri, Cesare Covino, Davide Zanon, Natalia Maximova, and Roberto Sala. 2023. "Iron Bioavailability in the Extracellular Environment Is More Relevant Than the Intracellular One in Viability and Gene Expression: A Lesson from Oligodendroglioma Cells" Biomedicines 11, no. 11: 2940. https://doi.org/10.3390/biomedicines11112940
APA StyleBraidotti, S., Curci, D., Zampieri, D., Covino, C., Zanon, D., Maximova, N., & Sala, R. (2023). Iron Bioavailability in the Extracellular Environment Is More Relevant Than the Intracellular One in Viability and Gene Expression: A Lesson from Oligodendroglioma Cells. Biomedicines, 11(11), 2940. https://doi.org/10.3390/biomedicines11112940








