The Expression of Two Distinct Sets of Glycolytic Enzymes Reveals Differential Effects of Glycolytic Reprogramming on Pancreatic Ductal Tumorigenesis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Generation and Animal Care
2.2. Southern Blot
2.3. Cell Culture and Treatment
2.4. Immunohistochemistry (IHC)
2.5. Assessment of Primary Disease Burden
2.6. Masson Trichrome Staining
2.7. Western Blot (WB)
2.8. RNA Extraction and Quantitative Real-Time RCR (q-PCR)
2.9. Measurement of Oxygen Consumption and Extracellular Acidification
2.10. Measurement of Glucose Uptake and Lactate Production
2.11. Statistical Analysis
3. Results
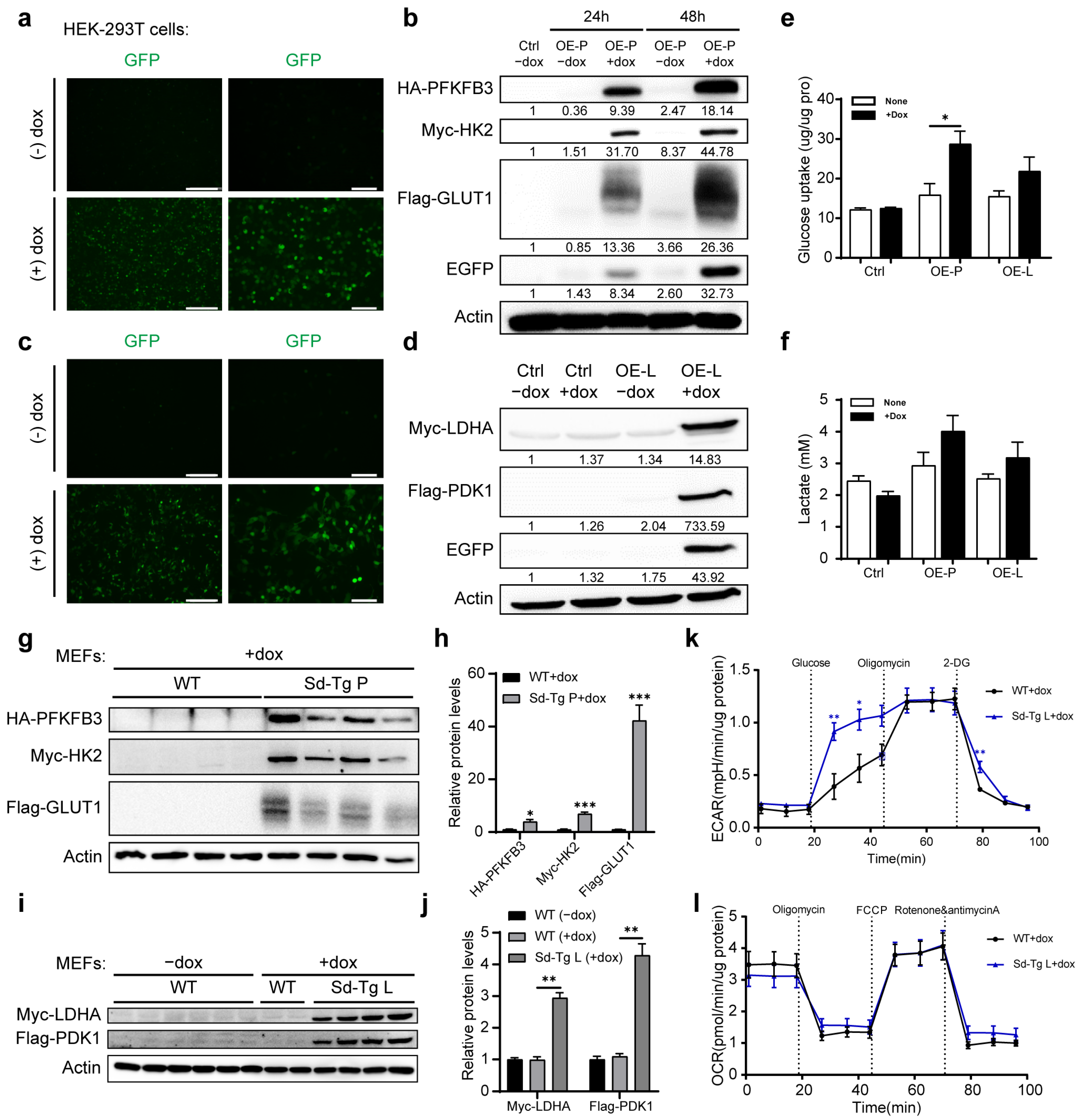
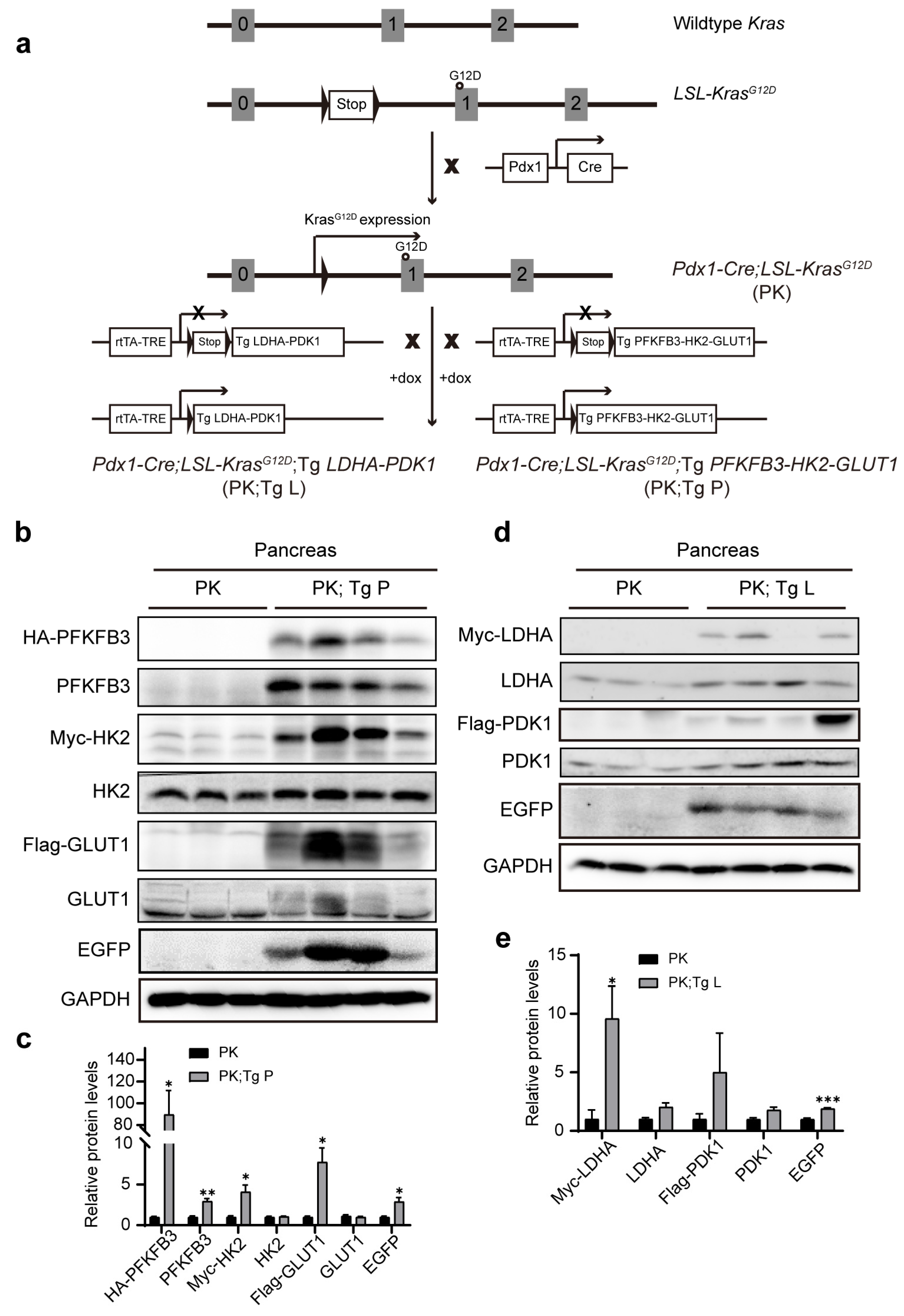
3.1. Generation of BAC Transgenic Mice with the Controlled Expression of Two Sets of Glycolytic Enzymes
3.2. The Overexpression of Glycolytic Enzymes Did Not Influence Tumor Development at PanIN2 Stage
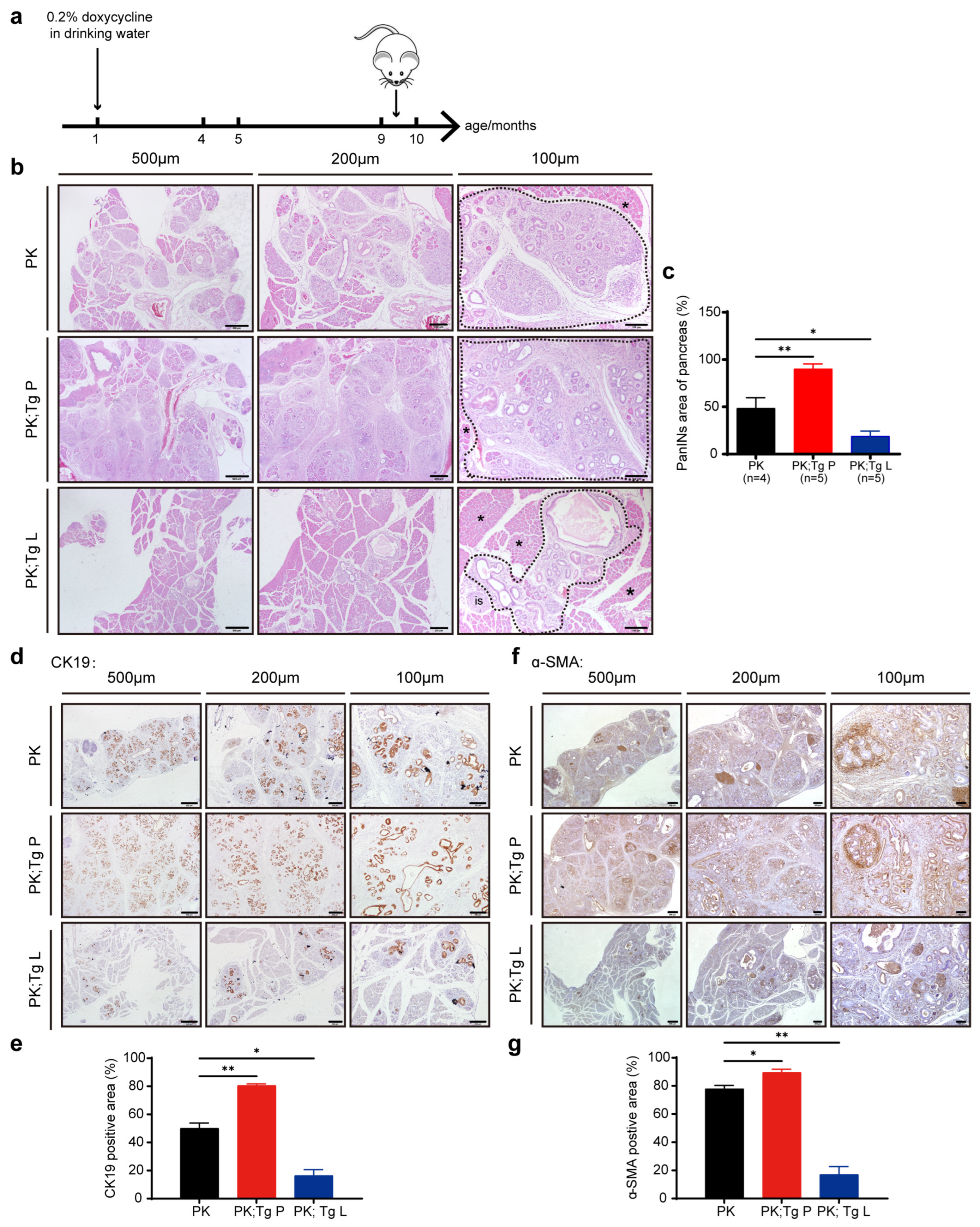
3.3. The Overexpression of the Two Sets of Glycolytic Enzymes Exhibited Completely Different Influences on the PanIN3 Stage of Tumor Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.-J.; Yang, F.; Jin, C.; Fu, D.-L. Current status and progress of pancreatic cancer in China. World J. Gastroenterol. 2015, 21, 7988–8003. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Rishi, A.; Goggins, M.; Wood, L.D.; Hruban, R.H. Pathological and Molecular Evaluation of Pancreatic Neoplasms. Semin. Oncol. 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Ponz-Sarvise, M.; Tuveson, D.A.; Yu, K.H. Mouse Models of Pancreatic Ductal Adenocarcinoma. Hematol. Clin. N. Am. 2015, 29, 609–617. [Google Scholar] [CrossRef]
- Aguirre, A.J.; Bardeesy, N.; Sinha, M.; Lopez, L.; Tuveson, D.A.; Horner, J.; Redstone, M.S.; de Pingo, R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003, 17, 3112–3126. [Google Scholar] [CrossRef]
- Shen, R.; Wang, Q.; Cheng, S.; Liu, T.; Jiang, H.; Zhu, J.; Wu, Y.; Wang, L. The biological features of PanIN initiated from oncogenic Kras mutation in genetically engineered mouse models. Cancer Lett. 2013, 339, 135–143. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef]
- Tandon, M.; Coudriet, G.M.; Criscimanna, A.; Socorro, M.; Eliliwi, M.; Singhi, A.D.; Cruz-Monserrate, Z.; Bailey, P.; Lotze, M.T.; Zeh, H.; et al. Prolactin Promotes Fibrosis and Pancreatic Cancer Progression. Cancer Res. 2019, 79, 5316–5327. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Park, S.; Phillips, P.A.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; Pirola, R.; Wilson, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef]
- Pothula, S.P.; Xu, Z.H.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. 2016, 381, 194–200. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, J.; Yang, S.; Wang, H.; Wu, C.-J.; Sugimoto, H.; LeBleu, V.S.; Kalluri, R. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 2021, 39, 548–565.e6. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Finley, L.W.; Zhang, J.; Ye, J.; Ward, P.S.; Thompson, C.B. SnapShot: Cancer Metabolism Pathways. Cell Metab. 2013, 17, 466–466.e2. [Google Scholar] [CrossRef]
- Rong, Y.; Wu, W.; Ni, X.; Kuang, T.; Jin, D.; Wang, D.; Lou, W. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumor Biol. 2013, 34, 1523–1530. [Google Scholar] [CrossRef]
- Zhou, W.; Capello, M.; Fredolini, C.; Racanicchi, L.; Piemonti, L.; Liotta, L.A.; Novelli, F.; Petricoin, E.F. Proteomic Analysis Reveals Warburg Effect and Anomalous Metabolism of Glutamine in Pancreatic Cancer Cells. J. Proteome Res. 2012, 11, 554–563. [Google Scholar] [CrossRef]
- Ambrosetti, D.; Dufies, M.; Dadone, B.; Durand, M.; Borchiellini, D.; Amiel, J.; Pouyssegur, J.; Rioux-Leclercq, N.; Pages, G.; Burel-Vandenbos, F.; et al. The two glycolytic markers GLUT1 and MCT1 correlate with tumor grade and survival in clear-cell renal cell carcinoma. PLoS ONE 2018, 13, e0193477. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, Y.; Wang, J.; Deng, Y.; Lin, D. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016, 16, 9. [Google Scholar] [CrossRef]
- Hu, K.Y.; Wang, D.G.; Liu, P.F.; Cao, Y.W.; Wang, Y.H.; Yang, X.C.; Hu, C.X.; Sun, L.J.; Niu, H.T. Targeting of MCT1 and PFKFB3 influences cell proliferation and apoptosis in bladder cancer by altering the tumor microenvironment. Oncol. Rep. 2016, 36, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Leca, J.; Olivares, O.; Lavaut, M.-N.; Vidal, N.; Berthezène, P.; Dusetti, N.J.; Loncle, C.; Calvo, E.; Turrini, O.; et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 3919–3924. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Zhang, Y.; Chen, Q.; Sun, A.; Peng, Y.; Zhang, G.; Zhou, D.; Xie, Y.; Hou, X.; Zheng, F.; et al. Increased glycolysis in skeletal muscle coordinates with adipose tissue in systemic metabolic homeostasis. J. Cell. Mol. Med. 2021, 25, 7840–7854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Chen, Q.; Li, M.; Feng, J.; Sun, Y.; Zhao, R.; Zhu, Y.; Lv, Y.; Zhu, Z.; et al. PGC1beta regulates multiple myeloma tumor growth through LDHA-mediated glycolytic metabolism. Mol. Oncol. 2018, 12, 1579–1595. [Google Scholar] [CrossRef]
- Newington, J.T.; Rappon, T.; Albers, S.; Wong, D.Y.; Rylett, R.; Cumming, R.C. Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid beta and other toxins by decreasing mitochondrial respiration and reactive oxygen species production. J. Biol. Chem. 2012, 287, 37245–37258. [Google Scholar] [CrossRef]
- Tan, Z.; Xie, N.; Cui, H.; Moellering, D.R.; Abraham, E.; Thannickal, V.J.; Liu, G. Pyruvate Dehydrogenase Kinase 1 Participates in Macrophage Polarization via Regulating Glucose Metabolism. J. Immunol. 2015, 194, 6082–6089. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, O.; Wall, J.B.J.; Zheng, M.; Zhou, Y.; Wang, L.; Vaseghi, H.R.; Qian, L.; Liu, J. Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci. Rep. 2017, 7, 2193. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Southern Blotting: Capillary Transfer of DNA to Membranes. Cold Spring Harb. Protoc. 2006, 2006, 40. [Google Scholar] [CrossRef]
- Zhang, G.; Xie, Y.; Zhou, Y.; Xiang, C.; Chen, L.; Zhang, C.; Hou, X.; Chen, J.; Zong, H.; Liu, G. p53 pathway is involved in cell competition during mouse embryogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, 498–503. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Sun, A.; Peng, Y.; Hou, W.; Xiang, C.; Zhang, G.; Lai, B.; Hou, X.; Zheng, F.; et al. The coupling of mitoproteolysis and oxidative phosphorylation enables tracking of an active mitochondrial state through MitoTimer fluorescence. Redox Biol. 2022, 56, 102447. [Google Scholar] [CrossRef]
- Niwa, H.; Yamamura, K.; Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [PubMed]
- Gossen, M.; Freundlieb, S.; Bender, G.; Müller, G.; Hillen, W.; Bujard, H. Transcriptional Activation by Tetracyclines in Mammalian Cells. Science 1995, 268, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.K.; Rivera-Soto, R.; Gray, S.J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015, 19, 49–57. [Google Scholar]
- Gaszner, M.; Felsenfeld, G. Insulators: Exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 2006, 7, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Workman, A.L.; Vignali, K.M.; Vignali, D.A. Design and Construction of 2A Peptide-Linked Multicistronic Vectors. Cold Spring Harb. Protoc. 2012, 2012, 199–204. [Google Scholar] [CrossRef]
- Jain, R.; Fischer, S.; Serra, S.; Chetty, R. The Use of Cytokeratin 19 (CK19) Immunohistochemistry in Lesions of the Pancreas, Gastrointestinal Tract, and Liver. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 9–15. [Google Scholar] [CrossRef]
- Levine, A.J.; Puzio-Kuter, A.M. The Control of the Metabolic Switch in Cancers by Oncogenes and Tumor Suppressor Genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef]
- Ross, S.J.; Critchlow, S.E. Emerging approaches to target tumor metabolism. Curr. Opin. Pharmacol. 2014, 17, 22–29. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, H.; Wang, N.; Chen, L.; Meng, Z.; Chen, Z.; Feng, Y. Functional inhibition of lactate dehydrogenase suppresses pancreatic adenocarcinoma progression. Clin. Transl. Med. 2021, 11, e467. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Chakravarty, H.; Yang, Z.; Tam, K.Y. Identification of Novel Pyruvate Dehydrogenase Kinase 1 (PDK1) Inhibitors by Kinase Activity-Based High-Throughput Screening for Anticancer Therapeutics. ACS Comb. Sci. 2018, 20, 660–671. [Google Scholar] [CrossRef]
- Stacpoole, P.W. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. JNCI J. Natl. Cancer Inst. 2017, 109, 71. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.; Thompson, C.B. Cancer’s sweet tooth. Cancer Cell 2006, 9, 419–420. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Kishton, R.J.; Rathmell, J.C. Novel Therapeutic Targets of Tumor Metabolism. Cancer J. 2015, 21, 62–69. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zheng, F.; Wang, F.; Liu, X.; Xiang, C.; Fu, S.; Shen, K.; Liu, G. The Expression of Two Distinct Sets of Glycolytic Enzymes Reveals Differential Effects of Glycolytic Reprogramming on Pancreatic Ductal Tumorigenesis in Mice. Biomedicines 2023, 11, 2962. https://doi.org/10.3390/biomedicines11112962
Zhang Y, Zheng F, Wang F, Liu X, Xiang C, Fu S, Shen K, Liu G. The Expression of Two Distinct Sets of Glycolytic Enzymes Reveals Differential Effects of Glycolytic Reprogramming on Pancreatic Ductal Tumorigenesis in Mice. Biomedicines. 2023; 11(11):2962. https://doi.org/10.3390/biomedicines11112962
Chicago/Turabian StyleZhang, Yannan, Fangfang Zheng, Fan Wang, Xingqian Liu, Cong Xiang, Shiyu Fu, Kun Shen, and Geng Liu. 2023. "The Expression of Two Distinct Sets of Glycolytic Enzymes Reveals Differential Effects of Glycolytic Reprogramming on Pancreatic Ductal Tumorigenesis in Mice" Biomedicines 11, no. 11: 2962. https://doi.org/10.3390/biomedicines11112962
APA StyleZhang, Y., Zheng, F., Wang, F., Liu, X., Xiang, C., Fu, S., Shen, K., & Liu, G. (2023). The Expression of Two Distinct Sets of Glycolytic Enzymes Reveals Differential Effects of Glycolytic Reprogramming on Pancreatic Ductal Tumorigenesis in Mice. Biomedicines, 11(11), 2962. https://doi.org/10.3390/biomedicines11112962





