Comparison of the Surface Roughness of CAD/CAM Metal-Free Materials Used for Complete-Arch Implant-Supported Prostheses: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
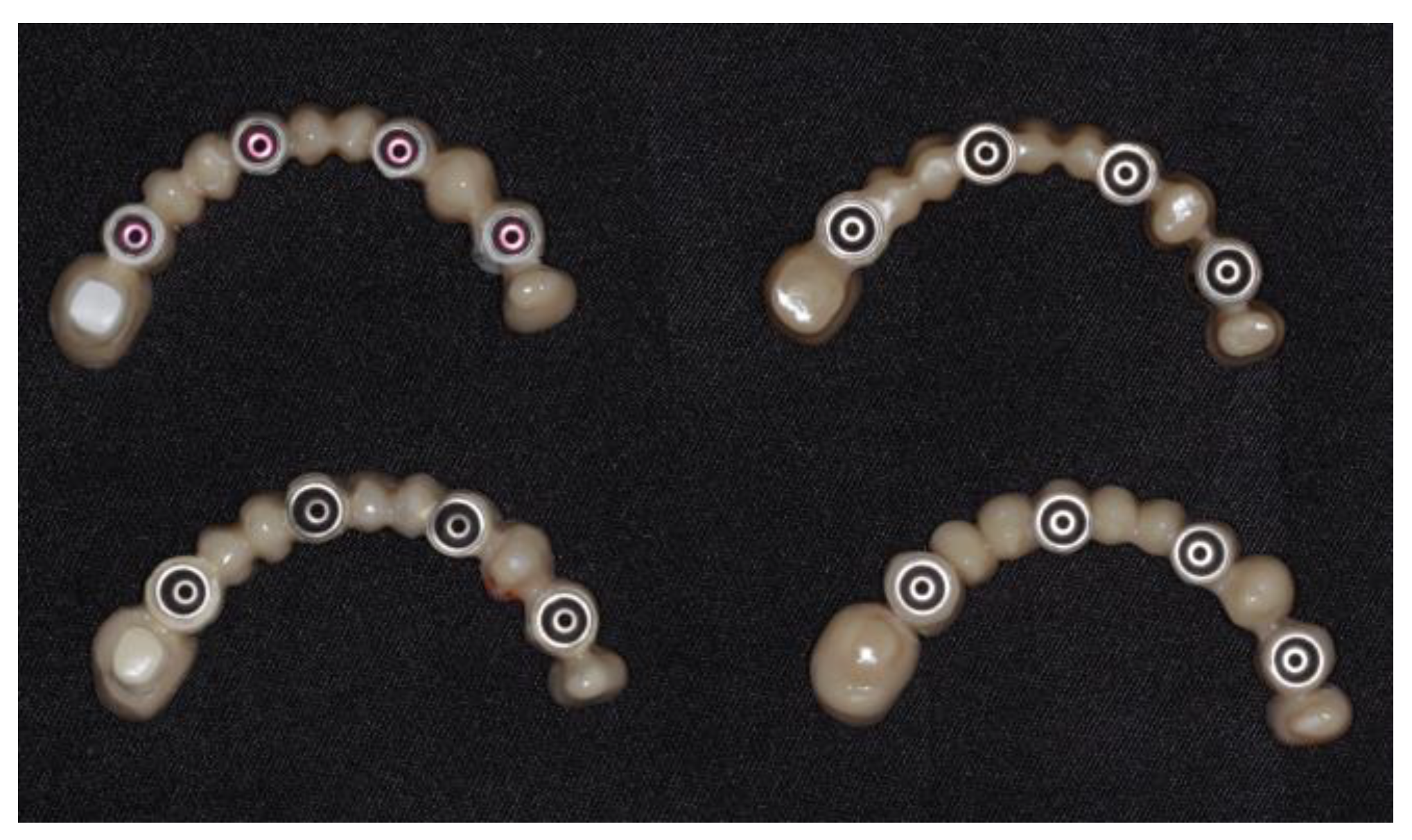
2.2. Specimen Preparation: Materials, Design, and Laboratory Manufacturing
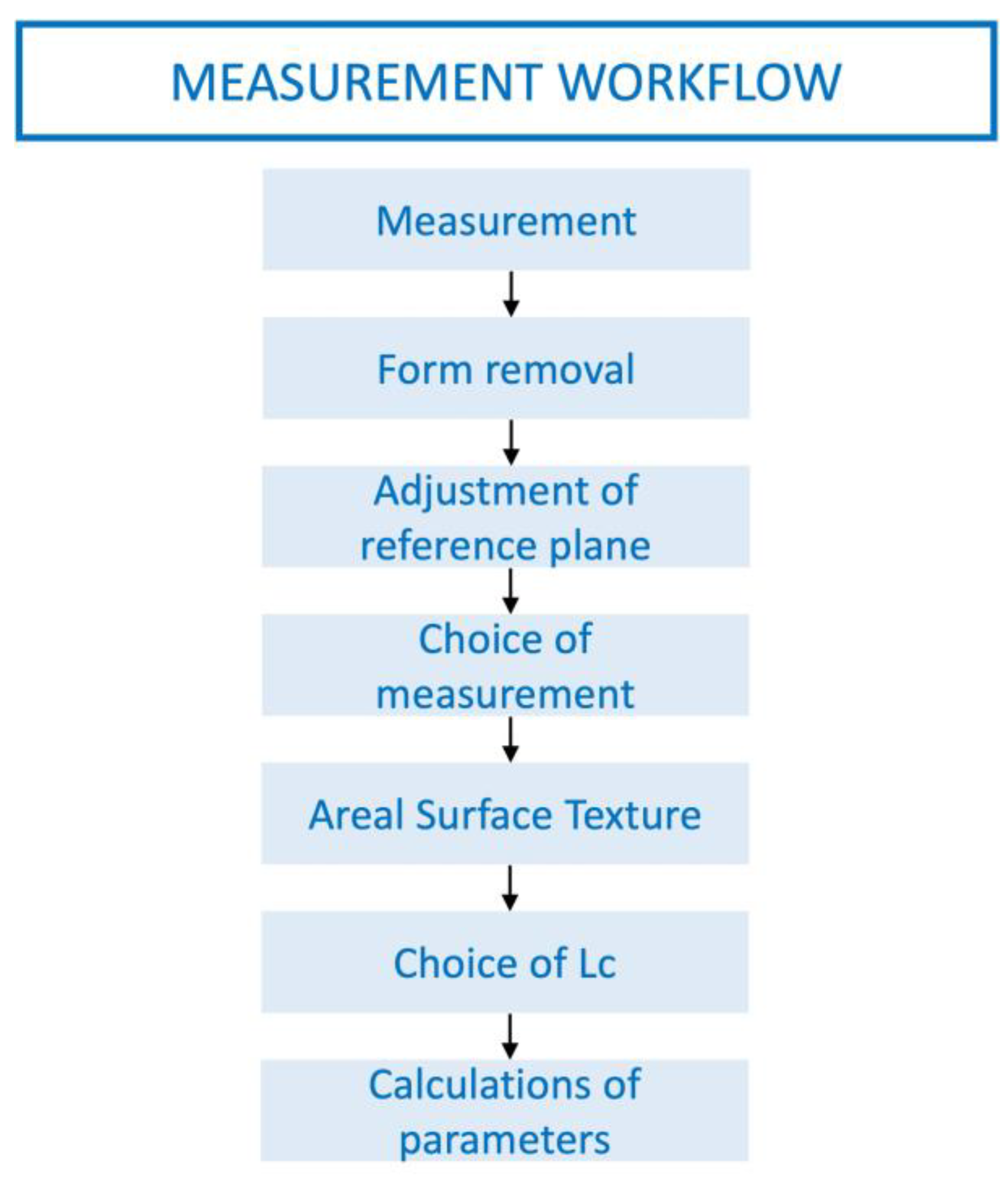
2.3. Surface Texture Analysis
2.4. Hydrothermal Aging Process of Specimens
2.5. Microscopic Observation
2.6. Statistical Analysis
3. Results
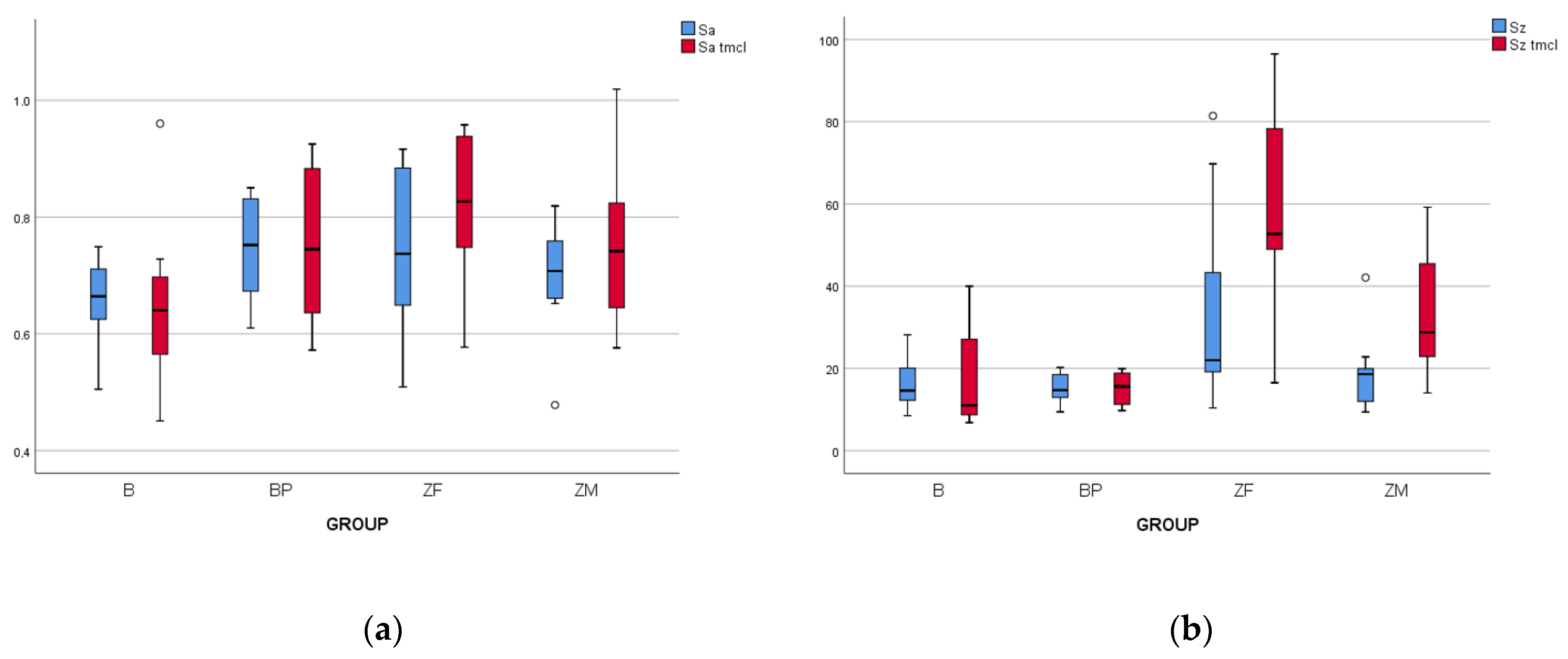
3.1. Surface Roughness Measurements
3.1.1. Baseline Data
3.1.2. Data after Aging Process
3.1.3. Effect of Thermocycling
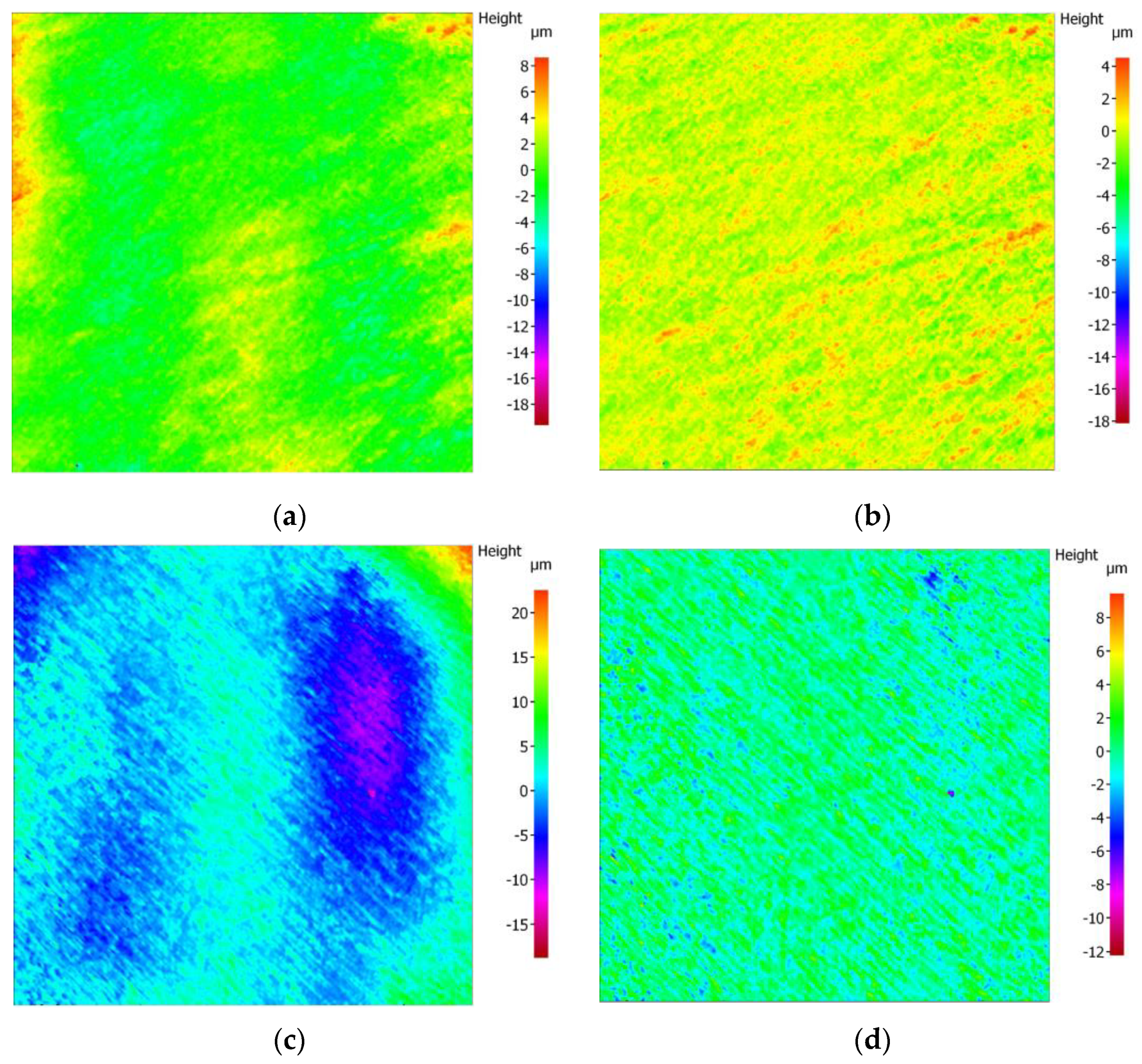
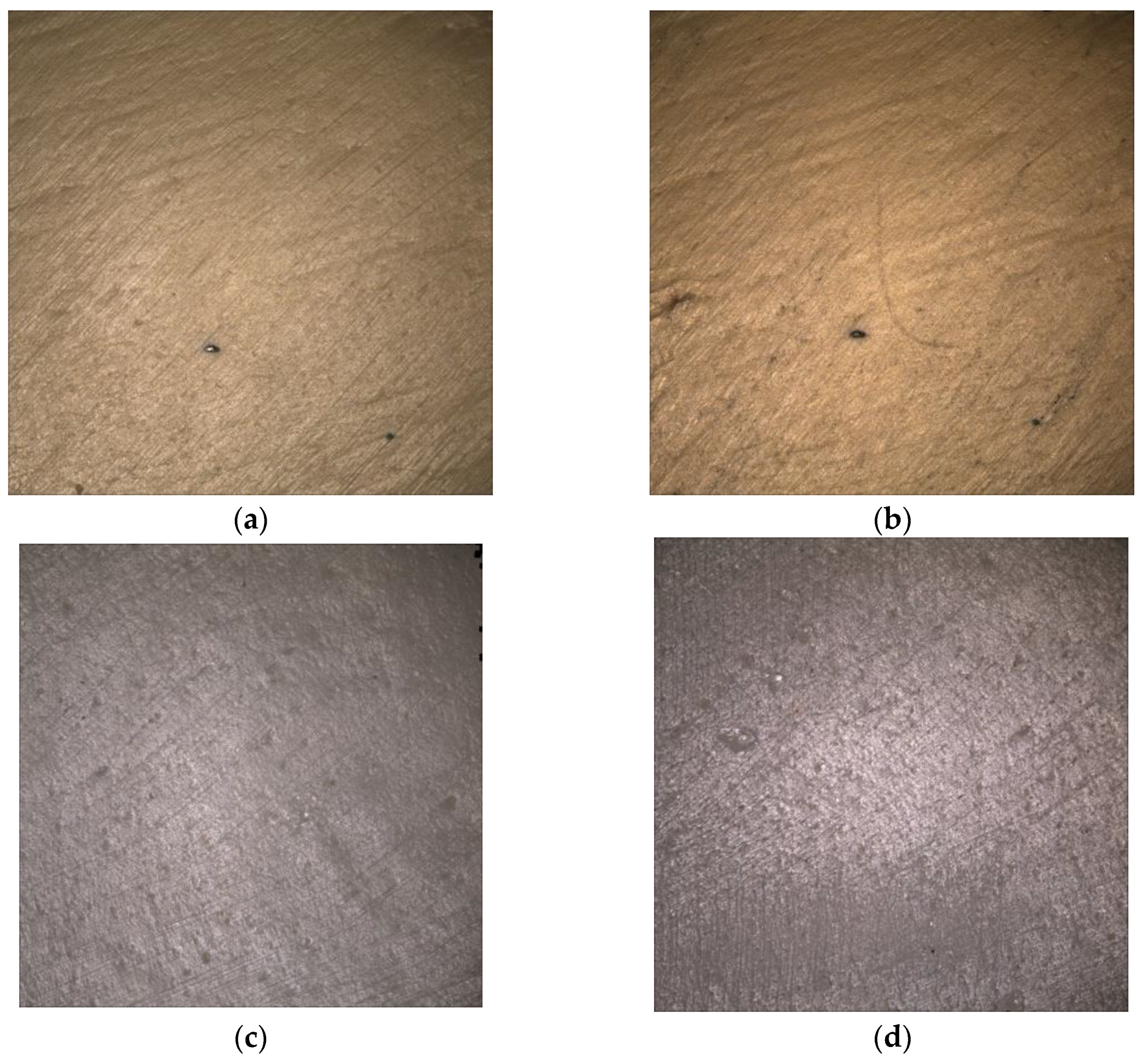
3.2. Surface Microscopic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valente, M.; Da Silva, G.; Bachmann, L.; Agnelli, J.; Dos Reis, A. An in Vitro Analysis of the Physical and Mechanical Behavior of a Polyetheretherketone (PEEK) Component for an Implant-Supported and Retained Removable Dental Prosthesis (I-RDP). Int. J. Prosthodont. 2021, 36, 612–619. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of Surface Roughness of Oral Hard Materials to the Threshold Surface Roughness for Bacterial Plaque Retention: A Review of the Literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Batak, B.; Çakmak, G.; Johnston, W.M.; Yilmaz, B. Surface Roughness of High-Performance Polymers Used for Fixed Implant-Supported Prostheses. J. Prosthet. Dent. 2021, 126, 254.e1–254.e6. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, A.; Wimmer, T.; Schmidlin, P.R.; Scherer, H.; Löffler, P.; Roos, M.; Stawarczyk, B. Physicomechanical Characterization of Polyetheretherketone and Current Esthetic Dental CAD/CAM Polymers after Aging in Different Storage Media. J. Prosthet. Dent. 2016, 115, 321–328.e2. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Jebaseelan, D. 3-D Surface Morphological Characterization of CAD/CAM Milled Dental Zirconia: An In Vitro Study of the Effect of Post-Fabrication Processes. Materials 2022, 15, 4685. [Google Scholar] [CrossRef]
- Pradíes, G.; Godoy-Ruiz, L.; Özcan, M.; Moreno-Hay, I.; Martínez-Rus, F. Analysis of Surface Roughness, Fracture Toughness, and Weibull Characteristics of Different Framework-Veneer Dental Ceramic Assemblies after Grinding, Polishing, and Glazing: Influence of Grinding, Polishing, and Glazing on Ceramics Systems. J. Prosthodont. 2019, 28, e216–e221. [Google Scholar] [CrossRef]
- Hahnel, S.; Wieser, A.; Lang, R.; Rosentritt, M. Biofilm Formation on the Surface of Modern Implant Abutment Materials. Clin. Oral. Implant. Res. 2015, 26, 1297–1301. [Google Scholar] [CrossRef]
- Vulović, S.; Popovac, A.; Radunović, M.; Petrović, S.; Todorović, M.; Milić-Lemić, A. Microbial Adhesion and Viability on Novel CAD/CAM Framework Materials for Implant-Supported Hybrid Prostheses. Eur. J. Oral. Sci. 2022, 131, e12911. [Google Scholar] [CrossRef]
- Rashid, H. The Effect of Surface Roughness on Ceramics Used in Dentistry: A Review of Literature. Eur. J. Dent. 2014, 8, 571–579. [Google Scholar] [CrossRef]
- Hafezeqoran, A.; Sabanik, P.; Koodaryan, R.; Ghalili, K.M. Effect of Sintering Speed, Aging Processes, and Different Surface Treatments on the Optical and Surface Properties of Monolithic Zirconia Restorations. J. Prosthet. Dent. 2022, S0022-3913(21)00690-9. [Google Scholar] [CrossRef] [PubMed]
- Schabbach, L.M.; Dos Santos, B.C.; De Bortoli, L.S.; Fabris, D.; Fredel, M.C.; Henriques, B. Translucent Multi-Layered Zirconia: Sandblasting Effect on Optical and Mechanical Properties. Dent. Mater. 2023, 39, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Erjavec, A.K.; Črešnar, K.P.; Švab, I.; Vuherer, T.; Žigon, M.; Brunčko, M. Determination of Shear Bond Strength between PEEK Composites and Veneering Composites for the Production of Dental Restorations. Materials 2023, 16, 3286. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A. Materials in Digital Dentistry—A Review. J. Esthet. Restor. Dent. 2020, 32, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabab’ah, M.; Hamadneh, W.; Alsalem, I.; Khraisat, A.; Abu Karaky, A. Use of High Performance Polymers as Dental Implant Abutments and Frameworks: A Case Series Report. J. Prosthodont. 2019, 28, 365–372. [Google Scholar] [CrossRef]
- Wiesli, M.G.; Özcan, M. High-Performance Polymers and Their Potential Application as Medical and Oral Implant Materials: A Review. Implant. Dent. 2015, 24, 448–457. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of Polyetheretherketone (PEEK) in Oral Implantology and Prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Jin, H.-Y.; Teng, M.-H.; Wang, Z.-J.; Li, X.; Liang, J.-Y.; Wang, W.-X.; Jiang, S.; Zhao, B.-D. Comparative Evaluation of BioHPP and Titanium as a Framework Veneered with Composite Resin for Implant-Supported Fixed Dental Prostheses. J. Prosthet. Dent. 2019, 122, 383–388. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef]
- Zoidis, P.; Papathanasiou, I. Modified PEEK Resin-Bonded Fixed Dental Prosthesis as an Interim Restoration after Implant Placement. J. Prosthet. Dent. 2016, 116, 637–641. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Eichberger, M.; Uhrenbacher, J.; Wimmer, T.; Edelhoff, D.; Schmidlin, P.R. Three-Unit Reinforced Polyetheretherketone Composite FDPs: Influence of Fabrication Method on Load-Bearing Capacity and Failure Types. Dent. Mater. J. 2015, 34, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef] [PubMed]
- Paratelli, A.; Perrone, G.; Ortega, R.; Gómez-Polo, M. Polyetheretherketone in Implant Prosthodontics: A Scoping Review. Int. J. Prosthodont. 2020, 33, 671–679. [Google Scholar] [CrossRef]
- Limpuangthip, N.; Poosanthanasarn, E.; Salimee, P. Surface Roughness and Hardness of CAD/CAM Ceramic Materials after Polishing with a Multipurpose Polishing Kit: An In Vitro Study. Eur. J. Dent. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Spitznagel, F.A.; Boldt, J.; Gierthmuehlen, P.C. CAD/CAM Ceramic Restorative Materials for Natural Teeth. J. Dent. Res. 2018, 97, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Keul, C.; Eichberger, M.; Figge, D.; Edelhoff, D.; Lümkemann, N. Three Generations of Zirconia: From Veneered to Monolithic. Part I. Quintessence Int. 2017, 48, 369–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Evaluating Dental Zirconia. Dent. Mater. 2019, 35, 15–23. [Google Scholar] [CrossRef]
- Tong, H.; Tanaka, C.B.; Kaizer, M.R.; Zhang, Y. Characterization of Three Commercial Y-TZP Ceramics Produced for Their High-Translucency, High-Strength and High-Surface Area. Ceram. Int. 2016, 42, 1077–1085. [Google Scholar] [CrossRef]
- Alshiddi, I.F.; Habib, S.R.; Zafar, M.S.; Bajunaid, S.; Labban, N.; Alsarhan, M. Fracture Load of CAD/CAM Fabricated Cantilever Implant-Supported Zirconia Framework: An In Vitro Study. Molecules 2021, 26, 2259. [Google Scholar] [CrossRef]
- Herreño, F. Methodology for 3D Surface Roughness Characterization; National University of Colombia: Bogotá, Colombia, 2017. [Google Scholar]
- Abualsaud, R.; Abussaud, M.; Assudmi, Y.; Aljoaib, G.; Khaled, A.; Alalawi, H.; Akhtar, S.; Matin, A.; Gad, M.M. Physiomechanical and Surface Characteristics of 3D-Printed Zirconia: An In Vitro Study. Materials 2022, 15, 6988. [Google Scholar] [CrossRef]
- ISO 25178:2012(E); Surface Metrology—Areal—Part 1: Specification and Verification of Surface Texture Profiles. Interna-Tional Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- ISO 25178:2012(E); Surface Texture: Areal—Part 2: Terms, Definitions, and Surface Texture Parameters. International Or-Ganization for Standardization (ISO): Geneva, Switzerland, 2012.
- Alao, A.-R.; Stoll, R.; Song, X.-F.; Miyazaki, T.; Hotta, Y.; Shibata, Y.; Yin, L. Surface Quality of Yttria-Stabilized Tetragonal Zirconia Polycrystal in CAD/CAM Milling, Sintering, Polishing and Sandblasting Processes. J. Mech. Behav. Biomed. Mater. 2017, 65, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Delgado, L.; Molmeneu, M.; García, D.; Rodríguez, D. Analysis of the Misfit of Dental Implant-Supported Prostheses Made with Three Manufacturing Processes. J. Prosthet. Dent. 2014, 111, 116–123. [Google Scholar] [CrossRef]
- Matzinger, M.; Hahnel, S.; Preis, V.; Rosentritt, M. Polishing Effects and Wear Performance of Chairside CAD/CAM Materials. Clin. Oral. Investig. 2019, 23, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ji, Y. Low-Temperature Degradation of Zirconia-Based All-Ceramic Crowns Materials: A Mini Review and Outlook. J. Mater. Sci. Technol. 2016, 32, 593–596. [Google Scholar] [CrossRef]
- Ziębowicz, A.; Oßwald, B.; Kern, F.; Schwan, W. Effect of Simulated Mastication on Structural Stability of Prosthetic Zirconia Material after Thermocycling Aging. Materials 2023, 16, 1171. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the Art of Zirconia for Dental Applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Lawson, N.C.; McLaren, E.E.; Nejat, A.H.; Burgess, J.O. Comparison of the Mechanical Properties of Translucent Zirconia and Lithium Disilicate. J. Prosthet. Dent. 2018, 120, 132–137. [Google Scholar] [CrossRef]
- Ban, S. Classification and Properties of Dental Zirconia as Implant Fixtures and Superstructures. Materials 2021, 14, 4879. [Google Scholar] [CrossRef]
- Maminskas, J.; Pilipavicius, J.; Staisiunas, E.; Baranovas, G.; Alksne, M.; Daugela, P.; Juodzbalys, G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials 2020, 13, 2070. [Google Scholar] [CrossRef]
- ISO 4287:1999; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1999.
- Kaizer, M.R.; Gierthmuehlen, P.C.; dos Santos, M.B.; Cava, S.S.; Zhang, Y. Speed Sintering Translucent Zirconia for Chairside One-Visit Dental Restorations: Optical, Mechanical, and Wear Characteristics. Ceram. Int. 2017, 43, 10999–11005. [Google Scholar] [CrossRef]
- Wertz, M.; Hoelzig, H.; Kloess, G.; Hahnel, S.; Koenig, A. Influence of Manufacturing Regimes on the Phase Transformation of Dental Zirconia. Materials 2021, 14, 4980. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.-G.; Dimitriadi, M.; Tzoutzas, I.; Koidis, P.; Zinelis, S.; Eliades, G. Effect of Water Storage on Hardness and Interfacial Strength of Resin Composite Luting Agents Bonded to Surface-Treated Monolithic Zirconia. Dent. J. 2021, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Kim, S.-H. Effect of Hydrothermal Aging on the Optical Properties of Precolored Dental Monolithic Zirconia Ceramics. J. Prosthet. Dent. 2019, 121, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Schneider-Feyrer, S.; Behr, M.; Preis, V. In Vitro Shock Absorption Tests on Implant-Supported Crowns: Influence of Crown Materials and Luting Agents. Int. J. Oral. Maxillofac. Implant. 2018, 33, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Preis, V.; Behr, M.; Handel, G.; Schneider-Feyrer, S.; Hahnel, S.; Rosentritt, M. Wear Performance of Dental Ceramics after Grinding and Polishing Treatments. J. Mech. Behav. Biomed. Mater. 2012, 10, 13–22. [Google Scholar] [CrossRef]
- Kongkiatkamon, S.; Peampring, C. Effect of Speed Sintering on Low Temperature Degradation and Biaxial Flexural Strength of 5Y-TZP Zirconia. Molecules 2022, 27, 5272. [Google Scholar] [CrossRef]



| Framework Material | Groups | Chemical Composition | Brand Name |
|---|---|---|---|
| Polyether ether ketone | B | Polyether ether ketone with 20 wt.% inorganic fillers * | Bio.hpp |
| Polyether ether ketone | BP | Polyether ether ketone with 25 wt.% inorganic fillers * | Bio.hpp Plus |
| Zirconia frame | ZF | 94–95% ZrO2, 4.5–5.5% Y2O3, <0.5% Al2O3, <0.5% other oxide | Luxor Z Frame |
| Zirconia monolithic | ZM | 90–95% ZrO2, 4–10% Y2O3, ≤0.5% Al2O3, <0.5% other oxide | Luxor Z True Nature |
| Experimental Group | Sa | Sa | ||
|---|---|---|---|---|
| Baseline | tmcl | Baseline | tmcl | |
| B | 0.66 ± 0.07 aA | 0.65 ± 0.13 aA | 16.13 ± 5.84 aA | 16.56 ± 11.48 aA |
| BP | 0.75 ± 0.09 aA | 0.75 ± 0.14 aA | 15.08 ± 3.27 aA | 15.19 ± 4.01 aA |
| ZF | 0.75 ± 0.15 aA | 0.81 ± 0.14 aA | 33.63 ± 23.91 bA | 56.55 ± 23.92 bB |
| ZM | 0.70 ± 0.09 aA | 0.75 ± 0.14 aA | 18.98 ± 9.29 abA | 33.85 ± 14.90 aB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mory, N.; Cascos, R.; Celemín-Viñuela, A.; Gómez-Polo, C.; Agustín-Panadero, R.; Gómez-Polo, M. Comparison of the Surface Roughness of CAD/CAM Metal-Free Materials Used for Complete-Arch Implant-Supported Prostheses: An In Vitro Study. Biomedicines 2023, 11, 3036. https://doi.org/10.3390/biomedicines11113036
Mory N, Cascos R, Celemín-Viñuela A, Gómez-Polo C, Agustín-Panadero R, Gómez-Polo M. Comparison of the Surface Roughness of CAD/CAM Metal-Free Materials Used for Complete-Arch Implant-Supported Prostheses: An In Vitro Study. Biomedicines. 2023; 11(11):3036. https://doi.org/10.3390/biomedicines11113036
Chicago/Turabian StyleMory, Nataly, Rocío Cascos, Alicia Celemín-Viñuela, Cristina Gómez-Polo, Rubén Agustín-Panadero, and Miguel Gómez-Polo. 2023. "Comparison of the Surface Roughness of CAD/CAM Metal-Free Materials Used for Complete-Arch Implant-Supported Prostheses: An In Vitro Study" Biomedicines 11, no. 11: 3036. https://doi.org/10.3390/biomedicines11113036





