Prefrontal Dopamine in Flexible Adaptation to Environmental Changes: A Game for Two Players
Abstract
1. Introduction
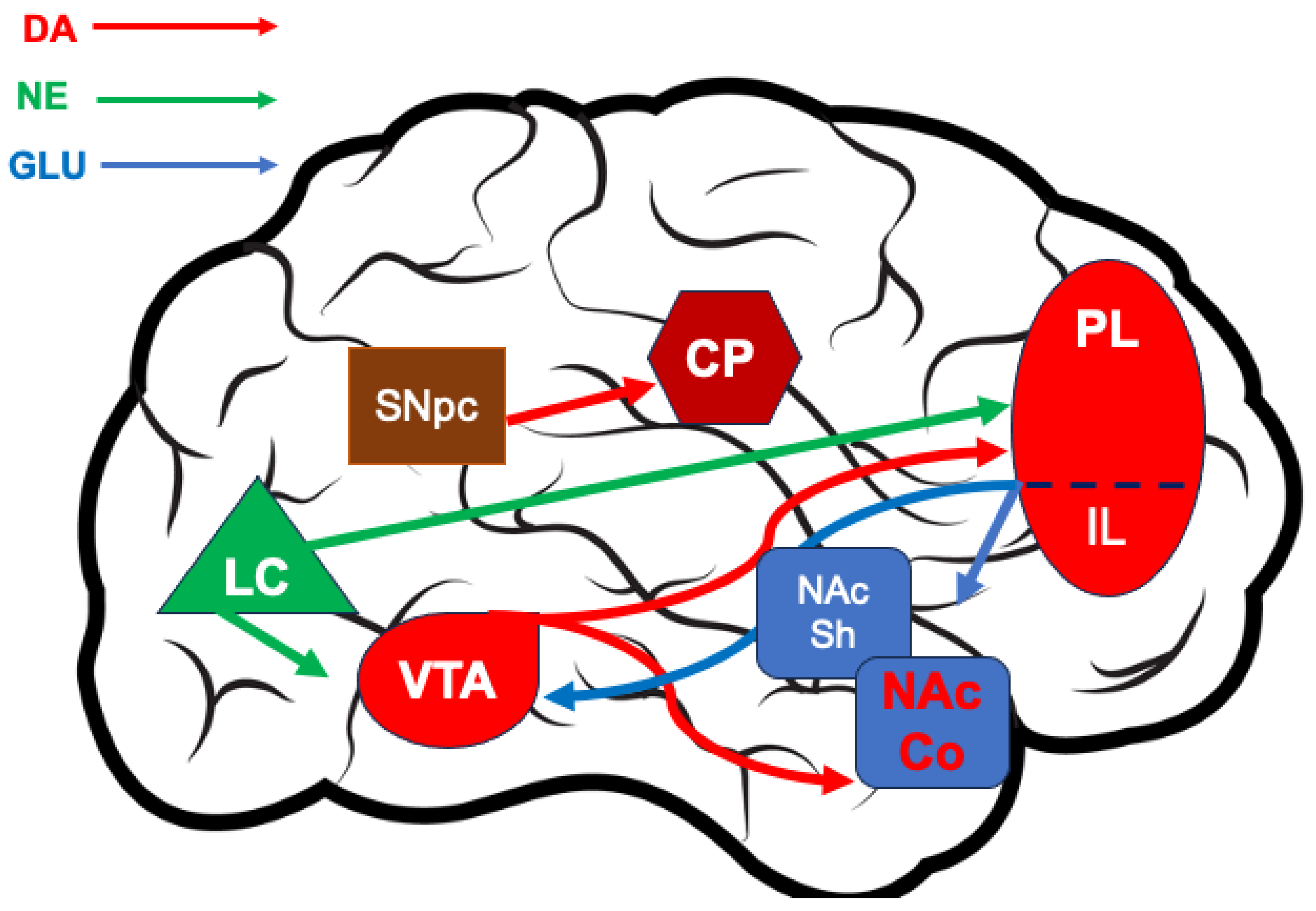
2. The Cortical–Striatal DA Transmission in Health and Disease
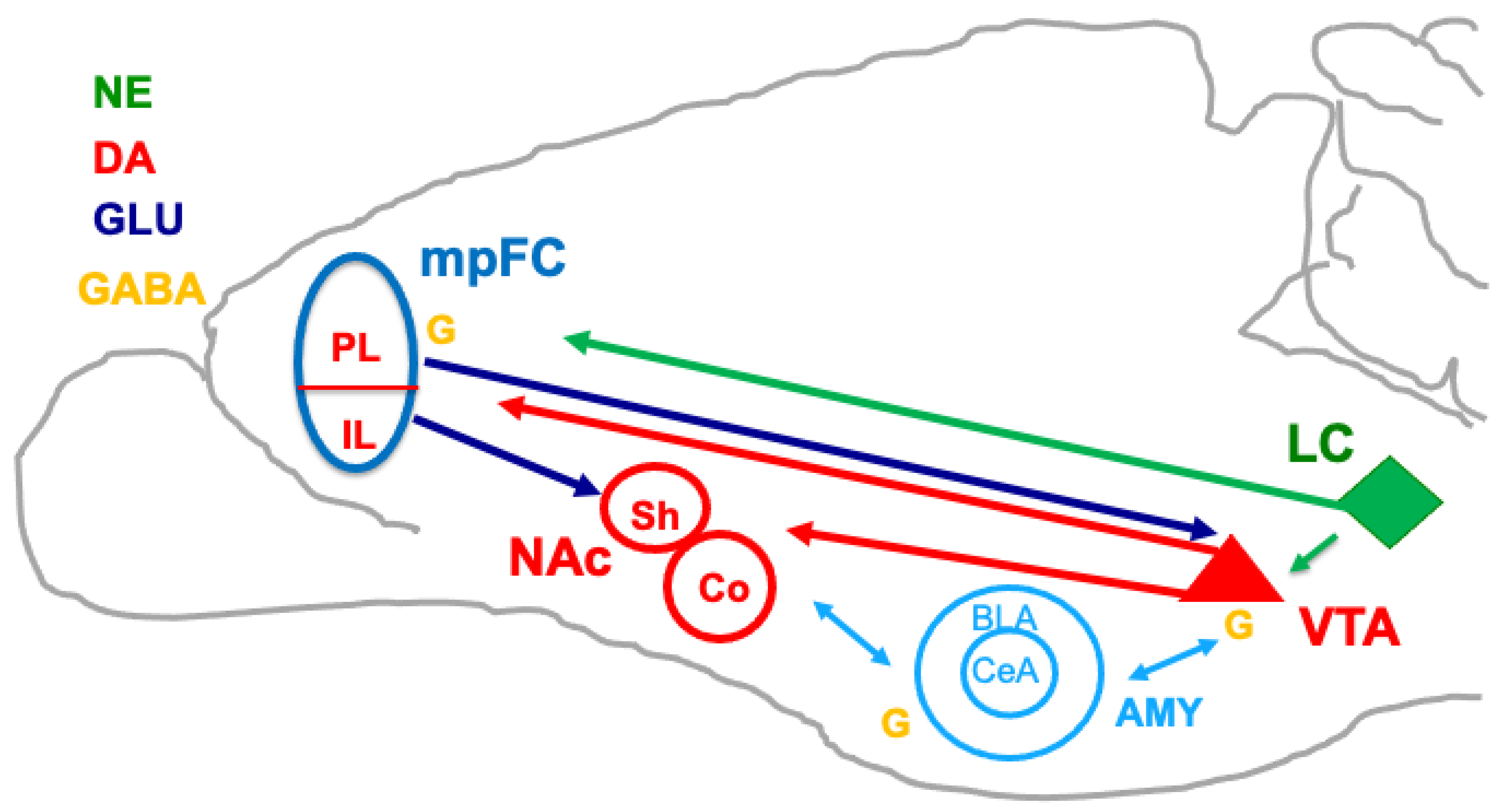
3. Cognitive/Behavioral Flexibility, Cortical–Striatal DA, and Goal Value
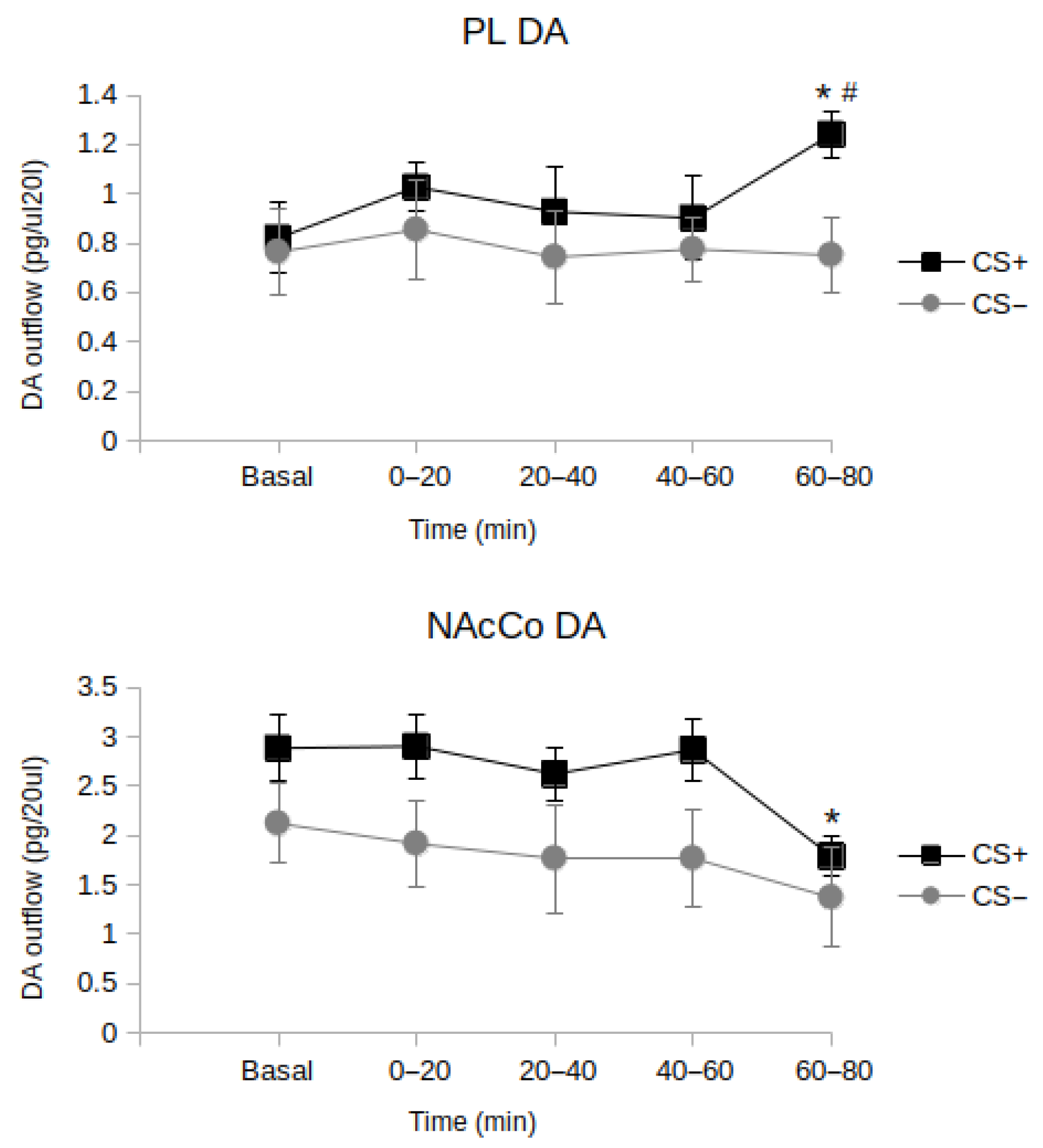
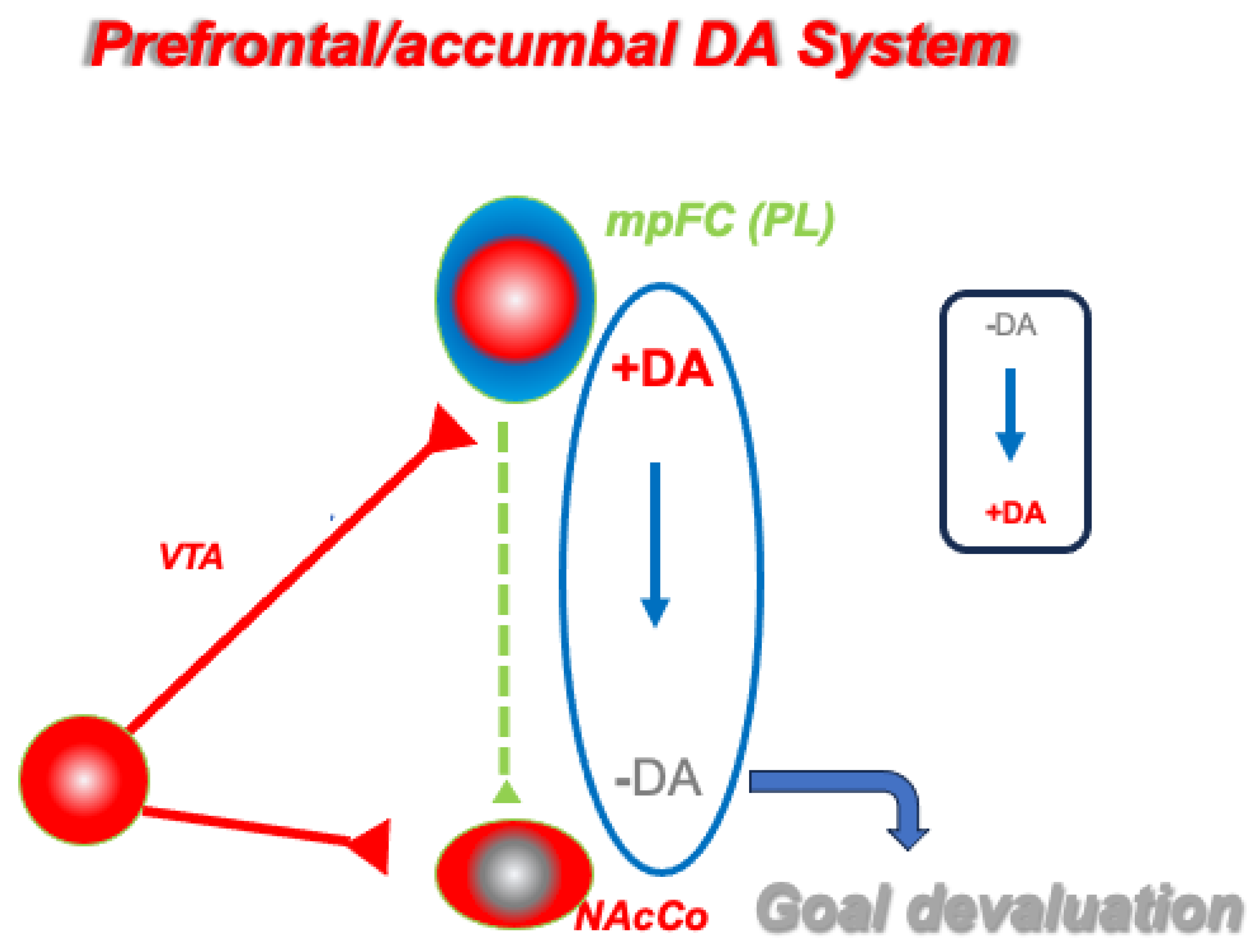
4. Updating Goal Value
5. General Discussion, Limitations, and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [CrossRef]
- Lange, F.; Seer, C.; Kopp, B. Cognitive flexibility in neurological disorders: Cognitive components and event-related potentials. Neurosci. Biobehav. Rev. 2017, 83, 496–507. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 6031478. [Google Scholar] [CrossRef]
- Jalal, B.; Chamberlain, S.R.; Sahakian, B.J. Obsessive-compulsive disorder: Etiology, neuropathology, and cognitive dysfunction. Brain Behav. 2023, 3, e3000. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A Neural Substrate of Prediction and Reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Schultz, W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000, 1, 199–207. [Google Scholar] [CrossRef]
- Sarno, S.; Beirán, M.; Falcó-Roget, J.; Diaz-Deleon, G.; Rossi-Pool, R.; Romo, R.; Parga, N. Dopamine firing plays a dual role in coding reward prediction errors and signaling motivation in a working memory task. Proc. Natl. Acad. Sci. USA 2022, 119, e2113311119. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.W.; Afjei, S.A.; Pollak Dorocic, I.; Peck, J.R.; Liu, C.; Kim, C.K.; Tian, L.; Deisseroth, K.; Lammel, S. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 2019, 101, 133–151.e7. [Google Scholar] [CrossRef] [PubMed]
- Papalini, S.; Beckers, T.; Vervliet, B. Dopamine: From prediction error to psychotherapy. Transl. Psychiatry 2020, 10, 164. [Google Scholar] [CrossRef]
- Esser, R.; Korn, C.W.; Ganzer, F.; Haaker, J. L-DOPA modulates activity in the vmPFC, nucleus accumbens, and VTA during threat extinction learning in humans. Elife 2021, 10, e65280. [Google Scholar] [CrossRef] [PubMed]
- Raczka, K.A.; Mechias, M.-L.; Gartmann, N.; Reif, A.; Deckert, J.; Pessiglione, M.; Kalisch, R. Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Transl. Psychiatry 2011, 1, e12. [Google Scholar] [CrossRef]
- Suzuki, M.; Pennartz, C.M.A.; Aru, J. How deep is the brain? The shallow brain hypothesis. Nat. Rev. Neurosci. 2023, 24, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.S.; Kundu, P.; Dowell, N.; Mechelmans, D.J.; Favre, P.; Irvine, M.A.; Robbins, T.W.; Daw, N.; Bullmore, E.T.; Harrison, N.A.; et al. Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex 2016, 74, 118–133. [Google Scholar] [CrossRef]
- Jeong, H.; Taylor, A.; Floeder, J.R.; Lohmann, M.; Mihalas, S.; Wu, B.; Zhou, M.; Burke, D.A.; Namboodiri, V.M.K. Mesolimbic dopamine release conveys causal associations. Science 2022, 378, eabq6740. [Google Scholar] [CrossRef] [PubMed]
- Day, J.J.; Roitman, M.F.; Wightman, R.M.; Carelli, R.M. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat. Neurosci. 2007, 10, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Webler, R.D.; Oathes, D.J.; van Rooij, S.J.; Gewirtz, J.C.; Nahas, Z.; Lissek, S.M.; Widge, A.S. Causally mapping human threat extinction relevant circuits with depolarizing brain stimulation methods. Neurosci. Biobehav. Rev. 2023, 144, 105005. [Google Scholar] [CrossRef] [PubMed]
- Massi, L.; Hagihara, K.M.; Courtin, J.; Hinz, J.; Müller, C.; Fustiñana, M.S.; Xu, C.; Karalis, N.; Lüthi, A. Disynaptic specificity of serial information flow for conditioned fear. Sci. Adv. 2023, 9, eabq1637. [Google Scholar] [CrossRef]
- Anderson, M.C.; Floresco, S.B. Prefrontal-hippocampal interactions supporting the extinction of emotional memories: The retrieval stopping model. Neuropsychopharmacology 2022, 47, 180–195. [Google Scholar] [CrossRef]
- Rusu, S.I.; Pennartz, C.M.A. Learning, memory and consolidation mechanisms for behavioral control in hierarchically organized cortico-basal ganglia systems. Hippocampus 2020, 30, 73–98. [Google Scholar] [CrossRef]
- Hamel, L.; Cavdaroglu, B.; Yeates, D.; Nguyen, D.; Riaz, S.; Patterson, D.; Khan, N.; Kirolos, N.; Roper, K.; Ha, Q.A.; et al. Cortico-Striatal Control over Adaptive Goal-Directed Responding Elicited by Cues Signaling Sucrose Reward or Punishment. J. Neurosci. 2022, 42, 3811–3822. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Jenni, N.L.; Rutledge, G.; Floresco, S.B. Distinct Medial Orbitofrontal-Striatal Circuits Support Dissociable Component Processes of Risk/Reward Decision-Making. J. Neurosci. 2022, 42, 2743–2755. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, C.; Orrú, M.; Rea, W.; Ciudad-Roberts, A.; Yepes, G.; Britt, J.P.; Ferré, S. Local Control of Extracellular Dopamine Levels in the Medial Nucleus Accumbens by a Glutamatergic Projection from the Infralimbic Cortex. J. Neurosci. 2016, 36, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Mateo, Y.; Johnson, K.A.; Covey, D.P.; Atwood, B.K.; Wang, H.-L.; Zhang, S.; Gildish, I.; Cachope, R.; Bellocchio, L.; Guzmán, M.; et al. Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 2017, 96, 1112–1126.e5. [Google Scholar] [CrossRef]
- Weele, C.M.V.; Siciliano, C.A.; Matthews, G.A.; Namburi, P.; Izadmehr, E.M.; Espinel, I.C.; Nieh, E.H.; Schut, E.H.S.; Padilla-Coreano, N.; Burgos-Robles, A.; et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 2018, 563, 397–401. [Google Scholar] [CrossRef]
- Ventura, R.; Morrone, C.; Puglisi-Allegra, S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. USA 2007, 104, 5181–5186. [Google Scholar] [CrossRef]
- Cabib, S.; Puglisi-Allegra, S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012, 36, 79–89. [Google Scholar] [CrossRef]
- Jauhar, S.; McCutcheon, R.A.; Veronese, M.; Borgan, F.; Nour, M.; Rogdaki, M.; Pepper, F.; Stone, J.M.; Egerton, A.; Vamvakas, G.; et al. The relationship between striatal dopamine and anterior cingulate glutamate in first episode psychosis changes with antipsychotic treatment. Transl. Psychiatry 2023, 13, 184. [Google Scholar] [CrossRef]
- Avram, M.; Brandl, F.; Knolle, F.; Cabello, J.; Leucht, C.; Scherr, M.; Mustafa, M.; Koutsouleris, N.; Leucht, S.; Ziegler, S.; et al. Aberrant striatal dopamine links topographically with cortico-thalamic dysconnectivity in schizophrenia. Brain 2020, 143, 3495–3505. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal cortex and depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef]
- Theis, H.; Probst, C.; Fernagut, P.-O.; van Eimeren, T. Unlucky punches: The vulnerability-stress model for the development of impulse control disorders in Parkinson’s disease. Npj Park. Dis. 2021, 7, 112. [Google Scholar] [CrossRef]
- Steidel, K.; Ruppert, M.C.; Palaghia, I.; Greuel, A.; Tahmasian, M.; Maier, F.; Hammes, J.; van Eimeren, T.; Timmermann, L.; Tittgemeyer, M.; et al. Dopaminergic pathways and resting-state functional connectivity in Parkinson’s disease with freezing of gait. NeuroImage Clin. 2021, 32, 102899. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Zhang, Q.; Zhou, Y.; Wang, R.; Tian, C.; Xiang, H. Impulsivity and Response Inhibition Related Brain Networks in Adolescents With Internet Gaming Disorder: A Preliminary Study Utilizing Resting-State fMRI. Front. Psychiatry 2020, 11, 618319. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter, K.A.; Wunsch, A.M.; Nakata, K.G.; Donckels, E.; Neumaier, J.F.; Ferguson, S.M. Corticostriatal Afferents Modulate Responsiveness to Psychostimulant Drugs and Drug-Associated Stimuli. Neuropsychopharmacology 2015, 41, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Bloomfield, M.A.P.; Dahoun, T.; Mehta, M.; Howes, O.D. Chronic psychosocial stressors are associated with alterations in salience processing and corticostriatal connectivity. Schizophr Res. 2019, 213, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.D.; Zald, D.H.; Felger, J.C.; Christman, S.; Claassen, D.O.; Horga, G.; Miller, J.M.; Gifford, K.; Rogers, B.; Szymkowicz, S.M.; et al. Influences of dopaminergic system dysfunction on late-life depression. Mol. Psychiatry 2021, 27, 180–191. [Google Scholar] [CrossRef]
- Hammes, J.; Theis, H.; Giehl, K.; Hoenig, M.C.; Greuel, A.; Tittgemeyer, M.; Timmermann, L.; Fink, G.R.; Drzezga, A.; Eggers, C.; et al. Dopamine metabolism of the nucleus accumbens and fronto-striatal connectivity modulate impulse control. Brain 2019, 142, 733–743. [Google Scholar] [CrossRef]
- Decourt, M.; Jiménez-Urbieta, H.; Benoit-Marand, M.; Fernagut, P.-O. Neuropsychiatric and Cognitive Deficits in Parkinson’s Disease and Their Modeling in Rodents. Biomedicines 2021, 9, 684. [Google Scholar] [CrossRef]
- Loftus, A.M.; Gasson, N.; Lopez, N.; Sellner, M.; Reid, C.; Cocks, N.; Lawrence, B.J. Cognitive Reserve, Executive Function, and Memory in Parkinson’s Disease. Brain Sci. 2021, 11, 992. [Google Scholar] [CrossRef]
- Laubach, M.; Amarante, L.M.; Swanson, K.; White, S.R. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 2018. [CrossRef]
- Wei, L.; Hu, X.; Yuan, Y.; Liu, W.; Chen, H. Abnormal ventral tegmental area-anterior cingulate cortex connectivity in Parkinson’s disease with depression. Behav. Brain Res. 2018, 347, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.J.; Miyasaki, J.M.; Zurowski, M.; Ko, J.H.; Cho, S.S.; Pellecchia, G.; Antonelli, F.; Houle, S.; Lang, A.E.; Strafella, A.P. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: A [11C] FLB-457 and PET study. Neurobiol. Dis. 2012, 48, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Cui, Q.; He, Z.; Sheng, W.; Pang, Y.; Chen, Y.; Tang, Q.; Yang, Y.; Luo, W.; Yu, Y.; et al. Prefrontal-limbic-striatum dysconnectivity associated with negative emotional endophenotypes in bipolar disorder during depressive episodes. J. Affect. Disord. 2021, 295, 422–430. [Google Scholar] [CrossRef]
- Watanabe, M.; Narita, M. Brain Reward Circuit and Pain. Adv. Exp. Med. Biol. 2018, 1099, 201–210. [Google Scholar] [PubMed]
- Ziółkowska, B. The Role of Mesostriatal Dopamine System and Corticostriatal Glutamatergic Transmission in Chronic Pain. Brain Sci. 2021, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Pereira, I.; Llorca-Torralba, M.; Bravo, L.; Camarena-Delgado, C.; Soriano-Mas, C.; Berrocoso, E. The Role of the Locus Coeruleus in Pain and Associated Stress-Related Disorders. Biol. Psychiatry 2022, 91, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.K.; Westlund, K.N. The noradrenergic locus coeruleus as a chronic pain generator. J. Neurosci. Res. 2017, 95, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Robbins, T.W.; Rowe, J.B. The role of noradrenaline in cognition and cognitive disorders. Brain 2021, 144, 2243–2256. [Google Scholar] [CrossRef]
- Beardmore, R.; Hou, R.; Darekar, A.; Holmes, C.; Boche, D. The Locus Coeruleus in Aging and Alzheimer’s Disease: A Postmortem and Brain Imaging Review. J. Alzheimer’s Dis. 2021, 83, 5–22. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Martini, N.; Lombardo, F.; Galgani, A.; Bastiani, L.; Della Latta, D.; Hlavata, H.; Busceti, C.L.; Biagioni, F.; Puglisi-Allegra, S.; et al. Locus Coeruleus magnetic resonance imaging: A comparison between native-space and template-space approach. J. Neural Transm. 2022, 129, 387–394. [Google Scholar] [CrossRef]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain 2001, 124, 2503–2512. [Google Scholar] [CrossRef]
- Levy-Gigi, E.; Haim-Nachum, S.; Hall, J.M.; Crouse, J.J.; Winwood-Smith, R.; Lewis, S.J.; Moustafa, A.A. The interactive effect of valence and context on reversal learning in individuals with Parkinson’s disease. Neurosci. Lett. 2019, 692, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Darvas, M.; Palmiter, R.D. Contributions of Striatal Dopamine Signaling to the Modulation of Cognitive Flexibility. Biol. Psychiatry 2011, 69, 704–707. [Google Scholar] [CrossRef]
- Sala-Bayo, J.; Fiddian, L.; Nilsson, S.R.O.; Hervig, M.E.; McKenzie, C.; Mareschi, A.; Boulos, M.; Zhukovsky, P.; Nicholson, J.; Dalley, J.W.; et al. Dorsal and ventral striatal dopamine D1 and D2 receptors differentially modulate distinct phases of serial visual reversal learning. Neuropsychopharmacology 2020, 45, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, I.T.; Patel, T.; Wadia, V.; Lee, A.T.; Liptak, A.T.; Bender, K.J.; Sohal, V.S. Tonic or Phasic Stimulation of Dopaminergic Projections to Prefrontal Cortex Causes Mice to Maintain or Deviate from Previously Learned Behavioral Strategies. J. Neurosci. 2017, 37, 8315–8329. [Google Scholar] [CrossRef]
- Fraser, K.M.; Pribut, H.J.; Janak, P.H.; Keiflin, R. From Prediction to Action: Dissociable Roles of Ventral Tegmental Area and Substantia Nigra Dopamine Neurons in Instrumental Reinforcement. J. Neurosci. 2023, 43, 3895–3908. [Google Scholar] [CrossRef]
- Belin, D.; Everitt, B.J. Cocaine Seeking Habits Depend upon Dopamine-Dependent Serial Connectivity Linking the Ventral with the Dorsal Striatum. Neuron 2008, 57, 432–441. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.W.; Fraser, K.M.; Lammel, S. Mesoaccumbal Dopamine Heterogeneity: What Do Dopamine Firing and Release Have to Do with It? Annu. Rev. Neurosci. 2022, 45, 109–129. [Google Scholar] [CrossRef]
- Lammel, S.; Lim, B.K.; Malenka, R.C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76, 351–359. [Google Scholar] [CrossRef]
- Haber, S.N.; Fudge, J.L.; McFarland, N.R. Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. J. Neurosci. 2000, 20, 2369–2382. [Google Scholar] [CrossRef]
- Poisson, C.L.; Engel, L.; Saunders, B.T. Dopamine Circuit Mechanisms of Addiction-Like Behaviors. Front. Neural Circuits 2021, 15, 752420. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W.; Vaghi, M.M.; Banca, P. Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron 2019, 102, 27–47. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu. Rev. Psychol. 2016, 67, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. Affective valence in the brain: Modules or modes? Nat. Rev. Neurosci. 2019, 20, 225–234. [Google Scholar] [CrossRef]
- Berridge, K.C. Evolving Concepts of Emotion and Motivation. Front. Psychol. 2018, 9, 1647. [Google Scholar] [CrossRef]
- Faure, A.; Reynolds, S.M.; Richard, J.M.; Berridge, K.C. Mesolimbic Dopamine in Desire and Dread: Enabling Motivation to Be Generated by Localized Glutamate Disruptions in Nucleus Accumbens. J. Neurosci. 2008, 28, 7184–7192. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, M.G.; Zachry, J.E.; Melugin, P.R.; Cajigas, S.A.; Chevee, M.F.; Kelley, S.J.; Kutlu, B.; Tian, L.; Siciliano, C.A.; Calipari, E.S. Dopamine release in the nucleus accumbens core signals perceived saliency. Curr. Biol. 2021, 31, 4748–4761.e8. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Nour, M.M.; Dahoun, T.; Jauhar, S.; Pepper, F.; Expert, P.; Veronese, M.; Adams, R.A.; Turkheimer, F.; Mehta, M.A.; et al. Mesolimbic Dopamine Function Is Related to Salience Network Connectivity: An Integrative Positron Emission Tomography and Magnetic Resonance Study. Biol. Psychiatry 2019, 85, 368–378. [Google Scholar] [CrossRef]
- Robinson, M.J.; Berridge, K.C. Instant transformation of learned repulsion into motivational “wanting”. Curr. Biol. 2013, 23, 282–289. [Google Scholar] [CrossRef]
- Zbukvic, I.C.; Kim, J.H. Divergent prefrontal dopaminergic mechanisms mediate drug- and fear-associated cue extinction during adolescence versus adulthood. Eur. Neuropsychopharmacol. 2018, 28, 1–12. [Google Scholar] [CrossRef]
- Latagliata, E.C.; Iacono, L.L.; Chiacchierini, G.; Sancandi, M.; Rava, A.; Oliva, V.; Puglisi-Allegra, S. Single Prazosin Infusion in Prelimbic Cortex Fosters Extinction of Amphetamine-Induced Conditioned Place Preference. Front. Pharmacol. 2017, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, M.G.; Zachry, J.E.; Melugin, P.R.; Tat, J.; Cajigas, S.; Isiktas, A.U.; Patel, D.D.; Siciliano, C.A.; Schoenbaum, G.; Sharpe, M.J.; et al. Dopamine signaling in the nucleus accumbens core mediates latent inhibition. Nat. Neurosci. 2022, 25, 1071–1081. [Google Scholar] [CrossRef]
- Siemsen, B.M.; Denton, A.R.; Parrila-Carrero, J.; Hooker, K.N.; Carpenter, E.A.; Prescot, M.E.; Brock, A.G.; Westphal, A.M.; Leath, M.-N.; McFaddin, J.A.; et al. Heroin Self-Administration and Extinction Increase Prelimbic Cortical Astrocyte–Synapse Proximity and Alter Dendritic Spine Morphometrics That Are Reversed by N-Acetylcysteine. Cells 2023, 12, 1812. [Google Scholar] [CrossRef]
- Bobadilla, A.-C.; Dereschewitz, E.; Vaccaro, L.; Heinsbroek, J.A.; Scofield, M.D.; Kalivas, P.W. Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol. Psychiatry 2020, 25, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D.; Linnman, C.; Faria, V.; Strassman, A.; Becerra, L.; Elman, I. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 2016, 68, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Salzman, C.D.; Fusi, S. Emotion, Cognition, and Mental State Representation in Amygdala and Prefrontal Cortex. Annu. Rev. Neurosci. 2010, 33, 173–202. [Google Scholar] [CrossRef]
- Seymour, B.; Dolan, R. Emotion, decision making, and the amygdala. Neuron 2008, 58, 662–671. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L. Emotional arousal and enhanced amygdala activity: New evidence for the old perseveration-consolidation hypothesis. Learn. Mem. 2005, 12, 77–79. [Google Scholar] [CrossRef][Green Version]
- Douma, E.H.; de Kloet, E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Piazza, P.V.; Le Moal, M. Glucocorticoids as a biological substrate of reward: Physiological and pathophysiological implications. Brain Res. Rev. 1997, 25, 359–372. [Google Scholar] [CrossRef]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Puglisi-Allegra, S.; Lazzeri, G.; Busceti, C.L.; Giorgi, F.S.; Biagioni, F.; Fornai, F. Lithium engages autophagy for neuroprotection and neuroplasticity: Translational evidence for therapy. Neurosci. Biobehav. Rev. 2023, 148, 105148. [Google Scholar] [CrossRef]
- Park, J.W.; Bhimani, R.V.; Park, J. Noradrenergic Modulation of Dopamine Transmission Evoked by Electrical Stimulation of the Locus Coeruleus in the Rat Brain. ACS Chem. Neurosci. 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Sevenster, D.; Visser, R.M.; D’Hooge, R. A translational perspective on neural circuits of fear extinction: Current promises and challenges. Neurobiol. Learn. Mem. 2018, 155, 113–126. [Google Scholar] [CrossRef]
- Xu, Z.; Adler, A.; Li, H.; Pérez-Cuesta, L.M.; Lai, B.; Li, W.; Gan, W.-B. Fear conditioning and extinction induce opposing changes in dendritic spine remodeling and somatic activity of layer 5 pyramidal neurons in the mouse motor cortex. Sci. Rep. 2019, 9, 4619. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, T.; Zhou, Q. Elevated dopamine signaling from ventral tegmental area to prefrontal cortical parvalbumin neurons drives conditioned inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 13077–13086. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Hernández, X.I.; Zafiri, D.; Sigurdsson, T.; Duvarci, S. Functional architecture of dopamine neurons driving fear extinction learning. Neuron 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Cui, X.; Tong, Q.; Xu, H.; Xie, C.; Xiao, L. A putative loop connection between VTA dopamine neurons and nucleus accumbens encodes positive valence to compensate for hunger. Prog. Neurobiol. 2023, 229, 102503. [Google Scholar] [CrossRef]
- Bunai, T.; Hirosawa, T.; Kikuchi, M.; Fukai, M.; Yokokura, M.; Ito, S.; Takata, Y.; Terada, T.; Ouchi, Y. tDCS-induced modulation of GABA concentration and dopamine release in the human brain: A combination study of magnetic resonance spectroscopy and positron emission tomography. Brain Stimul. 2021, 14, 154–160. [Google Scholar] [CrossRef]
- Ishikuro, K.; Hattori, N.; Imanishi, R.; Furuya, K.; Nakata, T.; Dougu, N.; Yamamoto, M.; Konishi, H.; Nukui, T.; Hayashi, T.; et al. Parkinson’s disease patient displaying increased neuromelanin-sensitive areas in the substantia nigra after rehabilitation with tDCS: A case report. Neurocase 2021, 27, 407–414. [Google Scholar] [CrossRef]
- Leow, L.-A.; Marcos, A.; Nielsen, E.; Sewell, D.K.; Ballard, T.; Dux, P.E.; Filmer, H.L. Dopamine alters the effect of brain stimulation on decision-making. J. Neurosci. 2023, 43, 6909–6919. [Google Scholar] [CrossRef] [PubMed]
- Borwick, C.; Lal, R.; Lim, L.W.; Stagg, C.J.; Aquili, L. Dopamine depletion effects on cognitive flexibility as modulated by tDCS of the dlPFC. Brain Stimul. 2020, 13, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Dennison, O.; Gao, J.; Lim, L.W.; Stagg, C.J.; Aquili, L. Catecholaminergic modulation of indices of cognitive flexibility: A pharmaco-tDCS study. Brain Stimul. 2019, 12, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Bunai, T.; Hirosawa, T.; Kikuchi, M.; Ito, S.; Minabe, Y.; Ouchi, Y. Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: A study with positron emission tomography. Transl. Psychiatry 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Cabib, S.; Orsini, C.; Le Moal, M.; Piazza, P.V. Abolition and reversal of straindifferences in behavioral responses to drugs of abuse after a brief experience. Science 2000, 289, 463–465. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic: New York, NY, YSA, 1998. [Google Scholar]
- LeCocq, M.R.; Sun, S.; Chaudhri, N. The role of context conditioning in the reinstatement of responding to an alcohol-predictive conditioned stimulus. Behav. Brain Res. 2022, 423, 113686. [Google Scholar] [CrossRef]
- Latagliata, E.C.; Valzania, A.; Pascucci, T.; Campus, P.; Cabib, S.; Puglisi-Allegra, S. Stress-induced activation of ventral tegmental mu-opioid receptors reduces accumbens dopamine tone by enhancing dopamine transmission in the medial pre-frontal cortex. Psychopharmacology 2014, 231, 4099–4108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latagliata, E.C.; Orsini, C.; Cabib, S.; Biagioni, F.; Fornai, F.; Puglisi-Allegra, S. Prefrontal Dopamine in Flexible Adaptation to Environmental Changes: A Game for Two Players. Biomedicines 2023, 11, 3189. https://doi.org/10.3390/biomedicines11123189
Latagliata EC, Orsini C, Cabib S, Biagioni F, Fornai F, Puglisi-Allegra S. Prefrontal Dopamine in Flexible Adaptation to Environmental Changes: A Game for Two Players. Biomedicines. 2023; 11(12):3189. https://doi.org/10.3390/biomedicines11123189
Chicago/Turabian StyleLatagliata, Emanuele Claudio, Cristina Orsini, Simona Cabib, Francesca Biagioni, Francesco Fornai, and Stefano Puglisi-Allegra. 2023. "Prefrontal Dopamine in Flexible Adaptation to Environmental Changes: A Game for Two Players" Biomedicines 11, no. 12: 3189. https://doi.org/10.3390/biomedicines11123189
APA StyleLatagliata, E. C., Orsini, C., Cabib, S., Biagioni, F., Fornai, F., & Puglisi-Allegra, S. (2023). Prefrontal Dopamine in Flexible Adaptation to Environmental Changes: A Game for Two Players. Biomedicines, 11(12), 3189. https://doi.org/10.3390/biomedicines11123189






