KDF1 Promoted Proliferation, Migration and Invasion of Lung Adenocarcinoma Cells through Activating STAT3 and AKT Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Cell Culture and Treatment
2.4. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.5. Western Blot Analysis
2.6. Cell Vitality Assay
2.7. Colony-Forming Assay
2.8. EdU Incorporation Test
2.9. Wound-Healing Assay
2.10. Matrigel Invasion Assay
2.11. Tumorigenicity Assay
2.12. Statistical Analysis
3. Results
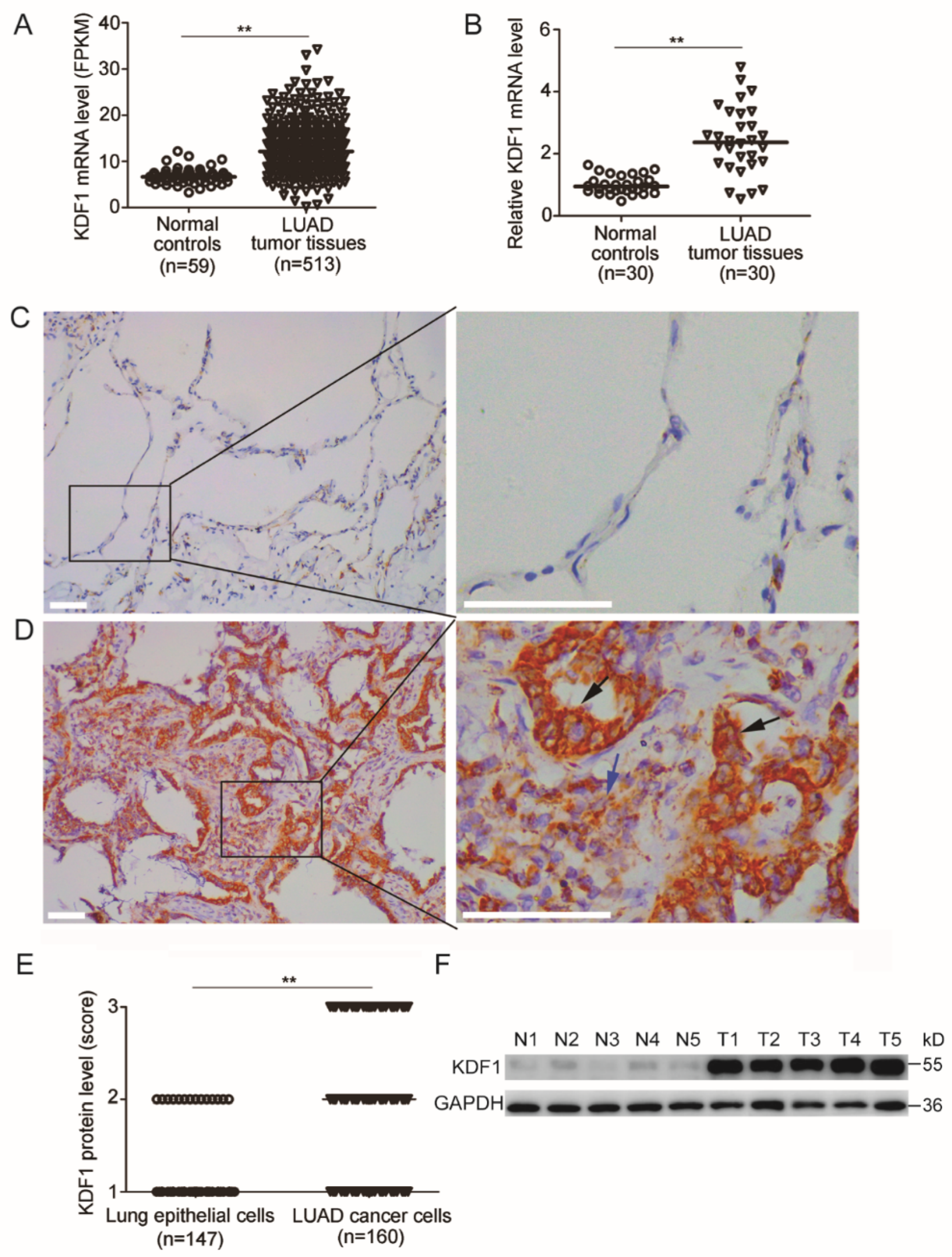
3.1. KDF1 Was Increasingly Expressed in the Tumor Tissues of LUAD Patients
3.2. Expression of KDF1 in Other Cancers
3.3. Association between the Level of KDF1 and Clinicopathological Parameters
3.4. Patients with Increased KDF1 in the Cancer Cells Had Shorter OS
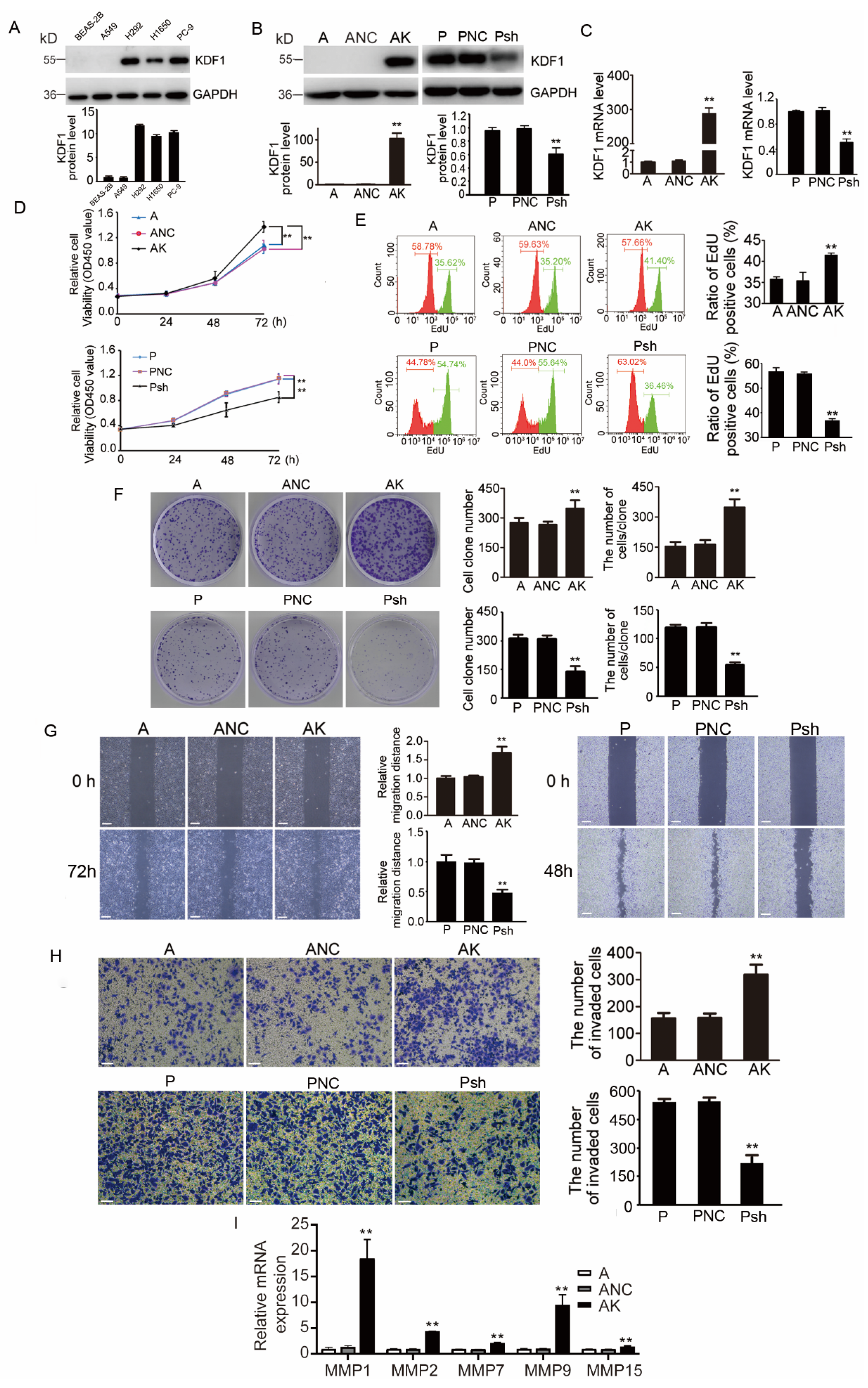
3.5. KDF1 Promoted the Proliferation, Migration and Invasion of LUAD Cells
3.6. Re-Knockdown of KDF1 in KDF1-Overexpressing A549 Cells Reversed the Cell Phenotype
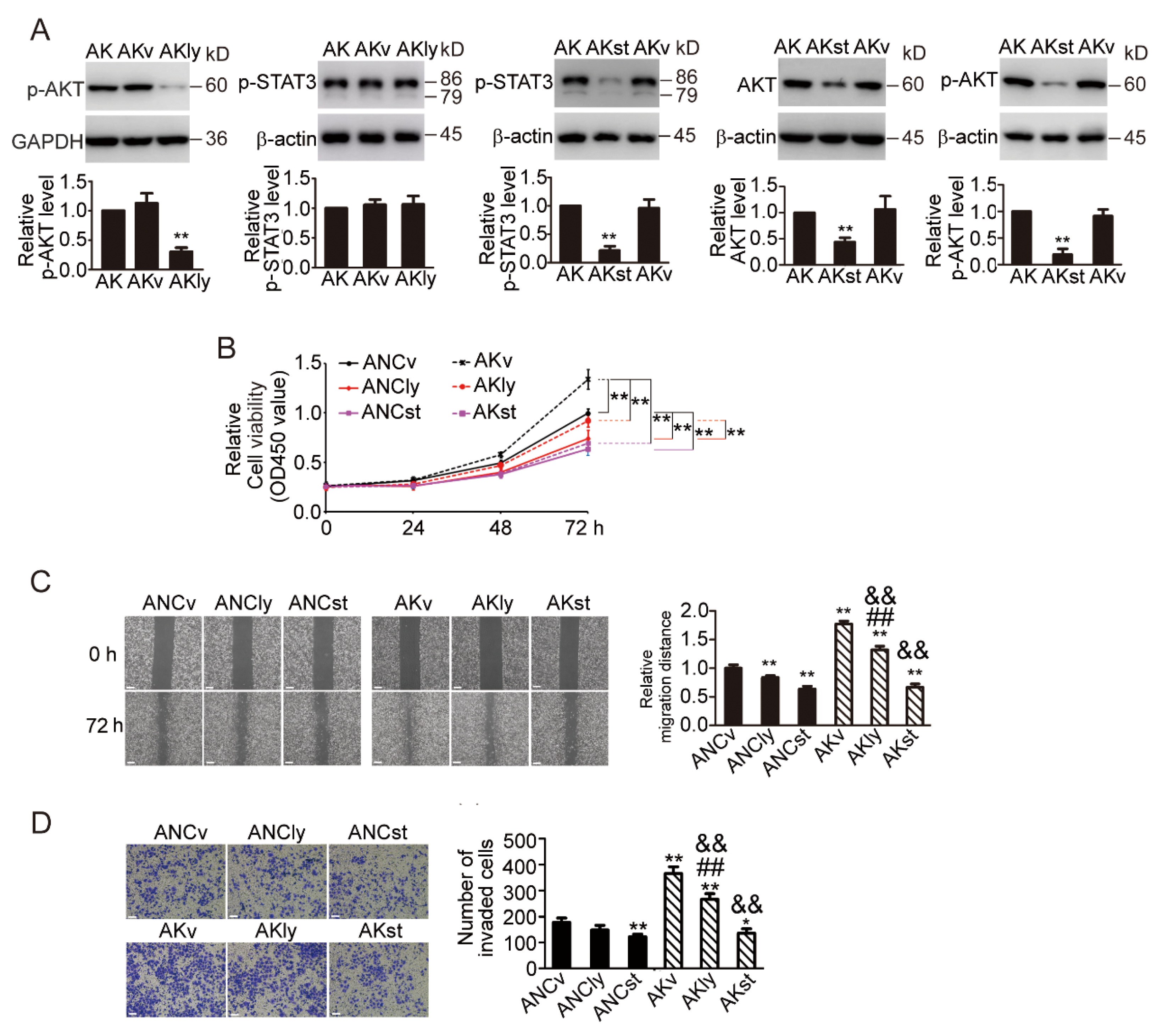
3.7. KDF1 Overexpression Significantly Upregulates the Level of AKT, p-AKT and p-STAT3 but Had No Effect on the Level of Iκκα, p38, p-p38 ERK, p-ERK and STAT3
3.8. STAT3 Inhibitor Inhibited AKT Signaling Pathway and Prevented the Phenotypic Changes Induced by KDF1 Overexpression
3.9. Overexpression of KDF1 Increased Xenograft Tumor Growth
3.10. Correlation of KDF1 and p-STAT3 Level in Human LUAD Tumor Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.H.; Zhang, Z.T.; Wei, H.X.; Ning, L.; Ai, J.S.; Li, W.H.; Zhang, H.; Wang, S.Q. LncRNA ST7-AS1, by regulating miR-181b-5p/KPNA4 axis, promotes the malignancy of lung adenocarcinoma. Cancer Cell Int. 2020, 20, 568. [Google Scholar] [CrossRef]
- Seguin, L.; Durandy, M.; Feral, C.C. Lung Adenocarcinoma Tumor Origin: A Guide for Personalized Medicine. Cancers 2022, 14, 1759. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kong, Y.; Weatherbee, S.D. Forward genetics identifies Kdf1/1810019J16Rik as an essential regulator of the proliferation-differentiation decision in epidermal progenitor cells. Dev. Biol. 2013, 383, 201–213. [Google Scholar] [CrossRef]
- Zheng, J.M.; Gan, M.F.; Yu, H.Y.; Ye, L.X.; Yu, Q.X.; Xia, Y.H.; Zhou, H.X.; Bao, J.Q.; Guo, Y.Q. KDF1 a Novel Tumor Suppressor in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 686678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, Y.; Tong, R.; Guan, J. KDF1 Promoted Proliferation and Metastasis of Epithelial Ovarian Cancer via Wnt/Beta-Catenin Pathway: TCGA-Based Data Mining and Experimental Validation. Front. Genet. 2022, 13, 808100. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Yue, J.; Gou, X.; Lin, A.; Weatherbee, S.D.; Wu, X. Regulation of epidermal differentiation through KDF1-mediated deubiquitination of IKKalpha. EMBO Rep. 2020, 21, e48566. [Google Scholar] [CrossRef]
- Kohler, J.; Catalano, M.; Ambrogio, C. Back to the Bench? MEK and ERK Inhibitors for the Treatment of KRAS Mutant Lung Adenocarcinoma. Curr. Med. Chem. 2018, 25, 558–574. [Google Scholar] [CrossRef]
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Tsai, P.J.; Lai, Y.H.; Manne, R.K.; Tsai, Y.S.; Sarbassov, D.; Lin, H.K. Akt: A key transducer in cancer. J. Biomed. Sci. 2022, 29, 76. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Razi, S.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl. Oncol. 2022, 18, 101364. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xiao, K. Nanoparticle-Mediated Delivery of STAT3 Inhibitors in the Treatment of Lung Cancer. Pharmaceutics 2022, 14, 2787. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Ernst, M.; Poh, A.R. Multicellular Effects of STAT3 in Non-small Cell Lung Cancer: Mechanistic Insights and Therapeutic Opportunities. Cancers 2021, 13, 6228. [Google Scholar] [CrossRef] [PubMed]
- Mohrherr, J.; Uras, I.Z.; Moll, H.P.; Casanova, E. STAT3: Versatile Functions in Non-Small Cell Lung Cancer. Cancers 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, H.; Liu, Y.; Zheng, J.; Wu, J.; Sun, K.; Feng, H.; Liu, H.; Han, D. KDF1 Novel Variant Causes Unique Dental and Oral Epithelial Defects. Int. J. Mol. Sci. 2022, 23, 12465. [Google Scholar] [CrossRef]
- Pan, Y.; Yi, S.; Chen, D.; Du, X.; Yao, X.; He, F.; Xiong, F. Identification of a novel missense heterozygous mutation in the KDF1 gene for non-syndromic congenital anodontia. Clin. Oral Investig. 2022, 26, 5171–5179. [Google Scholar] [CrossRef]
- Manaspon, C.; Thaweesapphithak, S.; Osathanon, T.; Suphapeetiporn, K.; Porntaveetus, T.; Shotelersuk, V. A novel de novo mutation substantiates KDF1 as a gene causing ectodermal dysplasia. Br. J. Dermatol. 2019, 181, 419–420. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Khalifa, O.; Binamer, Y.M.; Almutawa, A.; Arold, S.T.; Zaidan, H.; Alkuraya, F.S. KDF1, encoding keratinocyte differentiation factor 1, is mutated in a multigenerational family with ectodermal dysplasia. Hum. Genet. 2017, 136, 99–105. [Google Scholar] [CrossRef]
- Pezzuto, A.; Cappuzzo, F.; D’Arcangelo, M.; Ciccozzi, M.; Navarini, L.; Guerrini, S.; Ricci, A.; D’Ascanio, M.; Carico, E. Prognostic Value of p16 Protein in Patients With Surgically Treated Non-small Cell Lung Cancer; Relationship With Ki-67 and PD-L1. Anticancer Res. 2020, 40, 983–990. [Google Scholar] [CrossRef]
- Tong, L.; Li, J.; Li, Q.; Wang, X.; Medikonda, R.; Zhao, T.; Li, T.; Ma, H.; Yi, L.; Liu, P.; et al. ACT001 reduces the expression of PD-L1 by inhibiting the phosphorylation of STAT3 in glioblastoma. Theranostics 2020, 10, 5943–5956. [Google Scholar] [CrossRef]
- Kaminskiy, Y.; Melenhorst, J.J. STAT3 Role in T-Cell Memory Formation. Int. J. Mol. Sci. 2022, 23, 2878. [Google Scholar] [CrossRef]
- Nikolskii, A.A.; Shilovskiy, I.P.; Barvinskaia, E.D.; Korneev, A.V.; Sundukova, M.S.; Khaitov, M.R. Role of STAT3 Transcription Factor in Pathogenesis of Bronchial Asthma. Biochemistry 2021, 86, 1489–1501. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.C.; Song, W. Involvement of IL-10R/STAT3 pathway in amyloid beta clearance by microlgia in Alzheimer’s disease. Int. Immunopharmacol. 2021, 101, 108263. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Liu, D.; Sun, M.; Lu, C.; Yang, W.; Wang, C.; Cheng, Y.; Zhang, M.; Shen, M.; Yang, Z.; et al. Targeting STAT3: A crucial modulator of sepsis. J. Cell Physiol. 2021, 236, 7814–7831. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yin, B.; Ye, Y.; Dekhel, O.; Xiong, X.; Jian, Z.; Gu, L. The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia-reperfusion injury. Exp. Neurol. 2021, 341, 113690. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Hashimoto, A.; Muromoto, R.; Kitai, Y.; Oritani, K.; Matsuda, T. Central Roles of STAT3-Mediated Signals in Onset and Development of Cancers: Tumorigenesis and Immunosurveillance. Cells 2022, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, Y.; Li, Y.; Lv, T.; Liu, J.; Wang, X. Prognostic significance of STAT3 expression and its correlation with chemoresistance of non-small cell lung cancer cells. Acta Histochem. 2012, 114, 151–158. [Google Scholar] [CrossRef]
- Yin, Z.J.; Jin, F.G.; Liu, T.G.; Fu, E.Q.; Xie, Y.H.; Sun, R.L. Overexpression of STAT3 potentiates growth, survival, and radioresistance of non-small-cell lung cancer (NSCLC) cells. J. Surg. Res. 2011, 171, 675–683. [Google Scholar] [CrossRef]
- Lee, H.J.; Zhuang, G.; Cao, Y.; Du, P.; Kim, H.J.; Settleman, J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 2014, 26, 207–221. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018, 428, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhu, J.; Hui, B.; Jia, C.; Yan, X.; Jiang, T.; Wang, X. Circ-HSP90A expedites cell growth, stemness, and immune evasion in non-small cell lung cancer by regulating STAT3 signaling and PD-1/PD-L1 checkpoint. Cancer Immunol. Immunother. 2023, 72, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, X.; Zhang, Y.; Wu, B.; Fang, L.; Wang, N.; Yi, H.; Chang, N.; Chen, L.; Zhang, J. Aryl hydrocarbon receptor mediates Jak2/STAT3 signaling for non-small cell lung cancer stem cell maintenance. Exp. Cell Res. 2020, 396, 112288. [Google Scholar] [CrossRef] [PubMed]
- Sadrkhanloo, M.; Entezari, M.; Orouei, S.; Ghollasi, M.; Fathi, N.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Saebfar, H.; Hashemi, M.; et al. STAT3-EMT axis in tumors: Modulation of cancer metastasis, stemness and therapy response. Pharmacol. Res. 2022, 182, 106311. [Google Scholar] [CrossRef]






| Variable | KDF1 Level in Tumor Cells (Immunostaining Score) | p | ||
|---|---|---|---|---|
| 1 Number (%) | 2 Number (%) | 3 Number (%) | ||
| Age (years) | ||||
| ≤median | 17 (21.0) | 31 (38.3) | 33 (40.7) | 0.724 |
| >median | 15 (19.0) | 36 (45.6) | 28 (35.4) | |
| Gender | ||||
| Male | 9 (16.1) | 25 (44.6) | 22 (39.3) | 0.562 |
| Female | 23 (22.1) | 42 (40.4) | 39 (37.5) | |
| Tumor size | ||||
| ≤median | 22 (27.8) | 34 (43.0) | 23 (29.1) | 0.010 |
| >median | 10 (12.8) | 33 (42.3) | 35 (44.9) | |
| T (primary tumor extent) | ||||
| T0 or T1 | 25 (24.0) | 41 (39.4) | 38 (36.5) | 0.227 |
| T2–4 | 7 (12.5) | 26 (46.4) | 23 (41.1) | |
| With lymph node or distant metastasis | ||||
| Yes | 4 (13.8) | 10 (31.0) | 15 (55.2) | 0.104 |
| No | 28 (21.4) | 57 (43.5) | 46 (35.1) | |
| TNM stage | ||||
| Carcinoma in situ/I | 27 (21.4) | 56 (44.4) | 43 (34.1) | 0.065 |
| II–IV | 5 (14.7) | 11 (32.4) | 18 (52.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-Q.; Gan, M.-F.; Bao, J.-Q.; Zhou, H.-X.; Yang, J.; Dai, C.-J.; Zheng, J.-M. KDF1 Promoted Proliferation, Migration and Invasion of Lung Adenocarcinoma Cells through Activating STAT3 and AKT Pathway. Biomedicines 2023, 11, 3194. https://doi.org/10.3390/biomedicines11123194
Guo Y-Q, Gan M-F, Bao J-Q, Zhou H-X, Yang J, Dai C-J, Zheng J-M. KDF1 Promoted Proliferation, Migration and Invasion of Lung Adenocarcinoma Cells through Activating STAT3 and AKT Pathway. Biomedicines. 2023; 11(12):3194. https://doi.org/10.3390/biomedicines11123194
Chicago/Turabian StyleGuo, Yi-Qing, Mei-Fu Gan, Jia-Qian Bao, Han-Xi Zhou, Jing Yang, Chuan-Jing Dai, and Jing-Min Zheng. 2023. "KDF1 Promoted Proliferation, Migration and Invasion of Lung Adenocarcinoma Cells through Activating STAT3 and AKT Pathway" Biomedicines 11, no. 12: 3194. https://doi.org/10.3390/biomedicines11123194






