Application of a Receptor-Binding-Domain-Based Simple Immunoassay for Assessing Humoral Immunity against Emerging SARS-CoV-2 Virus Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recombinant RBD Production
2.2. ELISA Method
2.3. Virus Neutralization Assay
3. Results and Discussion
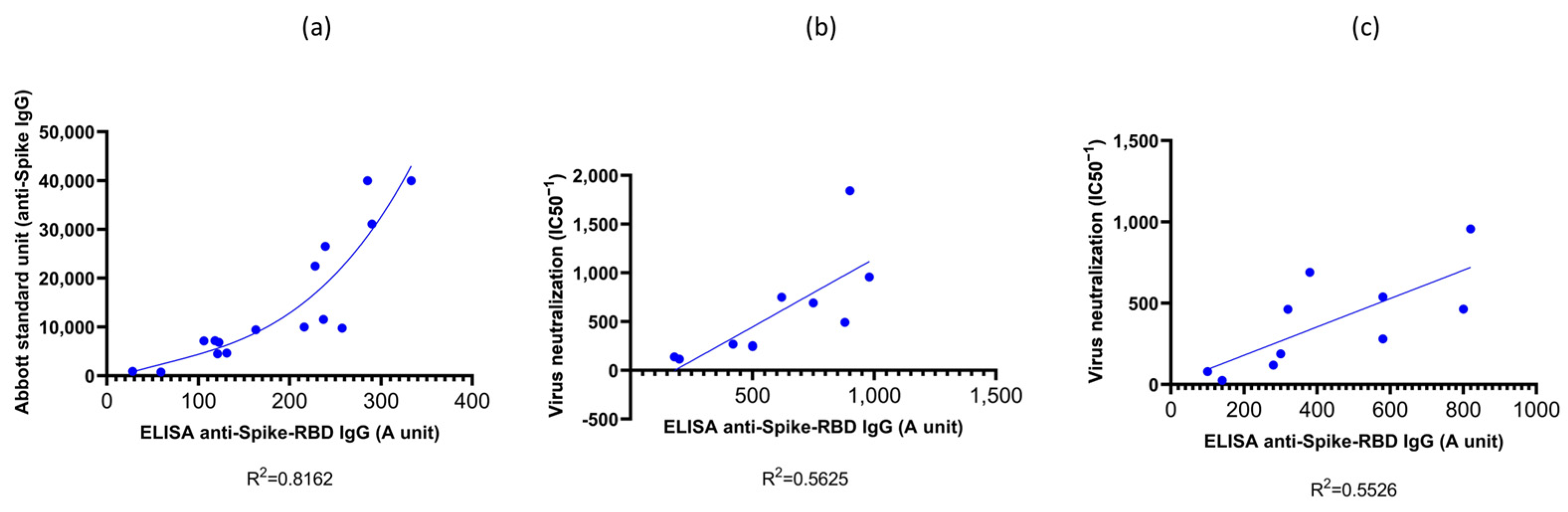
3.1. Validation of the RBD-ELISA Results with Commercially Available Quantitative Immunoassay and Virus Neutralization Assay
3.2. Results of RBD-ELISA for Three Virus Variants in Vaccinated Donors and Convalescent Patients
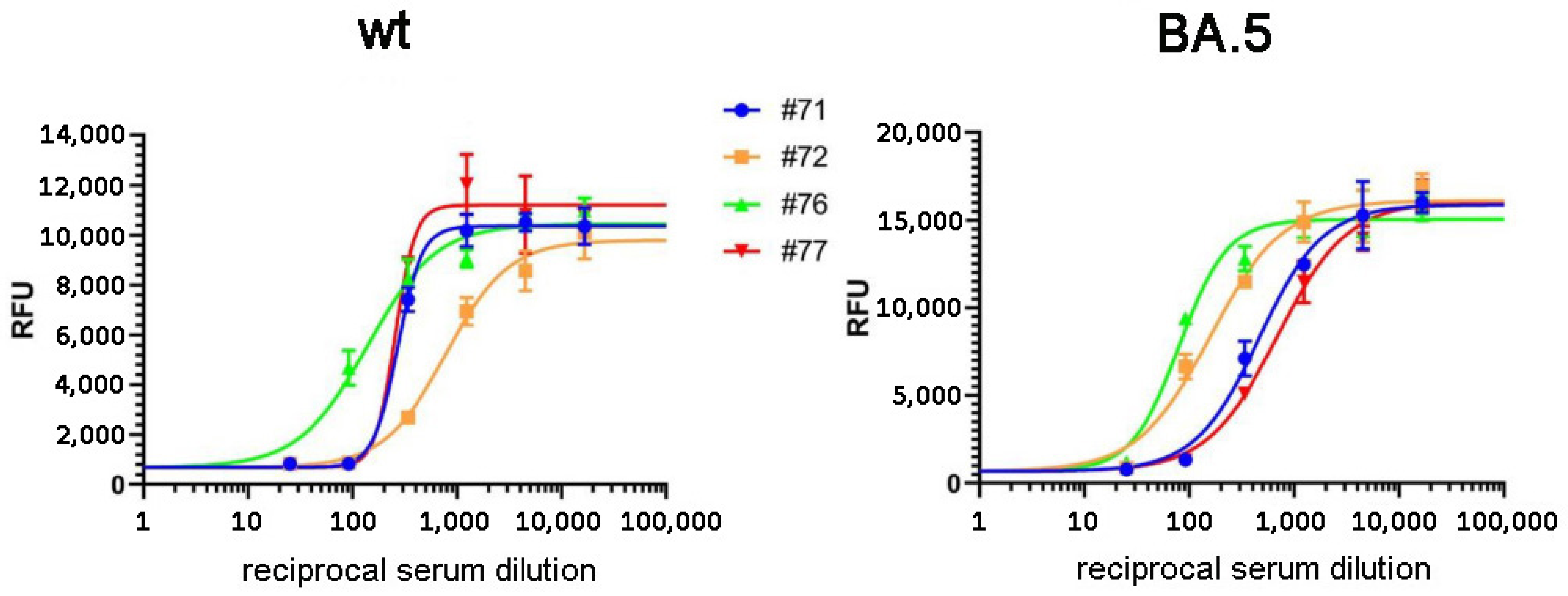
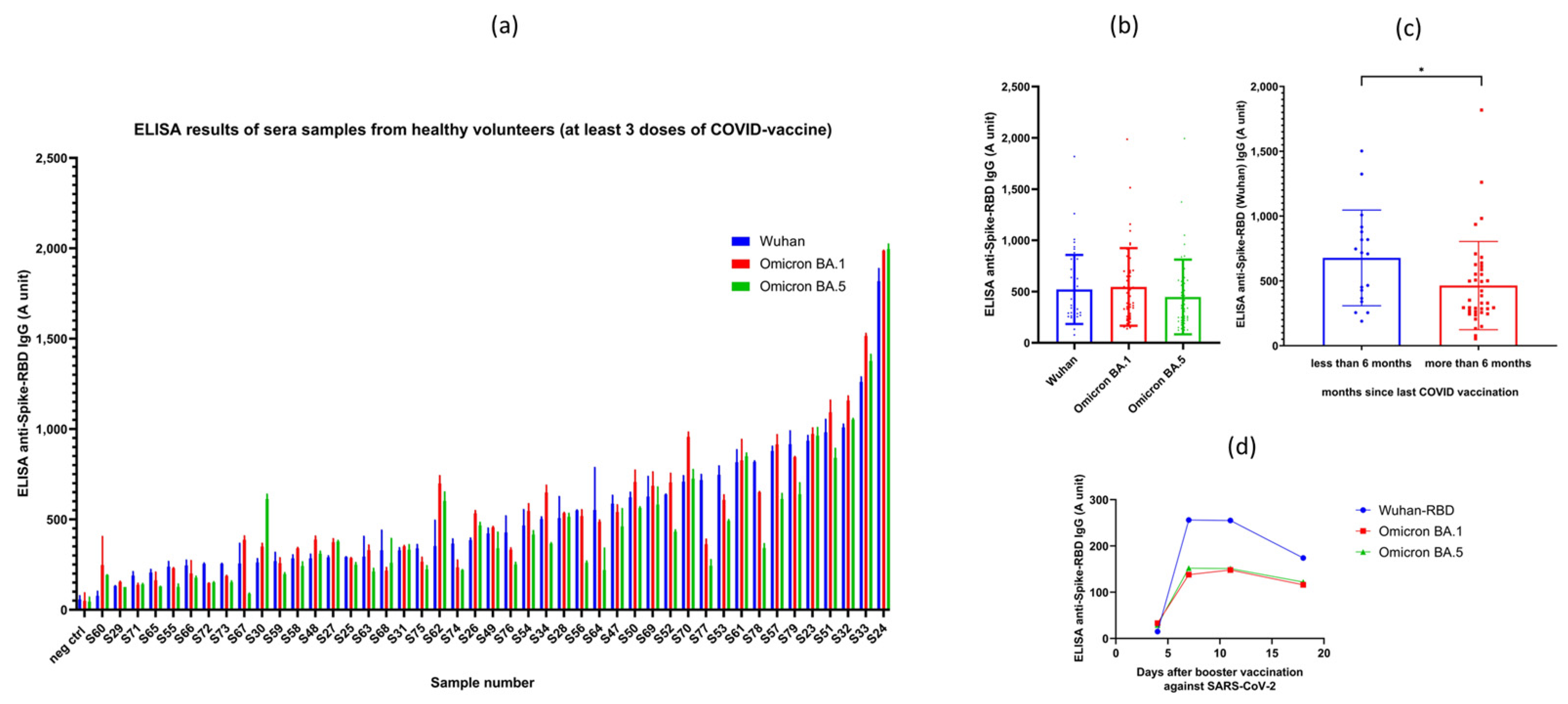
3.2.1. Results Obtained in Vaccinated Volunteer Donors
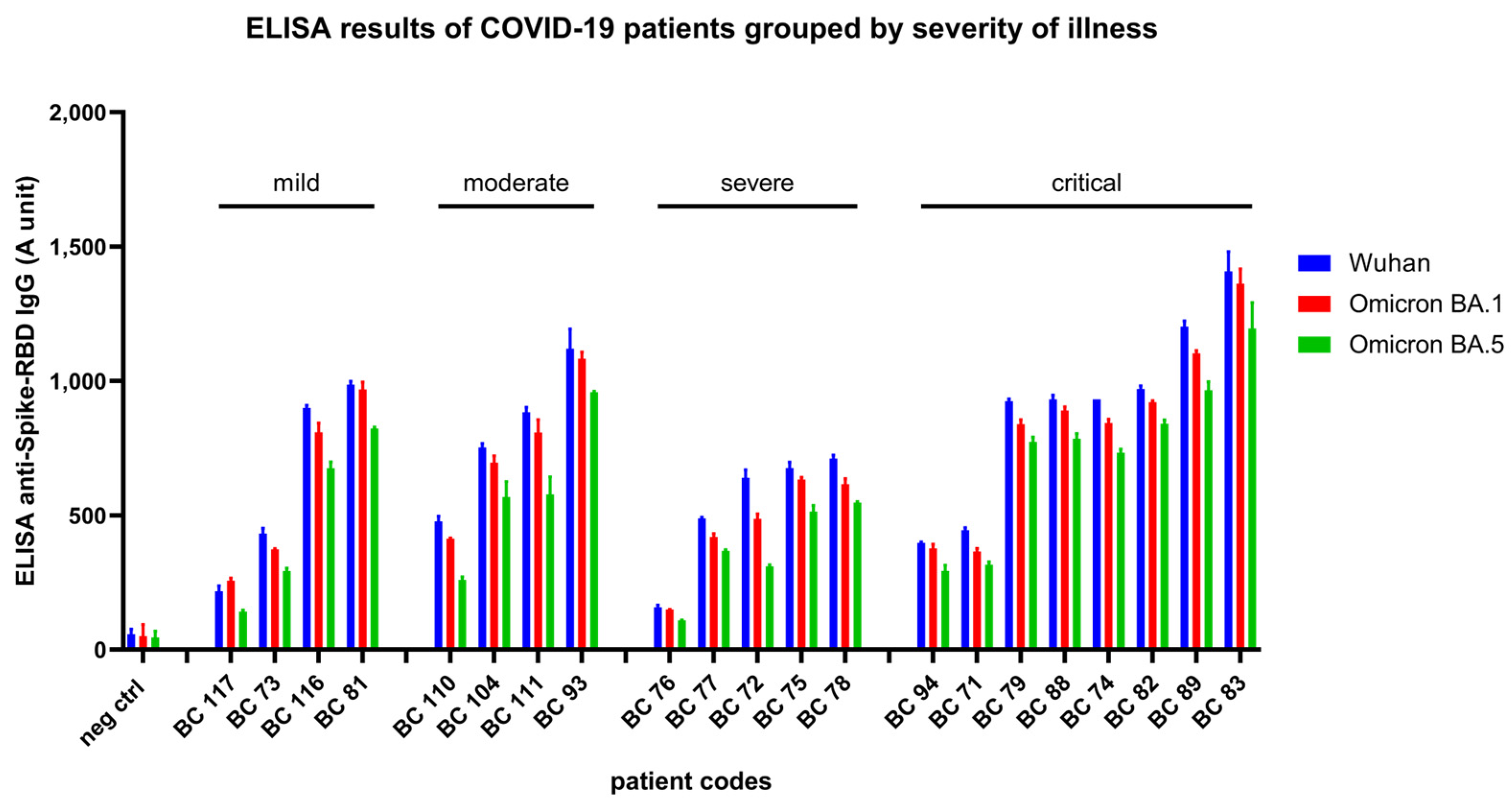
3.2.2. Results Obtained in Convalescent Patients after COVID-19 Disease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep Immune Profiling of COVID-19 Patients Reveals Distinct Immunotypes with Therapeutic Implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal Analysis Shows Durable and Broad Immune Memory after SARS-CoV-2 Infection with Persisting Antibody Responses and Memory B and T Cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, P158–168.E14. [Google Scholar] [CrossRef]
- Díaz-Espada, F.; Matheu, V.; Barrios, Y. A Review of Hypersensitivity Methods to Detect Immune Responses to SARS-CoV-2. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 50. [Google Scholar]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Case, J.B.; Winkler, E.S.; Thackray, L.B.; Kafai, N.M.; Bailey, A.L.; McCune, B.T.; Fox, J.M.; Chen, R.E.; Alsoussi, W.B.; et al. A SARS-CoV-2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell 2020, 182, P744–753.E4. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently Neutralizing and Protective Human Antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.M.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 Neutralizing Antibody Structures Inform Therapeutic Strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, L.; Zhang, G.; Yao, Y.; Zhou, H.; Shen, S.; Shen, B.; Li, B.; Li, X.; Zhang, Q.; et al. A SARS-CoV-2 Neutralizing Antibody with Extensive Spike Binding Coverage and Modified for Optimal Therapeutic Outcomes. Nat. Commun. 2021, 12, 2623. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.L.; Feng, Y.; Deng, Y.Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977.e3. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-Neutralizing Antibodies Predict Disease Severity and Survival. Cell 2021, 184, P476–488.E11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, J.; Zeng, Y.; Huang, C.; Chen, F.; Cao, Y.; Wu, S.; Wei, D.; Lin, Z.; Zhang, Y.; et al. Effectiveness of SARS-CoV-2-Inactivated Vaccine and the Correlation to Neutralizing Antibodies: A Test-Negative Case–Control Study. J. Med. Virol. 2023, 95, e28280. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zody, M.C.; Di Germanio, C.; Martinelli, R.; Mediavilla, J.R.; Cunningham, M.H.; Composto, K.; Chow, K.F.; Kordalewska, M.; Corvelo, A.; et al. Emergence of Multiple SARS-CoV-2 Antibody Escape Variants in an Immunocompromised Host Undergoing Convalescent Plasma Treatment. mSphere 2021, 6, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Jaki, L.; Weigang, S.; Kern, L.; Kramme, S.; Wrobel, A.G.; Grawitz, A.B.; Nawrath, P.; Martin, S.R.; Dähne, T.; Beer, J.; et al. Total Escape of SARS-CoV-2 from Dual Monoclonal Antibody Therapy in an Immunocompromised Patient. Nat. Commun. 2023, 14, 1999. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Priyanka; Parmar, M.; Angural, S.; Choudhary, O.P. Convalescent Plasma Therapy against the Emerging SARS-CoV-2 Variants: Delineation of the Potentialities and Risks. Int. J. Surg. 2022, 97, 106204. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab on Viral Load in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Kempf, A.; Nehlmeier, I.; Schulz, S.R.; Jäck, H.M.; Pöhlmann, S.; Hoffmann, M. Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. Lancet Infect Dis. 2022, 23, 22–23. [Google Scholar] [CrossRef]
- Tada, T.; Zhou, H.; Dcosta, B.M.; Samanovic, M.I.; Chivukula, V.; Herati, R.S.; Hubbard, S.R.; Mulligan, M.J.; Landau, N.R. Increased Resistance of SARS-CoV-2 Omicron Variant to Neutralization by Vaccine-Elicited and Therapeutic Antibodies. EBioMedicine 2022, 78, 103944. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Sharma, M.; Emran, T.B.; Rabaan, A.A. A Rapid Surge of the Omicron Variant’s Sublineages BQ.1/BQ.1.1: A Matter of Worry amid the Crucial Trajectory of the COVID-19 Pandemic. Int. J. Surg. 2023, 109, 504–506. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Zámbó, B.; Mózner, O.; Bartos, Z.; Török, G.; Várady, G.; Telbisz, Á.; Homolya, L.; Orbán, T.I.; Sarkadi, B. Cellular Expression and Function of Naturally Occurring Variants of the Human ABCG2 Multidrug Transporter. Cell. Mol. Life Sci. 2020, 77, 365–378. [Google Scholar] [CrossRef]
- Barda, N.; Canetti, M.; Gilboa, M.; Asraf, K.; Indenboim, V.; Weiss-Ottolenghi, Y.; Amit, S.; Zubli, D.; Doolman, R.; Mendelson, E.; et al. The Association Between Prebooster Vaccination Antibody Levels and the Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin. Infect. Dis. 2022, 76, 1315–1317. [Google Scholar] [CrossRef]
- Khoury, D.S.; Schlub, T.E.; Cromer, D.; Steain, M.; Fong, Y.; Gilbert, P.B.; Subbarao, K.; Triccas, J.A.; Kent, S.J.; Davenport, M.P. Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection. Emerg. Infect. Dis. 2023, 29, 381. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Hachmann, N.P.; Collier, A.Y.; Lasrado, N.; Mazurek, C.R.; Patio, R.C.; Powers, O.; Surve, N.; Theiler, J.; Korber, B.; et al. Substantial Neutralization Escape by SARS-CoV-2 Omicron Variants BQ.1.1 and XBB.1. N. Engl. J. Med. 2023, 388, 662–664. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mózner, O.; Moldvay, J.; Szabó, K.S.; Vaskó, D.; Domján, J.; Ács, D.; Ligeti, Z.; Fehér, C.; Hirsch, E.; Puskás, L.; et al. Application of a Receptor-Binding-Domain-Based Simple Immunoassay for Assessing Humoral Immunity against Emerging SARS-CoV-2 Virus Variants. Biomedicines 2023, 11, 3193. https://doi.org/10.3390/biomedicines11123193
Mózner O, Moldvay J, Szabó KS, Vaskó D, Domján J, Ács D, Ligeti Z, Fehér C, Hirsch E, Puskás L, et al. Application of a Receptor-Binding-Domain-Based Simple Immunoassay for Assessing Humoral Immunity against Emerging SARS-CoV-2 Virus Variants. Biomedicines. 2023; 11(12):3193. https://doi.org/10.3390/biomedicines11123193
Chicago/Turabian StyleMózner, Orsolya, Judit Moldvay, Kata Sára Szabó, Dorottya Vaskó, Júlia Domján, Dorottya Ács, Zoltán Ligeti, Csaba Fehér, Edit Hirsch, László Puskás, and et al. 2023. "Application of a Receptor-Binding-Domain-Based Simple Immunoassay for Assessing Humoral Immunity against Emerging SARS-CoV-2 Virus Variants" Biomedicines 11, no. 12: 3193. https://doi.org/10.3390/biomedicines11123193
APA StyleMózner, O., Moldvay, J., Szabó, K. S., Vaskó, D., Domján, J., Ács, D., Ligeti, Z., Fehér, C., Hirsch, E., Puskás, L., Stahl, C., Frey, M., & Sarkadi, B. (2023). Application of a Receptor-Binding-Domain-Based Simple Immunoassay for Assessing Humoral Immunity against Emerging SARS-CoV-2 Virus Variants. Biomedicines, 11(12), 3193. https://doi.org/10.3390/biomedicines11123193











