Role of Mangiferin in Management of Cancers through Modulation of Signal Transduction Pathways
Abstract
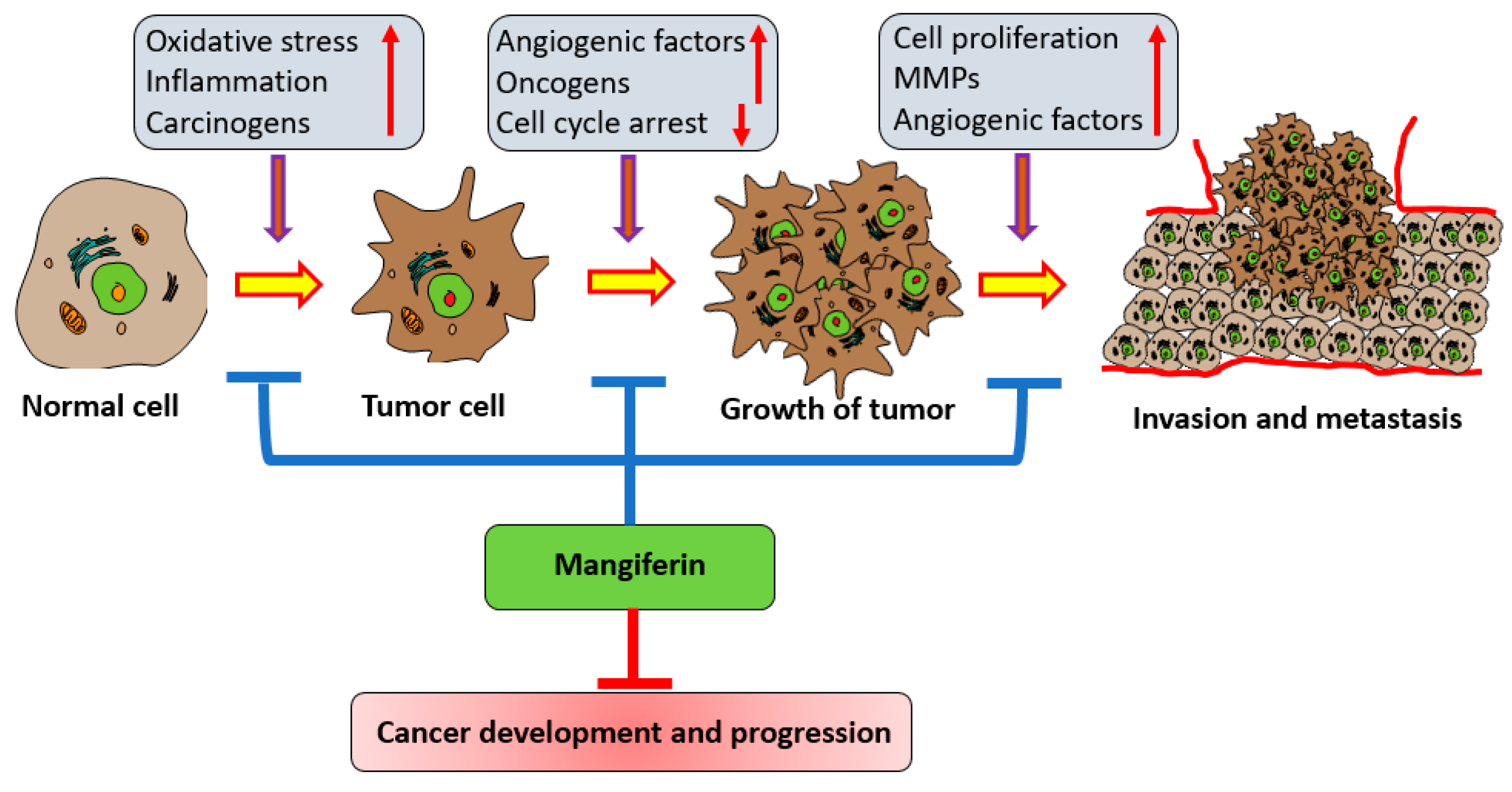
:1. Introduction
2. Methodology
3. Mechanism of Action of Mangiferin in Cancer Management
3.1. Inflammation
3.2. Oxidative Stress
3.3. Cell Cycle
3.4. Apoptosis
3.5. Angiogenesis
3.6. PI3K/Akt Pathway
3.7. Invasion and Metastasis
3.8. NRF2 Transcription Factor
4. Mangiferin: Potential Role in Several Types of Cancer
4.1. Lung Cancer
| Cancer | Study Types | Outcome of Study | Refs. |
|---|---|---|---|
| Lung | A549, H1299 and H2030 | Mangiferin controls the proliferation of adenocarcinoma cells and initiates apoptosis. | [84] |
| A549 | Mangiferin showed apoptosis-induction and growth-inhibitory effects. It was capable of increasing the anti-proliferative potential of cisplatin. | [28] | |
| Breast | MDA-MB-231 | Following the administration with mangiferin, this cancer cell decreased Rac1/Cdc42, WAVE2, phospho-Rac1/Cdc42, Arp2, and Arp3. | [87] |
| MCF7 and MCF10A | Mangiferin promotes apoptosis and stops cell proliferation. | [88] | |
| MCF-7 | Mangiferin addition to doxorubicin has a tendency to enhance the sensitivity of the cells to doxorubicin. Adding mangiferin to doxorubicin somewhat reduced the level of expression of P-gp mRNA. | [89] | |
| Cervix | HT29 and HeLa | Mangiferin decreased oxaliplatin IC50 values in cancer cells. DNA fragmentation, enhanced caspase 3 activation, retarted S-phase of cell cycle. | [41] |
| Prostate | PC3 | Mangiferin treatment reduced cancer cells proliferation. Its administration was found to induce apoptosis and enhance the activity of caspase-3. | [29] |
| Colorectal | Allograft mouse model of murine CT26 | Mangiferin treatments brings dose-dependent tumor regression and reduces metastasis. This compound inhibits tumour growth, angiogenesis, and metastasis. | [90] |
| Liver | Diethynitrosamine-Induced Hepatocellular Carcinoma | Mangiferin shows anticarcinogenic properties against this carcinoma. | [91] |
| HCC implantation murine model MHCC97L and HLF | Delay in G1/S transition was dependent on the amounts of mangiferin administered to HCC cells. | [92] | |
| Gastric | SGC-7901 | Elevation in Bad, Bax, and cleaved caspase-3,-9 and decrease in Mcl-1, Bcl-xL, and Bcl-2 activities was noticed by mangiferin. In addition, mangiferin lowered p-PI3K, p-mTOR, and p-Akt quantities. | [31] |
| Brain | U-87 MG and U-118 MG | Mangiferin enhanced the radiosensitivity of cancer cells towards radiation. Cancer cells treated with mangiferin revealed a greater amount of DNA damage, particularly corresponding to the elevated degree of radio sensitization. | [93] |
| U373MG, U87MG and CRT-MG | Inhibition of MMP-9 encouraged by mangiferin is associated with the suppression of glioma cell invasion. | [71] | |
| Ovarian | ES-2 and A2780 | The proliferation of cancer cells was suppressed by mangiferin. This compound decreases both the cancer cell invasion and migration. | [94] |
| Bone | Saos-2 and U2OS | Mangiferin decrease the cancer cell viability and proliferative potential. | |
| Oral | 7, 12-dimethylbenz [a] anthracene induced oral cancer | Orally administered mangiferin effectively prevented body weight gain and tumour progression. | [95] |
| Thyroid | TPC-1 | Viability of TPC-1 cells was decreased by mangiferin in a dose-dependent manner and mangiferin brings apoptosis. | [96] |
| Head and neck | CNE2 | Mangiferin inhibits cancer cell proliferation through induction of early apoptosis and G2/M arrest. Furthermore, mRNA and protein levels of Bax were up-regulated and Bcl-2 was to be down-regulated. | [62] |
| Blood | HL-60 | Mangiferin stops cancer cell growth, and cells in the G2/M stage increased in number, and the G2/M phase was arrested. | [27] |
| HL-60 | Mangiferin led to a decrease in the NF-κB p65 and suppressed the expressions of Bcl-xL as well as XIAP. | [97] | |
| HL-60 | Mangiferin increases the accumulation of the Nrf2 protein in HL-60, primarily in the nucleus. | [35] | |
| Multiple myeloma | IM9 cells, RPMI8226 and RPMI1788 | Mangiferin caused a decrease in the mitochondrial membrane potential and increased the number of apoptotic cells. | [36] |
| IM9 cells and RPMI8226 | Mangiferin in combination with an anti-cancer agent decreased the viability of multiple myeloma stem cell lines. | [98] | |
| Pancreas | Mia-PACa2 | Mangiferin increased the expression of LC3 II; in addition, Beclin-1, Bcl-2 decreased, and Bax expression increased dose dependently. | [99] |
4.2. Breast Cancer
4.3. Cervical Cancer
4.4. Prostate Cancer
4.5. Colon Cancer
4.6. Liver Cancer
4.7. Gastric Cancer
4.8. Brain Cancer
4.9. Ovarian Cancer
4.10. Bone Cancer
4.11. Oral Cancer
4.12. Thyroid Caner
4.13. Nasopharyngeal Cancer
4.14. Leukemia
4.15. Multiple Myeloma
4.16. Pancreatic Cancer
5. Synergistic Effects of Mangiferin with Other Therapeutic Agents in Cancer Cells
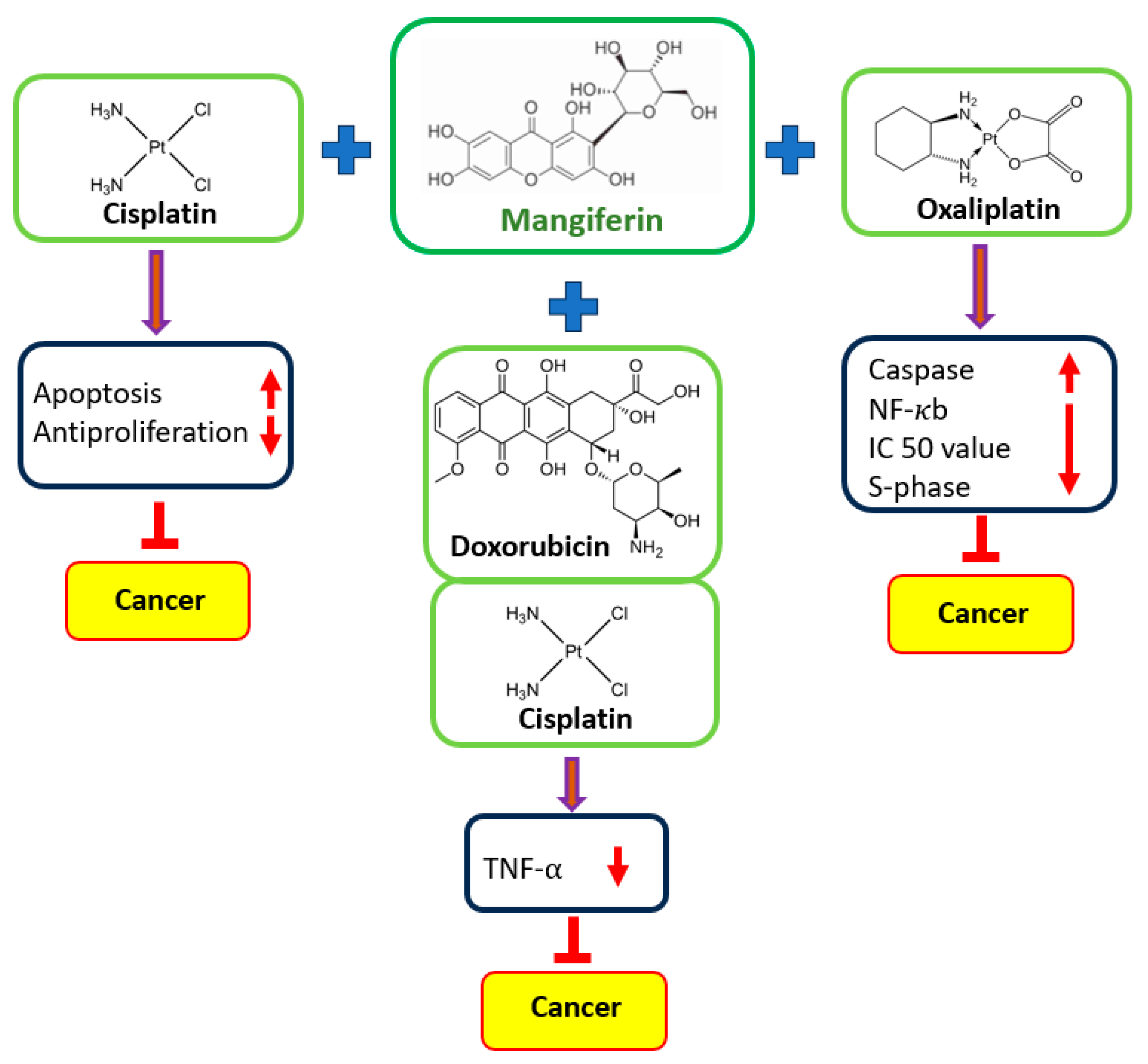
5.1. Acute Myeloid Leukemia
5.2. Colorectal Cancer
5.3. Cervical Cancer
5.4. Breast Cancer
5.5. Colon Cancer
5.6. Lung Cancer
6. Approaches to Improve the Mangiferin Delivery
7. Clinical Studies on Mangiferin
8. Conclusions, Challenges and Future Prospective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Małyszko, J.; Kozłowska, K.; Kozłowski, L.; Małyszko, J. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2016, 32, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Pompa, G.; Saccucci, M.; Di Carlo, G.; Brauner, E.; Valentini, V.; Di Carlo, S.; Gentile, T.; Guarino, G.; Polimeni, A. Survival of dental implants in patients with oral cancer treated by surgery and radiotherapy: A retrospective study. BMC Oral Health 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The potential role of apigenin in cancer prevention and treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, an active compound of Nigella sativa: Role in prevention and treatment of cancer. Curr. Pharm. Biotechnol. 2020, 21, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural products: Implication in cancer prevention and treatment through modulating various biological activities. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Anti-Cancer Agents) 2020, 20, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotective effects of garlic extract against carbon tetrachloride (CCl4)-induced liver injury via modulation of antioxidant, anti-inflammatory activities and hepatocyte architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Matkowski, A.; Kus, P.; Goralska, E.; Wozniak, D. Mangiferin—A bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013, 13, 439–455. [Google Scholar]
- Dong, M.; Li, L.; Li, G.; Song, J.; Liu, B.; Liu, X.; Wang, M. Mangiferin protects against alcoholic liver injury via suppression of inflammation-induced adipose hyperlipolysis. Food Funct. 2020, 11, 8837–8851. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Yin, L.; Huang, C.; Fan, S. Mangiferin relieves CCl4-induced liver fibrosis in mice. Sci. Rep. 2023, 13, 4172. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and cancer: Mechanisms of action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- González, J.C.; Guerra, I.R.; Balmaceda, I.H.; Hernández, R.D. Evaluation of combination treatment effect of mangiferin with cisplatin and 5-fluorouracil in CT26. WT and CKO-K1 cells. Rev. Cuba. Farm. 2014, 48, 658–671. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef]
- Dupré, A.; Malik, H.Z. Inflammation and cancer: What a surgical oncologist should know. Eur. J. Surg. Oncol. 2018, 44, 566–570. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2012; Volume 22, pp. 33–40. [Google Scholar]
- Hemmat, N.; Bannazadeh Baghi, H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog. Dis. 2019, 77, ftz048. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Alzohairy, M.A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. Protective effects of thymoquinone, an active compound of nigella sativa, on rats with Benzo (a) pyrene-Induced Lung injury through regulation of oxidative stress and inflammation. Molecules 2021, 26, 3218. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa date fruit pulp and seed in the management of diseases through in vitro and in silico analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of ginger on inflammatory diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Hadagali, M.D.; Chua, L.S. The anti-inflammatory and wound healing properties of honey. Eur. Food Res. Technol. 2014, 239, 1003–1014. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Khan, A.A.; Aloliqi, A.A.; Ali Syed, M.; Rahmani, A.H. Therapeutic Potential of Ajwa Dates (Phoenix dactylifera) Extract in Prevention of Benzo (a) pyrene-Induced Lung Injury through the Modulation of Oxidative Stress, Inflammation, and Cell Signalling Molecules. Appl. Sci. 2022, 12, 6784. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Yue, L. Inhibitory activity of mangiferin on helicobacter pylori-induced inflammation in human gastric carcinoma cells. Afr. J. Tradit. Complement. Altern. Med. 2016, 14, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.G.; Yao, Y.B.; Yang, J.; Tang, Y.L.; Huang, X. Mangiferin induces cell cycle arrest at G2/M phase through ATR-Chk1 pathway in HL-60 leukemia cells. Genet. Mol. Res. 2015, 14, 4989–5002. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Deng, J.; Tong, R.; Yang, Y.; He, X.; Lv, J.; Wang, H.; Deng, S.; Qi, P.; Zhang, D.; et al. Molecular mechanisms underlying mangiferin-induced apoptosis and cell cycle arrest in A549 human lung carcinoma cells. Mol. Med. Rep. 2016, 13, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, H.; Yang, L.; Li, P. Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA-182. Oncol. Lett. 2016, 11, 817–822. [Google Scholar] [CrossRef]
- Zou, B.; Wang, H.; Liu, Y.; Qi, P.; Lei, T.; Sun, M.; Wang, Y. Mangiferin induces apoptosis in human ovarian adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol. Rep. 2017, 38, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wen, G.; Jin, J.; Chen, Y.; Cao, J.; Xu, A. Mangiferin prevents the growth of gastric carcinoma by blocking the PI3K-Akt signalling pathway. Anti-Cancer Drugs 2018, 29, 167–175. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Kang, C.H.; Choi, Y.H.; Kim, G.Y. Mangiferin inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-κB activity. BMB Rep. 2015, 48, 559. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Li, S.; Zeng, L.; Chen, Y.; Fang, J. Mangiferin increases Nrf2 protein stability by inhibiting its ubiquitination and degradation in human HL60 myeloid leukemia cells. Int. J. Mol. Med. 2014, 33, 1348–1354. [Google Scholar] [CrossRef]
- Zhang, B.P.; Zhao, J.; Li, S.S.; Yang, L.J.; Zeng, L.L.; Chen, Y.; Fang, J. Mangiferin activates Nrf2-antioxidant response element signaling without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro. Acta Pharmacol. Sin. 2014, 35, 257–266. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Kino, T.; Yamagishi, M.; Iida, M.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; Satou, T.; et al. Mangiferin induces apoptosis in multiple myeloma cell lines by suppressing the activation of nuclear factor kappa B-inducing kinase. Chem. Biol. Interact. 2016, 251, 26–33. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Sakamoto, K.; Ichimura, E.; Enomoto, A.; Suzuki, Y.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; et al. Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol. Appl. Pharmacol. 2016, 306, 105–112. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, L.; Chen, Y.; Bao, C.; Deng, J.; Deng, J. Mangiferin inhibits macrophage classical activation via downregulating interferon regulatory factor 5 expression. Mol. Med. Rep. 2016, 14, 1091–1098. [Google Scholar] [CrossRef]
- Qu, S.; Wang, W.; Li, D.; Li, S.; Zhang, L.; Fu, Y.; Zhang, N. Mangiferin inhibits mastitis induced by LPS via suppressing NF-ĸB and NLRP3 signaling pathways. Int. Immunopharmacol. 2017, 43, 85–90. [Google Scholar] [CrossRef]
- Szandruk, M.; Merwid-Ląd, A.; Szeląg, A. The impact of mangiferin from Belamcanda chinensis on experimental colitis in rats. Inflammopharmacology 2018, 26, 571–581. [Google Scholar] [CrossRef] [PubMed]
- du Plessis-Stoman, D.; du Preez, J.; van de Venter, M. Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFκB in cancer cell lines. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 177–184. [Google Scholar] [PubMed]
- García-Rivera, D.; Delgado, R.; Bougarne, N.; Haegeman, G.; Berghe, W.V. Gallic acid indanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDA-MB231 breast cancer cells. Cancer Lett. 2011, 305, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; and Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Aminjan, H.H.; Abtahi, S.R.; Hazrati, E.; Chamanara, M.; Jalili, M.; Paknejad, B. Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation. Life Sci. 2019, 232, 116607. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martinez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR screen identifies a pathway required for paraquat-induced cell deat. Nat. Chem. Biol. 2017, 13, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS ONE 2014, 9, e115364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Han, S.; Zhou, Y.; Qi, B.; Wang, X. Therapeutic effects of mangiferin on sepsis-associated acute lung and kidney injuries via the downregulation of vascular permeability and protection of inflammatory and oxidative damages. Eur. J. Pharm. Sci. 2020, 152, 105400. [Google Scholar] [CrossRef] [PubMed]
- Viswanadh, E.K.; Rao, B.N.; Rao, B.S. Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Hum. Exp. Toxicol. 2010, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Carvalho, A.C.; Trevisan, M.T.; Andrade, G.M.; Nobre-Júnior, H.V.; Moraes, M.O.; Magalhães, H.I.; Morais, T.C.; Santos, F.A. Mangiferin ameliorates 6-hydroxydopamine-induced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Pharmacol. Rep. 2012, 64, 848–856. [Google Scholar] [CrossRef]
- Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Cytoprotective Effect of Mangiferin on Benzo (a) pyrene-Induced Lung Carcinogenesis in Swiss Albino Mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Jayakumar, T.; Nishigaki, I.; Ekambaram, G.; Nishigaki, Y.; Vetriselvi, J.; Sakthisekaran, D. Immunomodulatory effect of mangiferin in experimental animals with benzo (a) pyrene-induced lung carcinogenesis. Int. J. Biomed. Sci. IJBS 2013, 9, 68. [Google Scholar] [CrossRef]
- Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Protective role of mangiferin against Benzo (a) pyrene induced lung carcinogenesis in experimental animals. Biol. Pharm. Bull. 2008, 31, 1053–1058. [Google Scholar] [CrossRef]
- Bisteau, X.; Caldez, M.J.; Kaldis, P. The Complex Relationship Between Liver Cancer and the Cell Cycle: A Story of Multiple Regulations. Cancers 2014, 6, 79–111. [Google Scholar] [CrossRef]
- Sava, G.P.; Fan, H.; Coombes, R.C.; Buluwela, L.; Ali, S. CDK7 Inhibitors as Anticancer Drugs. Cancer Metastasis Rev. 2020, 39, 805–823. [Google Scholar] [CrossRef]
- Shen, Y.; Sherman, J.W.; Chen, X.; Wang, R. Phosphorylation of CDC25C by AMP-Activated Protein Kinase Mediates a Metabolic Checkpoint During Cell-Cycle G/M-Phase Transition. J. Biol. Chem. 2018, 293, 5185–5199. [Google Scholar] [CrossRef]
- Yao, Y.B.; Peng, Z.G.; Liu, Z.F.; Yang, J.; Luo, J. Effects of mangiferin on cell cycle status and CDC2/Cyclin B1 expression of HL-60 cells. Zhong Yao Cai 2010, 33, 81–85. [Google Scholar]
- Yu, L.; Chen, M.; Zhang, R.; Jin, Z. Inhibition of cancer cell growth in gemcitabine-resistant pancreatic carcinoma by mangiferin phytochemical involves induction of autophagy, endogenous ROS production, cell cycle disruption, mitochondrial mediated apoptosis and suppression of cancer cell migration and invasion. J. BUON 2019, 24, 1581–1586. [Google Scholar] [PubMed]
- Arbiser, J.L.; Bonner, M.Y.; Gilbert, L.C. Targeting the duality of cancer. NPJ Precis. Oncol. 2017, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jing, L.; Wang, Q.; Lin, C.-C.; Chen, X.; Diao, J.; Liu, Y.; Sun, X. Bas-PGAM5L-Drp1 complex is required for intrinsic apoptosis execution. Oncotarget 2015, 6, 30017–30034. [Google Scholar] [CrossRef]
- Jan, R. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e187925. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Wang, A.Y.; Huang, Y.Q.; Luo, Y.; Ling, M. Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac. J. Cancer Prev. 2014, 15, 7065–7068. [Google Scholar] [CrossRef]
- Wang, X.; Yuwen, T.; Yanqin, T. Mangiferin inhibits inflammation and cell proliferation, and activates proapoptotic events via NF-κB inhibition in DMBA-induced mammary carcinogenesis in rats. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Matte, A.; Jung, Y.; Ryu, J.; Anand, W.B.; Han, E.Y.; Liu, M.; Carbone, C.; Melisi, D.; Nagasawa, T.; et al. Pathologic angiogenesis in the bone marrow of humanized sickle cell mice is reversed by blood transfusion. Blood J. Am. Soc. Hematol. 2020, 135, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lv, H.; Tang, C.; Li, Y.; Huang, J.; Zhang, H. Mangiferin inhibits cell migration and angiogenesis via PI3K/AKT/mTOR signaling in high glucose- and hypoxia-induced RRCECs. Mol. Med. Rep. 2021, 23, 473. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Hernández, R.; Hernández-Balmaseda, I.; Rodeiro-Guerra, I.; Gonzalez, J.C.; De Wever, O.; Logie, E.; Declerck, K.; Pérez-Novo, C.; Berghe, W.V. Anti-angiogenic effects of mangiferin and mechanism of action in metastatic melanoma. Melanoma Res. 2020, 30, 39–51. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Jung, J.S.; Jung, K.; Kim, D.H.; Kim, H.S. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol. Res. 2012, 66, 95–103. [Google Scholar] [CrossRef]
- Meirson, T.; Gil-Henn, H.; Samson, A.O. Invasion and metastasis: The elusive hallmark of cancer. Oncogene 2020, 39, 2024–2026. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Yun, H.J.; Kim, H.G.; Han, E.H.; Lee, G.W.; Jeong, H.G. Suppression of PMA-induced tumor cell invasion by dihydroartemisinin via inhibition of PKCα/Raf/MAPKs and NF-κB/AP-1-dependent mechanisms. Biochem. Pharmacol. 2010, 79, 1714–1726. [Google Scholar] [CrossRef]
- Horejs, C.M. Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. Eur. J. Cell Biol. 2016, 95, 427–440. [Google Scholar] [CrossRef]
- Fidler, I.J. The role of the organ microenvironment in brain metastasis. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 21, pp. 107–112. [Google Scholar]
- Xiao, J.; Liu, L.; Zhong, Z.; Xiao, C.; Zhang, J. Mangiferin regulates proliferation and apoptosis in glioma cells by induction of microRNA-15b and inhibition of MMP-9 expression. Oncol. Rep. 2015, 33, 2815–2820. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Menegon, S.; Columbano, A.; Giordano, S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, P.; Petracci, E.; Canale, M.; Priano, I.; Capelli, L.; Calistri, D.; Chiadini, E.; Cravero, P.; Rossi, A.; Delmonte, A.; et al. Liquid biopsy for EGFR mutation analysis in advanced non-small-cell lung cancer patients: Thoughts drawn from a real-life experience. Biomedicines 2021, 9, 1299. [Google Scholar] [CrossRef]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef]
- Zhang, C.; Leighl, N.B.; Wu, Y.L.; Zhong, W.Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45. [Google Scholar] [CrossRef]
- Chi, X.J.; Meng, J.J.; Lin, C.Y.; Su, Q.S.; Qin, Y.Y.; Wei, R.H.; Lan, D.; Huang, C. Mangiferin inhibits human lung adenocarcinoma by suppressing MiR-27b and MiR-92a. Evid. -Based Complement. Altern. Med. 2021, 2021, 2822950. [Google Scholar] [CrossRef]
- Zhou, Q.; Hou, K.; Fu, Z. Transferrin-Modified Mangiferin-Loaded SLNs: Preparation, Characterization, and Application in A549 Lung Cancer Cell. Drug Des. Dev. Ther. 2022, 10, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nishigaki, I.; Ekambaram, G.; Sakthisekaran, D. Potent chemopreventive effect of mangiferin on lung carcinogenesis in experimental Swiss albino mice. J. Cancer Res. Ther. 2014, 10, 1033–1039. [Google Scholar] [PubMed]
- Deng, Q.; Tian, Y.X.; Liang, J. Mangiferin inhibits cell migration and invasion through Rac1/WAVE2 signalling in breast cancer. Cytotechnology 2018, 70, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Cuccioloni, M.; Bonfili, L.; Mozzicafreddo, M.; Cecarini, V.; Scuri, S.; Cocchioni, M.; Nabissi, M.; Santoni, G.; Eleuteri, A.M.; Angeletti, M. Mangiferin blocks proliferation and induces apoptosis of breast cancer cells via suppression of the mevalonate pathway and by proteasome inhibition. Food Funct. 2016, 7, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Louisa, M.; Soediro, T.M.; Suyatna, F.D. In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac. J. Cancer Prev. 2014, 15, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, J.C.; Hernández-Balmaseda, I.; Declerck, K.; Pérez-Novo, C.; Logie, E.; Theys, C.; Jakubek, P.; Quiñones-Maza, O.L.; Dantas-Cassali, G.; Carlos dos Reis, D.; et al. Antiproliferative, antiangiogenic, and antimetastatic therapy response by mangiferin in a syngeneic immunocompetent colorectal cancer mouse model involves changes in mitochondrial energy metabolism. Front. Pharmacol. 2021, 12, 670167. [Google Scholar] [CrossRef]
- Yang, G.; Shang, X.; Cui, G.; Zhao, L.; Zhao, H.; Wang, N. Mangiferin attenuated diethynitrosamine-induced hepatocellular carcinoma in Sprague-Dawley rats via alteration of oxidative stress and apoptotic pathway. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Guo, W.; Man, K.; Cheng, C.S.; Chen, Z.; Feng, Y. Repression of WT1-mediated LEF1 transcription by mangiferin governs β-catenin-independent Wnt signalling inactivation in hepatocellular carcinoma. Cell. Physiol. Biochem. 2018, 47, 1819–1834. [Google Scholar] [CrossRef]
- Mu, F.; Liu, T.; Zheng, H.; Xie, X.; Lei, T.; He, X.; Du, S.; Tong, R.; Wang, Y. Mangiferin induces radiosensitization in glioblastoma cells by inhibiting nonhomologous end joining. Oncol. Rep. 2018, 40, 3663–3673. [Google Scholar] [CrossRef]
- Zeng, Z.; Lin, C.; Wang, S.; Wang, P.; Xu, W.; Ma, W.; Wang, J.; Xiang, Q.; Liu, Y.; Yang, J.; et al. Suppressive activities of mangiferin on human epithelial ovarian cancer. Phytomedicine 2020, 76, 153267. [Google Scholar] [CrossRef]
- Liu, M.; Wen, C.; Pan, S. Modulator effect of mangiferin on biochemical characterization in 7, 12-dimethylbenz [a] anthracene induced oral cancer in experimental hamsters. Vet. Med. Sci. 2021, 7, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M. Growth inhibitory effect of mangiferin on thyroid cancer cell line TPC1. Biotechnol. Bioprocess Eng. 2018, 23, 649–654. [Google Scholar] [CrossRef]
- Shoji, K.; Tsubaki, M.; Yamazoe, Y.; Satou, T.; Itoh, T.; Kidera, Y.; Tanimori, Y.; Yanae, M.; Matsuda, H.; Taga, A.; et al. Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-κB in HL-60 cells. Arch. Pharmacal Res. 2011, 34, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsubaki, M.; Kino, T.; Kawamura, A.; Isoyama, S.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; Matsuda, H.; et al. Mangiferin enhances the sensitivity of human multiple myeloma cells to anticancer drugs through suppression of the nuclear factor κB pathway. Int. J. Oncol. 2016, 48, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Pizot, C.; Dragomir, M.; Macacu, A.; Koechlin, A.; Bota, M.; Boyle, P. Global burden of pancreas cancer: Regional disparities in incidence, mortality, and survival. J. Health Inequal. 2019, 5, 96–112. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 70, 593–601. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Mosaddik, A.; Gyawali, R.; Ahn, K.S.; Cho, S.K. Induction of apoptosis by ethanolic extract of mango peel and comparative analysis of the chemical constitutes of mango peel and flesh. Food Chem. 2012, 133, 416–422. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Yan, W.; Yang, D.; Shen, B. Translational bioinformatics for diagnostic and prognostic prediction of prostate cancer in the next-generation sequencing era. BioMed Res. Int. 2013, 2013, 901578. [Google Scholar] [CrossRef]
- Yoshimi, N.; Matsunaga, K.; Katayama, M.; Yamada, Y.; Kuno, T.; Qiao, Z.; Hara, A.; Yamahara, J.; Mori, H. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001, 163, 163–170. [Google Scholar] [CrossRef]
- Saranya, M.; Maheswari, R. Mangiferin a bioactive compound of mangifera indica l on oxidative damage and antioxidant status in n-diethylnitrosoamine induced hepatocellular carcinoma in animal model. J. Pharm. Biol. Sci. 2018, 6, 114. [Google Scholar]
- Yusefi, A.R.; Lankarani, K.B.; Bastani, P.; Radinmanesh, M.; Kavosi, Z. Risk factors for gastric cancer: A systematic review. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 591. [Google Scholar] [PubMed]
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: A global study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Odri, G.A.; Tchicaya-Bouanga, J.; Yoon, D.J.; Modrowski, D. Metastatic progression of osteosarcomas: A review of current knowledge of environmental versus oncogenic drivers. Cancers 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Qin, Y.; Li, C.; Dai, X.; Wu, T.; Yin, W. Mangiferin suppresses human metastatic osteosarcoma cell growth by down-regulating the expression of metalloproteinases-1/2 and parathyroid hormone receptor 1. AMB Express 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.S.; Chen, C.J.; Shih, Y.L.; Peng, S.F.; Chen, Y.L.; Liu, K.C.; Huang, H.C.; Hsueh, S.C.; Chen, K.W.; Lu, H.F.; et al. Mangiferin induces immune responses and evaluates the survival rate in WEHI-3 cell generated mouse leukemia in vivo. Environ. Toxicol. 2021, 36, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.G.; Luo, J.; Xia, L.H.; Chen, Y.; Song, S.J. CML cell line K562 cell apoptosis induced by mangiferin. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2004, 12, 590–594. [Google Scholar] [PubMed]
- Cheng, P.; Peng, Z.G.; Yang, J.; Song, S.J. The effect of mangiferin on telomerase activity and apoptosis in leukemic K562 cells. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2007, 30, 306–309. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute Myeloid Leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- Han, D.; Chen, C.; Zhang, C.; Zhang, Y.; Tang, X. Determination of mangiferin in rat plasma by liquid-liquid extraction with UPLC-MS/MS. J. Pharm. Biomed. Anal. 2009, 51, 260–263. [Google Scholar] [CrossRef]
- Xiao, W.; Hou, J.; Ma, J.; Yu, B.; Ren, J.; Jin, W.; Wu, J.; Zheng, D.; Fan, K. Mangiferin loaded magnetic PCEC microspheres: Preparation, characterization and antitumor activity studies in vitro. Arch. Pharmacal Res 2014, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, H.; Sun, L.; Tong, L.; Zhang, T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 2014, 93, 54–61. [Google Scholar] [CrossRef]
- Razura-Carmona, F.F.; Pérez-Larios, A.; González-Silva, N.; Herrera-Martínez, M.; Medina-Torres, L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A. Mangiferin-loaded polymeric nanoparticles: Optical characterization, effect of Anti-Topoisomerase, I and Cytotoxicity. Cancers 2019, 11, 1965. [Google Scholar] [CrossRef] [PubMed]
- Khoobchandani, M.; Khan, A.; Katti, K.K.; Thipe, V.C.; Al-Yasiri, A.Y.; MohanDoss, D.K.; Nicholl, M.B.; Lugão, A.B.; Hans, C.P.; Katti, K.V. Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci. Rep. 2021, 11, 16797. [Google Scholar] [CrossRef]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugão, A.B.; Katti, K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-198 AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef]
- Yusri, P.Z.; Ghazali, N.F.; Mazlan, N.A.; Lum, P.T.; Noor, A.A.; Mani, S.; Sekar, M. Synthesis and characterization of mangiferin loaded n, o-cmc nanoparticles and its cytotoxic effect on osteosarcoma mg-63 cells. Int. J. Res. Pharm. Sci. 2020, 11, 2136–2145. [Google Scholar] [CrossRef]
- Harsha, P.J.; Thotakura, N.; Kumar, M.; Sharma, S.; Mittal, A.; Khurana, R.K.; Singh, B.; Negi, P.; Raza, K. A novel PEGylated carbon nanotube conjugated mangiferin: An explorative nanomedicine for brain cancer cells. J. Drug Deliv. Sci. Technol. 2019, 53, 101186. [Google Scholar] [CrossRef]
- Na, L.; Zhang, Q.; Jiang, S.; Du, S.; Zhang, W.; Li, Y.; Sun, C.; Niu, Y. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: A double-blind randomized controlled trial. Sci. Rep. 2015, 5, 10344. [Google Scholar] [CrossRef]
- Anaya-Loyola, M.A.; García-Marín, G.; García-Gutiérrez, D.G.; Castaño-Tostado, E.; Reynoso-Camacho, R.; López-Ramos, J.E.; Enciso-Moreno, J.A.; Pérez-Ramírez, I.F. A mango (Mangifera indica L.) juice by-product reduces gastrointestinal and upper respiratory tract infection symptoms in children. Food Res. Int. 2020, 136, 109492. [Google Scholar] [CrossRef]
- Kaliappan, I.; Kammala, A.K.; Ramasamy, M.K.; Dubey, G.P. Structural elucidation of possible metabolic profile of mangiferin by oral and intraperitoneal administration. J. Pharm. Drug Deliv. Res. 2015, 4, 2. [Google Scholar]
- Gu, P.C.; Wang, L.; Han, M.N.; Peng, J.; Shang, J.C.; Pan, Y.Q.; Han, W.L. Comparative pharmacokinetic study of mangiferin in normal and alloxan-induced diabetic rats after oral and intravenous administration by UPLC-MS/MS. Pharmacology 2019, 103, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, B.; Pan, G.; He, L.; Li, Z.; Fan, M.; Jian, L.; Chen, M.; Wang, K.; Huang, C. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab. Dispos. 2012, 40, 2109–2118. [Google Scholar] [CrossRef] [PubMed]

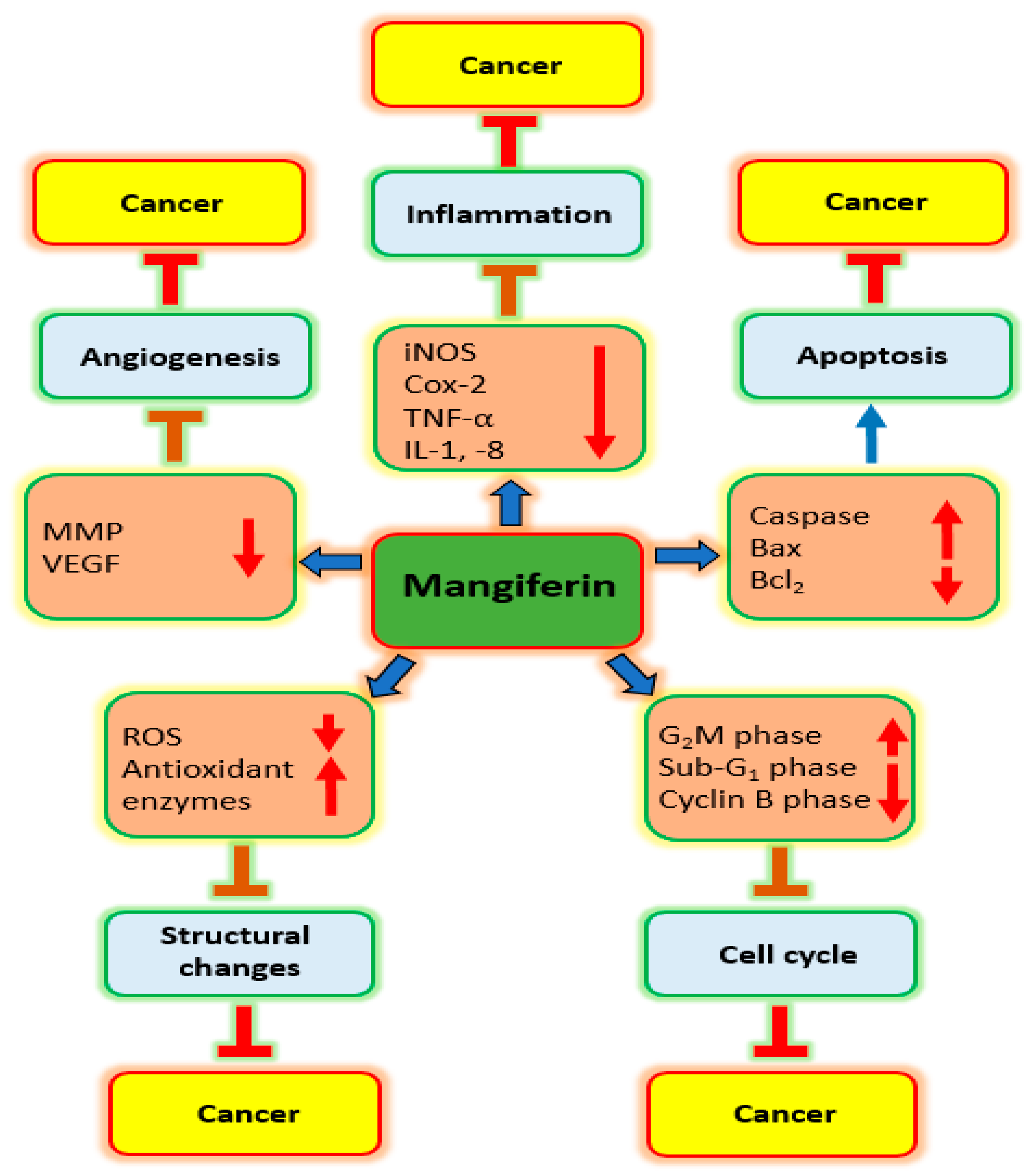

| Action on | Cancer | Cell Line | Dose | Outcome | Refs. |
|---|---|---|---|---|---|
| Inflammation | Gastric | AGS | 10, 20, 50 & 100 μg/mL | iNOS protein expression as well as COX-2 downregulated with increased concentration of mangiferin | [26] |
| Cell cycle | Blood | HL-60 | 20–250 µM | Mangiferin alters cell cycle arrest at the G2/M phase. | [27] |
| Lung | A549 | 25 µg/mL | Mangiferin administration, more cells were arrested in the sub-G1 phase | [28] | |
| Apoptosis | Prostate | PC3 | 20 & 40 µM | Apoptosis of prostate cancer cells was increased by mangiferin treatment | [29] |
| Ovarian | Ovcar3 | 25 µg/mL | Cells treated with mangiferin showed membrane blebbing, shrinkage of cytoplasm and nucleus | [30] | |
| PI3K/Akt | Gastric | SGC-7901 | 5 & 10 µmol/L | Expression of p-mTOR, p-PI3K, and p-Akt, were lowered by mangiferin treatment. | [31] |
| Metalloproteinase | Prostate | LNCaP | 400 µM | Cell stimulation with TNF-α enhanced MMP-9 expression; while mangiferin suppressed this effect | [32] |
| Breast | MDA-MB-231 | 12, 25 & 50 µM | Mangiferin was negatively regulate MMP-9 and -7 | [33] | |
| Epithelial to Mesenchymal Transition | Breast | MDA-MB-231 and BT-549 | 12, 25 & 50 µM | Anticancer potential induced by mangiferin via modulation of MMP-7 and -9, and EMT | [33] |
| Nrf2 | Blood | HL60 | 50, 100 or 200 μM | Dose-dependent increase in the Nrf2 protein level after mangiferin treatment | [34] |
| Blood | HL60 | 50, 100 & 200 mol/L | Mangiferin increased the whole-cell buildup of Nrf2 protein. | [35] |
| Cancer | Cell Lines | Anti-Cancer Drugs/Treatment Type | Outcome of the Study | Refs. |
|---|---|---|---|---|
| Myeloid leukemia | HL60 | Etoposide | Mangiferin decreases the cytotoxicity caused by etoposide in cancer cells, and when combined with a low concentration of etoposide, the treatment even increases the rate of cell inhibition. | [35] |
| Cervix, breast, and colon cancer | HeLa, HT29, and MCF7 | Oxaliplatin | Mangiferin decrease the oxaliplatin IC50 values. A prolonged S-phase of the cell cycle, increased caspase 3 activation. | [41] |
| Breast cancer | MCF-7 | Doxorubicin | Cell viability was decreased substantially when doxorubicin was administered in conjunction with mangiferin. | [102] |
| Colon cancer | CT26.WT | Cisplatin and 5-fluorouracil | Combining mangiferin with 5-fluorouracil and cisplatin promotes cell death and the cytotoxicity of drugs. | [14] |
| Lung cancer | A549 | Etoposide and cisplatin | Mangiferin demonstrated the promising potential of the combination therapy by increasing the antiproliferative capacity of cisplatin on cancer cells. | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alharbi, H.O.A.; Alwanian, W.M.; Alhunayhani, B.A.; Algahtani, M.; Theyab, A.; Almansour, N.M.; Algefary, A.N.; et al. Role of Mangiferin in Management of Cancers through Modulation of Signal Transduction Pathways. Biomedicines 2023, 11, 3205. https://doi.org/10.3390/biomedicines11123205
Rahmani AH, Almatroudi A, Allemailem KS, Alharbi HOA, Alwanian WM, Alhunayhani BA, Algahtani M, Theyab A, Almansour NM, Algefary AN, et al. Role of Mangiferin in Management of Cancers through Modulation of Signal Transduction Pathways. Biomedicines. 2023; 11(12):3205. https://doi.org/10.3390/biomedicines11123205
Chicago/Turabian StyleRahmani, Arshad Husain, Ahmad Almatroudi, Khaled S. Allemailem, Hajed Obaid A. Alharbi, Wanian M. Alwanian, Basmah Awwadh Alhunayhani, Mohammad Algahtani, Abdulrahman Theyab, Nahlah Makki Almansour, Ahmed N. Algefary, and et al. 2023. "Role of Mangiferin in Management of Cancers through Modulation of Signal Transduction Pathways" Biomedicines 11, no. 12: 3205. https://doi.org/10.3390/biomedicines11123205
APA StyleRahmani, A. H., Almatroudi, A., Allemailem, K. S., Alharbi, H. O. A., Alwanian, W. M., Alhunayhani, B. A., Algahtani, M., Theyab, A., Almansour, N. M., Algefary, A. N., Aldeghaim, S. S. A., & Khan, A. A. (2023). Role of Mangiferin in Management of Cancers through Modulation of Signal Transduction Pathways. Biomedicines, 11(12), 3205. https://doi.org/10.3390/biomedicines11123205







