The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens
Abstract
:1. Introduction
2. Estrogens in the Male
2.1. Circulating Levels
| E2 Concentration Range (nM) | Methodology of Measurement | Ref. | |
|---|---|---|---|
| Healthy Men | PCa Patients | ||
| 0.156 | 0.200 * | RIA | [61] |
| 0.110–0.160 | 0.120–0.160 | RIA | [63] |
| 0.114–0.125 | 0.110–0.128 * | Dextran-coated charcoal method | [55] |
| 0.110–0.160 | 0.120–0.160 | RIA | [63] |
| 0.200 | - | RIA | [64] |
| 0.093 | - | Chemiluminescence immunoassay | [65] |
| 0.200 | - | RIA | [66] |
| 0.066–0.221 | 0.066–0.233 | - | [67] |
| 0.125 | 0.121 | RIA | [68] |
| 0.070 | - | Chemiluminescence immunoassay | [69] |
| 0.100–0.150 | 0.050–0.300 | RIA | [70] |
| 0.235 | 0.247 | - | [71] |
| 0.089–0.120 | 0.086–0.106 | Gas chromatography-mass spectrometry | [54] |
| 0.062 | 0.066 | RIA | [72] |
| 0.028–0.167 | - | Chemiluminescence immunoassay | [73] |
| 0.106 | 0.106 | RIA | [74] |
| 0.122 | 0.122 | Heterogeneous competitive magnetic separation assay | [75] |
| 0.028–0.156 | 0.063–0.068 | Chemiluminescence immunoassay | [76] |
| 0.107 | 0.101–0.105 | Chemiluminescence immunoassay | [77] |
| 0.082–0.234 | 0.200 | - | [50] |
| 0.108 | 0.159 * | Enzyme linked immunosorbent assay (ELISA) | [62] |
| 0.132 | - | RIA | [78] |
| 0.103 | - | RIA | [79] |
2.2. Intraprostatic Production
3. Estrogens in Prostate Cancer Therapy
3.1. Old-Times and Withdrawal
| Route of Administration | Compound | Dosage | Ref. |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3×/day | [111] |
| Conjugated estrogens | 1.25–2.5 mg 3×/day | [112,113] | |
| Ethinylestradiol | 0.15–3 mg/day | [112,113] | |
| Ethinylestradiol sulfonate | 1–2 mg 1×/week | [114,115] | |
| DES | 1–3 mg/day | [116] | |
| Dienestrol | 5 mg/day | [117] | |
| Hexestrol | 5 mg/day | [117] | |
| Fosfestrol | 100–480 mg 1–3×/day | [118,119] | |
| Chlorotrianisene | 12–48 mg/day | [120] | |
| Quadrosilan | 900 mg/day | [117] | |
| Estramustine phosphate | 140–1400 mg/day | [121] | |
| Transdermal patch | Estradiol | 2–6× 100 μg/day Scrotal: 1× 100 μg/day | [122,123] |
| Intramuscular or subcutaneous injection | Estradiol benzoate | 1.66 mg 3×/week | |
| Estradiol dipropionate | 5 mg 1×/week | ||
| Estradiol valerate | 10–40 mg 1×/1–2 weeks | [89,124] | |
| Estradiol undecylate | 100 mg 1×/4 weeks | [125] | |
| Polyestradiol phosphate | Alone: 160–320 mg 1×/4 weeks With oral EE: 40–80 mg 1×/4 weeks | [126,127] | |
| Estrone | 2–4 mg 2–3×/week | [91] | |
| Intravenous injection | Fosfestrol | 300–1200 mg 1–7×/week | [118,119] |
| Estramustine phosphate | 240–450 mg/day | [128] |
3.2. Synthetic Estrogens
4. Estrogens as Prostate Carcinogens
4.1. Cell Survival and Neoplastic Transformation
| Type of Study | Cell Line/Animal Model | Compound | Concentration/Dose | Assay Model/Method of Administration | Time of Treatment | Assay | Effect | Activated Pathway | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| In vitro | BPH-1 | E2 | 1 µM | 6-well plates | Up to 6 days/6 weeks | Sulforhodamine-B Comet | ↑ Proliferation Neoplastic transformation | Genotoxic mechanism | [151] |
| BPH-1 | 2-OHE2 | 1 µM | 6-well plates | Up to 9 days/6 weeks | Sulforhodamine-B Comet | ↑ Proliferation Neoplastic transformation | Genotoxic mechanism | [151] | |
| BPH-1 | 2-OHE2 | 10 µM | 6-well plates | 2, 4, 7 and 10 days | Sulforhodamine-B | ↓ Cell viability | Cytotoxicity | [151] | |
| BPH-1 | 4-OHE2 | 1 µM | 6-well plates | Up to 9 days/6 weeks | Sulforhodamine-B Comet | ↑ Proliferation Neoplastic transformation | Genotoxic mechanism | [151] | |
| LNCaP | E2 | 0.01 µM | Multi-well plates | 24 h | BrdU incorporation | ↑ Proliferation | MAPK (ERK1/2) and association of AR, ERβ, and Src | [175] | |
| LNCaP | E2 | 0.01–5 µM | 96-well plates | 3 days | MTS PSA measurement | ↑ PSA expression ↑ Cell growth | ER mediated | [176] | |
| LNCaP | αE2 | 0.01–5 µM | 96-well plates | 3 days | MTS PSA measurement | ↑ PSA expression ↑ Cell growth | ER mediated | [176] | |
| LNCaP | E2 | 0.01 µM | 24 h/48 h | WST-1 based | ↑ Proliferation ↑ PSA expression | ↑ c-Myc | [177] | ||
| DU145 | E2 | 0.01 µM | 12-well plates | 24 h | Soft-agar colony formation TUNEL | ↑ Anchorage-independent growth ↓ Apoptosis | ↓ FOXO1 | [14] | |
| DU145 | E2 | 1 µM | 12-well plates | 24 h | Soft-agar colony formation TUNEL | ↑ Anchorage-independent growth ↓ Apoptosis | ↓ FOXO1 | [14] | |
| PC3 | E2 | 0.01 µM | 12-well plates | 24 h | Soft-agar colony formation TUNEL | ↑ Anchorage-independent growth ↓ Apoptosis | ↓ FOXO1 | [14] | |
| PC3 | E2 | 1 µM | 12-well plates | 24 h | Soft-agar colony formation TUNEL | ↑ Anchorage-independent growth ↓ Apoptosis | ↓ FOXO1 | [14] | |
| EPN | E2 | 0.01 µM | 60 mm dishes | 27 h/5 min | BrdU incorporation | ↑ Proliferation | MAPK (ERK1/2) and association of AR, ERα, and Src | [153] | |
| NRP-152 | E2 | 1 or 3 µM | 96-well plates/ 60 mm dishes | 2–6 weeks and 3–48 h | Soft agar colony formation Comet Flow cytometry | Neoplastic transformation | Genotoxic mechanism | [13] | |
| MDA-Pca 2b | βE2 | 0.01–5 µM | 96-well plates | 3 days | MTS PSA measurement | ↑ Cell growth | ER mediated | [176] | |
| In vivo | BALB/c mice | DES * | 2 mg DES (and 18 mg cholesterol) | Pellet s.c. implant | Up to 3 weeks | PCNA-immunohistochemistry | ↑ Proliferation | ERα | [164] |
| Nude mice | E2 | 1 or 3 µM | E2-NRP-152 cells s.c. injection in the flanks | 4–8 weeks | Tumor size measurement | ↑ Neoplastic transformation | Genotoxic mechanism | [13] | |
| Athymic nude mice | β-estradiol 3-benzoate * | 250 μg/kg (early exposure) + 2.5 mg pellet (secondary exposure) | S.c injection | 90 days (early exposure) + 110 days (secondary exposure) | Ki-67 quantification | ↑ Proliferation | PI3K-Akt pathway | [156] | |
| Xenograft BALB/ cA-nu castrated mice using DU145 and PC3 cells | E2 | 0.18 mg | Pellet s.c. implant | 25 to 35 days | Tumor volume and weight measurement TUNEL | ↑ Tumor growth | ↓ FOXO1 ERβ and KLF5 pathway | [14] | |
| Athymic nude mice Tissue recombinants composed of mouse urogenital mesenchyme plus an immortalized nontumorigenic human prostatic epithelial cell line (BPH-1) grown under the kidney capsule | E2 | 2.5 or 10 mg (plus Testosterone) | Silastic implants | 1–4 months | Immunohistochemistry Growth indices Determination of cancer incidence | ↑ Proliferation ↑ Apoptosis | Akt pathway | [152] | |
| NBL/Cr rats | 4-OHE2 | 5 μg/day | Silastic implants | 13 weeks | Measurement of DNA adducts Measurement of 8-hydroxyguanosine Measurement of lipid hydroperoxides | ↑ Inflammation ↑ Dysplasia | - | [160] | |
| NBL/Cr rats | 2F-E2 * | 5 μg/day | Silastic implants | 13 weeks | Measurement of DNA adducts Measurement of 8-hydroxyguanosine Measurement of lipid hydroperoxides | ↑ Inflammation ↑ Dysplasia | - | [160] | |
| NBL/Cr rats | E2 (+Testosterone) | S.c. silastic implants | 91 weeks | Hematoxylin and eosin staining | ↑ Prostate adenocarcinoma development | - | [159] | ||
| NBL/Cr rats | DES * (+Testosterone) | S.c. silastic implants | 91 weeks | Hematoxylin and eosin staining | ↑ Prostate adenocarcinoma development | - | [159] | ||
| Sprague- Dawley (Hsd:SD) rats | E2 (+Testosterone) | S.c. silastic implants | 75 weeks | Hematoxylin and eosin staining | ↑ Prostate adenocarcinoma development | - | [159] | ||
| Sprague- Dawley (Hsd:SD) rats | DES * (+Testosterone) | S.c. silastic implants | 75 weeks | Hematoxylin and eosin staining | ↑ Prostate adenocarcinoma development | - | [159] | ||
| CD-1 mice | E2 (+Testosterone) | Silastic implants | 4 months | Histopathological grading Immunohistochemistry Histological analysis | ↑ Prostate size | - | [144] | ||
| C57BL/6 mice | E2 (+Testosterone) | Silastic implants | 4 months | Histopathological grading Immunohistochemistry Histological analysis | ↑ Prostate size Prostatic intraepithelial neoplastic lesions’ induction | ↓ α-actin ↓ E-cadherin | [144] | ||
| C57BL/ 6 X J129 mice | E2 (+Testosterone) | Silastic implants | 4 months | Histopathological grading Immunohistochemistry Histological analysis | ↑ Prostate size | - | [144] |
4.2. Progression of Disease and Metastization
| Type of Study | Cell Line/Animal Model | Compound | Concentration/Dose | Assay Model/Method of Administration | Time of Treatment | Assay | Effect | Activated Pathway | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| In vitro | LNCaP | E2 | 0.01 µM | 24 h/72 h | Immunoblotting Wound healing Invasion | ↑ Migration ↑ Invasion EMT | ↑ N-cadherin ↑ Vimentin ↓ E-cadherin | [177] | |
| LNCaP | E2 | 0.01 µM | 12-well plates | 5 days | Alkaline phosphatase activity | ↑ Osteoblast-like properties | ERα signaling | [15] | |
| LNCaP | E2 | 0.01 µM | 96-well plates | 72 h | Wound healing Invasion | ↑ Migration ↑ Invasion | SOX4 up-regulation | [155] | |
| 22Rv1 | E2 | 0.01 µM | 24-well Boyden chambers | 18 h | Migration | ↑ Migration | ERα signaling | [15] | |
| RWPE-1 | E2 | 0.01 µM | 96-well plates | 48 h/72 h 8 days | RT-PCR analysis Western blot | EMT | ERα signaling | [182] | |
| C4–2 | E2 | 0.01 µM | 24-well Boyden chambers | 18 h | Migration | ↑ Migration | ERα signaling | [15] | |
| PacMetUT1 isolated from the lymph node metastasis | E2 | 0.01 µM | 24-well Boyden chambers | 18 h | Migration | ↑ Migration EMT | ERα signaling | [15] | |
| DU145 | Conditioned medium PrSC from BPH patients or WPMY-1 cells treated with E2 | 0.01 µM | 15-cm dishes | 48 h | Transwell migration Wound healing | ↑ Migration | ERα signaling ENO1 effects via its plasminogen binding domain | [186] | |
| DU145 | E2 | 0.01 µM | 24 h | Wound healing Invasion | ↑ Migration ↑ Invasion | ERα signaling ERβ signaling Galectin-3 signaling | [30] | ||
| DU145 | E2 | 0.01 µM | 48 h | Invasion analysis Colony formation analysis (soft agar) | ↑ Invasion | SRC | [197] | ||
| PC3 | E2 | 0.01 µM | Invasion analysis Colony formation analysis (soft agar) | ↑ Invasion | SRC | [197] | |||
| PC3 | Conditioned medium PrSC from BPH patients or WPMY-1 cells treated with E2 | 0.01 µM | 15-cm dishes | 48 h | Transwell migration Wound healing | ↑ Migration | ERα signaling ENO1 effects via its plasminogen binding domain | [186] | |
| PC3 | E2 | 0.01 µM | Incubation of cells 24-well, 8.0-μm pore size | Overnight | Matrigel invasion | ↑ Invasion | ERα/matrix metalloproteinase 12 axis activation | [193] | |
| PC3 | E2 | 0.0001 and 0.01 µM | 24 h | Wound healing Invasion Colony formation analysis (soft agar) | ↑ Migration ↑ Invasion | ERα and ERβ signaling β-catenin signaling | [198] | ||
| In vivo | CD-1, C57BL/6, and C57BL/ 6 x J129 mice | E2 | 2.5 or 10 mg (+Testosterone) | Silastic implants | 4 months | Histological analysis Immunohistochemistry | Carcinogenesis | ERα signaling | [144] |
| NBL/Cr rats | E2 | Silastic implants s.c. | 16 weeks | Measurement of DNA adducts Measurement of 8-hydroxyguanosine Measurement of lipid hydroperoxides | ↑ Tumor incidence | DNA adduct Oxidative DNA damage Lipid peroxidation | [160] | ||
| NBL/Cr rats | 4-OHE2 | 5 μg/day | Silastic implants | 13 weeks | Measurement of DNA adducts Measurement of 8-hydroxyguanosine Measurement of lipid hydroperoxides | Carcinogenesis | - | [160] | |
| NBL/Cr rats | 2F-E2 * | 5 μg/day | Silastic implants | 13 weeks | Measurement of DNA adducts Measurement of 8-hydroxyguanosine Measurement of lipid hydroperoxides | Carcinogenesis | - | [160] | |
| Athymic nude mice Tissue recombinants composed of mouse urogenital mesenchyme plus an immortalized nontumorigenic human prostatic epithelial cell line (BPH-1) grown under the kidney capsule | E2 | 2.5 or 10 mg (+Testosterone) | Silastic implants | 1–4 months | Immunohistochemistry Growth indices Determination of cancer incidence | ↑ Progression ↑ Metastization | Akt pathway | [152] |
5. Evidence of Estrogens as Protective Agents
5.1. Antiproliferative and Proapoptotic Effects
5.1.1. Endogenous Estrogens
5.1.2. Synthetic Estrogens
5.2. Suppression of Metastization
6. The Phytoestrogen Scope
6.1. Classification and Structure
6.2. Sources and Metabolism
6.3. Mechanisms of Action
6.4. Phytoestrogens Actions against Prostate Cancer
6.4.1. Apigenin
6.4.2. Chrysin
6.4.3. Biochanin A
6.4.4. Daidzein
6.4.5. Equol
6.4.6. Formononetin
6.4.7. Genistein
6.4.8. Naringenin
6.4.9. Kaempferol
6.4.10. Myricetin
6.4.11. Quercetin
6.4.12. Coumestrol
6.4.13. Resveratrol
6.4.14. Diosgenin
7. Lessons Learned, Conclusions, and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar] [CrossRef] [PubMed]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA A Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Res. 1941, 1, 293–297. [Google Scholar]
- Group, V.A.C.-o.U.R. Treatment and survival of patients with cancer of the prostate. Surg. Gynecol. Obstet. 1967, 124, 1011–1017. [Google Scholar]
- Bailar, J.C., III; Byar, D.P.; Veterans Administration Cooperative Urological Research Group. Estrogen treatment for cancer of the prostate. Early results with 3 doses of diethylstilbestrol and placebo. Cancer 1970, 26, 257–261. [Google Scholar] [CrossRef]
- Byar, D.P. The Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer 1973, 32, 1126–1130. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Isaacs, J.T. A history of prostate cancer treatment. Nat. Rev. Cancer 2002, 2, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K. The evolving role of estrogen therapy in prostate cancer. Clin. Prostate Cancer 2002, 1, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, Y.; Yuen, M.T.; Zou, C.; Danielpour, D.; Chan, F.L. 17-Beta-estradiol induces neoplastic transformation in prostatic epithelial cells. Cancer Lett. 2011, 304, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Osakabe, A.; Waku, T.; Suzuki, T.; Akaogi, K.; Fujimura, T.; Homma, Y.; Inoue, S.; Yanagisawa, J. Estrogen Exhibits a Biphasic Effect on Prostate Tumor Growth through the Estrogen Receptor beta-KLF5 Pathway. Mol. Cell. Biol. 2016, 36, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tai, Q.; Gu, X.; Schmitz, J.; Poullard, A.; Fajardo, R.J.; Mahalingam, D.; Chen, X.; Zhu, X.; Sun, L.Z. Estrogen and estrogen receptor alpha promotes malignancy and osteoblastic tumorigenesis in prostate cancer. Oncotarget 2015, 6, 44388–44402. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-de-Arellano, A.; Pereira-Suárez, A.L.; Rico-Fuentes, C.; López-Pulido, E.I.; Villegas-Pineda, J.C.; Sierra-Diaz, E. Distribution and Effects of Estrogen Receptors in Prostate Cancer: Associated Molecular Mechanisms. Front. Endocrinol. 2021, 12, 811578. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.G.; Wardan, H.; Davis, I.D.; Pezaro, C.; Sluka, P. Effects of estrogen receptor signaling on prostate cancer carcinogenesis. Transl. Res. 2020, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.I.; Cardoso, H.J.; Socorro, S. The Role of GPER Signaling in Carcinogenesis: A Focus on Prostate Cancer. In Recent Trends in Cancer Biology Spotlight on Signaling Cascades and microRNAs Cell Signaling Pathways and microRNAs in Cancer Biology; Fayyaz, S., Farooqi, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 59–117. [Google Scholar]
- Jensen, E.V.; DeSombre, E.R. Estrogen-receptor interaction. Science 1973, 182, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.-A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, B.W. A life-long search for the molecular pathways of steroid hormone action. Mol. Endocrinol. 2005, 19, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Göttlicher, M.; Heck, S.; Herrlich, P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J. Mol. Med. 1998, 76, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Risbridger, G.P. The dual, opposing roles of estrogen in the prostate. Ann. N. Y. Acad. Sci. 2009, 1155, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.P.; Ellem, S.J.; McPherson, S.J. Estrogen action on the prostate gland: A critical mix of endocrine and paracrine signaling. J. Mol. Endocrinol. 2007, 39, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Attia, D.M.; Ederveen, A.G. Opposing roles of ERα and ERβ in the genesis and progression of adenocarcinoma in the rat ventral prostate. Prostate 2012, 72, 1013–1022. [Google Scholar] [CrossRef]
- Souza, D.S.; Macheroni, C.; Vicente, C.M.; Cavalheiro, R.P.; Campo, V.L.; Porto, C.S. Estrogen receptors regulate galectin-3 in androgen-independent DU-145 prostate cancer cells. Oncol. Rep. 2023, 49, 8530. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, Y.; Tai, R.; Zhang, N. Estrogen receptor β suppresses inflammation and the progression of prostate cancer. Mol. Med. Rep. 2019, 19, 3555–3563. [Google Scholar] [CrossRef]
- Moorthy, H.K.; Laxman Prabhu, G.G.; Venugopal, P. The resurgence of estrogens in the treatment of castration-resistant prostate cancer. Indian J. Urol. IJU J. Urol. Soc. India 2019, 35, 189–196. [Google Scholar] [CrossRef]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Fu, B.C.; Bauer, S.R.; Pernar, C.H.; Chan, J.M.; Van Blarigan, E.L.; Giovannucci, E.L.; Kenfield, S.A.; Mucci, L.A. Association of plant-based diet index with prostate cancer risk. Am. J. Clin. Nutr. 2022, 115, 662–670. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, S.; Zhou, M.; Yu, W.; Zhang, Y.; He, X. Phytoestrogens and risk of prostate cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 2015, 13, 231. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Serttas, R.; Turkekul, K.; Dibirdik, I.; Bilir, A. The flavonoid apigenin reduces prostate cancer CD44+ stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016, 162, 77–86. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Serttas, R.; Erdogan, Z. The natural flavonoid apigenin sensitizes human CD44+ prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017, 88, 210–217. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Kanaan, Y.M.; Beyene, D.A.; Naab, T.J.; Copeland, R.L.; Tsai, H.L.; Kanarek, N.F.; Hudson, T.S. Risk of prostate cancer in African-American men: Evidence of mixed effects of dietary quercetin by serum vitamin D status. Prostate 2015, 75, 1376–1383. [Google Scholar] [CrossRef]
- van Die, M.D.; Williams, S.G.; Emery, J.; Bone, K.M.; Taylor, J.M.; Lusk, E.; Pirotta, M.V. A Placebo-Controlled Double-Blinded Randomized Pilot Study of Combination Phytotherapy in Biochemically Recurrent Prostate Cancer. Prostate 2017, 77, 765–775. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Siddiqui, I.A.; Panackal, J.E.; Mintie, C.A.; Ahmad, N. Quercetin-Resveratrol Combination for Prostate Cancer Management in TRAMP Mice. Cancers 2020, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Raut, P.W.; Baruah, M.M.; Sharma, A. Combination of quercetin and 2-methoxyestradiol inhibits epithelial-mesenchymal transition in PC-3 cell line via Wnt signaling pathway. Future Sci. OA 2021, 7, Fso747. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xie, B.; Liang, Z.; Chen, J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol. Lett. 2018, 15, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D.; Vadgama, J.V. Sensitization to docetaxel in prostate cancer cells by green tea and quercetin. J. Nutr. Biochem. 2015, 26, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Magyar, C.E.; Elshimali, Y.; Heber, D.; Vadgama, J.V. Green tea and quercetin sensitize PC-3 xenograft prostate tumors to docetaxel chemotherapy. J. Exp. Clin. Cancer Res. 2016, 35, 73. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Lou, W.; Gao, A.C.; Nadiminty, N. Quercetin targets hnRNPA1 to overcome enzalutamide resistance in prostate cancer cells. Mol. Cancer Ther. 2017, 16, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Marcsek, Z.; Kocsis, Z.; Szende, B.; Tompa, A. Effect of formaldehyde and resveratrol on the viability of Vero, HepG2 and MCF-7 cells. Cell Biol. Int. 2007, 31, 1214–1219. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Mukhtar, H.; Ahmad, N. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells. Photochem. Photobiol. 2008, 84, 415–421. [Google Scholar] [CrossRef]
- Gido, S.; Jenny, A.V.; Wiebke, A.; Johannes, H. Circulating steroid hormone variations throughout different stages of prostate cancer. Endocr.-Relat. Cancer 2017, 24, R403–R420. [Google Scholar] [CrossRef]
- Wang, Q.; Rangiah, K.; Mesaros, C.; Snyder, N.W.; Vachani, A.; Song, H.; Blair, I.A. Ultrasensitive quantification of serum estrogens in postmenopausal women and older men by liquid chromatography-tandem mass spectrometry. Steroids 2015, 96, 140–152. [Google Scholar] [CrossRef]
- MacKintosh, F.R.; Sprenkle, P.C.; Walter, L.C.; Rawson, L.; Karnes, R.J.; Morrell, C.H.; Kattan, M.W.; Nawaf, C.B.; Neville, T.B. Age and Prostate-Specific Antigen Level Prior to Diagnosis Predict Risk of Death from Prostate Cancer. Front. Oncol. 2016, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.M.; Lee, M.T.; Lam, H.M.; Leung, Y.K. Estrogens and prostate cancer: Etiology, mediators, prevention, and management. Endocrinol. Metab. Clin. North Am. 2011, 40, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, Y.; Raynaud, J.P.; Radulescu, C.; Fiet, J.; Giton, F.; Dreyfus, J.F.; Ghoneim, T.P.; Lebret, T.; Botto, H. Sexual steroids in serum and prostatic tissue of human non-cancerous prostate (STERPROSER trial). Prostate 2017, 77, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Jackson, M.A.; Jones, G.W.; Williams, A.O.; Rao, M.S.; Rajguru, S. Blood hormone profiles in prostate cancer patients in high-risk and low-risk populations. Cancer 1981, 48, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Chadid, S.; Barber, J.R.; Nelson, W.G.; Gurel, B.; Lucia, M.S.; Thompson, I.M.; Goodman, P.J.; Stanczyk, F.Z.; Parnes, H.L.; Lippman, S.M.; et al. The association between serum sex steroid hormone concentrations and intraprostatic inflammation in men without prostate cancer and irrespective of clinical indication for biopsy in the placebo arm of the Prostate Cancer Prevention Trial. Prostate 2020, 80, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Till, C.; Kristal, A.R.; Goodman, P.J.; Hsing, A.W.; Tangen, C.M.; Platz, E.A.; Stanczyk, F.Z.; Reichardt, J.K.; Tang, L.; et al. Serum estrogen levels and prostate cancer risk in the prostate cancer prevention trial: A nested case-control study. Cancer Causes Control CCC 2011, 22, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Mohr, B.A.; Feldman, H.A.; Kalish, L.A.; Longcope, C.; McKinlay, J.B. Are serum hormones associated with the risk of prostate cancer? Prospective results from the Massachusetts Male Aging Study. Urology 2001, 57, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Dorgan, J.F.; Albanes, D.; Virtamo, J.; Heinonen, O.P.; Chandler, D.W.; Galmarini, M.; McShane, L.M.; Barrett, M.J.; Tangrea, J.; Taylor, P.R. Relationships of serum androgens and estrogens to prostate cancer risk: Results from a prospective study in Finland. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 1998, 7, 1069–1074. [Google Scholar]
- Meunier, M.E.; Neuzillet, Y.; Raynaud, J.P.; Radulescu, C.; Ghoneim, T.; Fiet, J.; Giton, F.; Rouanne, M.; Dreyfus, J.F.; Lebret, T.; et al. Sex steroids in serum and prostatic tissue of human cancerous prostate (STERKPROSER trial). Prostate 2019, 79, 272–280. [Google Scholar] [CrossRef]
- Grosman, H.; Fabre, B.; Mesch, V.; Lopez, M.A.; Schreier, L.; Mazza, O.; Berg, G. Lipoproteins, sex hormones and inflammatory markers in association with prostate cancer. Aging Male Off. J. Int. Soc. Study Aging Male 2010, 13, 87–92. [Google Scholar] [CrossRef]
- Usoro, A.J.; Obot, A.S.; Ekaidem, I.S.; Akaiso, O.E.; Udoh, A.E.; Akinloye, O. Serum Testosterone, 17beta-Estradiol and PSA Levels in Subjects with Prostate Disorders. Indian J. Clin. Biochem. IJCB 2015, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Garland, C.; McPhillips, J.B.; Khaw, K.-T.; Wingard, D.L. A Prospective, Population-based Study of Androstenedione, Estrogens, and Prostatic Cancer. Cancer Res. 1990, 50, 169–173. [Google Scholar]
- Belanger, A.; Candas, B.; Dupont, A.; Cusan, L.; Diamond, P.; Gomez, J.L.; Labrie, F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J. Clin. Endocrinol. Metab. 1994, 79, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, T.M.; Hao, W.J.; Li, J.; Liu, L.; Zhu, B.P.; Li, X.Y. Correlation between sex hormone levels and obesity in the elderly male. Aging Male Off. J. Int. Soc. Study Aging Male 2012, 15, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Weiss, N.S.; Stanczyk, F.Z.; Lewis, S.K.; DiTommaso, D.; Etzioni, R.; Barnett, M.J.; Goodman, G.E. Endogenous sex hormones and prostate cancer risk: A case-control study nested within the Carotene and Retinol Efficacy Trial. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2003, 12, 1410–1416. [Google Scholar]
- Roddam, A.W.; Allen, N.E.; Appleby, P.; Key, T.J. Endogenous sex hormones and prostate cancer: A collaborative analysis of 18 prospective studies. J. Natl. Cancer Inst. 2008, 100, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Gann, P.H.; Hennekens, C.H.; Ma, J.; Longcope, C.; Stampfer, M.J. Prospective study of sex hormone levels and risk of prostate cancer. J. Natl. Cancer Inst. 1996, 88, 1118–1126. [Google Scholar] [CrossRef]
- Hagiuda, J.; Ishikawa, H.; Marumo, K. Serum oestradiol levels in male partners of infertile couples. Andrologia 2015, 47, 669–673. [Google Scholar] [CrossRef]
- Hammond, G.L.; Kontturi, M.; Vihko, P.; Vihko, R. Serum steroids in normal males and patients with prostatic diseases. Clin. Endocrinol. 1978, 9, 113–121. [Google Scholar] [CrossRef]
- Hsing, A.W.; Comstock, G.W. Serological precursors of cancer: Serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 1993, 2, 27–32. [Google Scholar]
- Nomura, A.; Heilbrun, L.K.; Stemmermann, G.N.; Judd, H.L. Prediagnostic serum hormones and the risk of prostate cancer. Cancer Res. 1988, 48, 3515–3517. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, S.; Olaizola, I.; Alastrue, A.; Martinez, E.; Granada, M.L.; Balibrea, J.M.; Moreno, P.; Serra, A.; Navarro-Diaz, M.; Romero, R.; et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes. Surg. 2012, 22, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Leitzmann, M.F.; Rifai, N.; Kantoff, P.W.; Chen, Y.C.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2005, 14, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Abdollah, F.; Capitanio, U.; Suardi, N.; Gallina, A.; Castagna, G.; Clementi, M.C.; Briganti, A.; Rigatti, P.; Montorsi, F. Circulating sex steroids and prostate cancer: Introducing the time-dependency theory. World J. Urol. 2013, 31, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Schnoeller, T.J.; Steinestel, J.; Zengerling, F.; Schrader, A.J.; Jentzmik, F. Serum 17beta-estradiol fails as a marker in identification of aggressive tumour disease in patients with localized prostate cancer. World J. Urol. 2015, 33, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Severi, G.; Morris, H.A.; MacInnis, R.J.; English, D.R.; Tilley, W.; Hopper, J.L.; Boyle, P.; Giles, G.G. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2006, 15, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Kaufman, J.M.; Deslypere, J.P.; Thomas, G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J. Clin. Endocrinol. Metab. 1993, 76, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Zumoff, B.; Strain, G.W.; Kream, J.; O’Connor, J.; Levin, J.; Fukushima, D.K. Obese young men have elevated plasma estrogen levels but obese premenopausal women do not. Metab. Clin. Exp. 1981, 30, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Davis, S.R. Minireview: Aromatase and the Regulation of Estrogen Biosynthesis—Some New Perspectives. Endocrinology 2001, 142, 4589–4594. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in Male Physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Miller, W.L. Molecular Biology of Steroid Hormone Synthesis*. Endocr. Rev. 1988, 9, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Krolik, M.; Milnerowicz, H. The effect of using estrogens in the light of scientific research. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2012, 21, 535–543. [Google Scholar]
- Farkas, S.; Szabó, A.; Hegyi, A.E.; Török, B.; Fazekas, C.L.; Ernszt, D.; Kovács, T.; Zelena, D. Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions. Biomedicines 2022, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Williams, G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol. Cell. Endocrinol. 2012, 351, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, H.; Soloway, M.S. Immunohistochemical evidence of the existence and localization of aromatase in human prostatic tissues. Prostate 1992, 21, 309–314. [Google Scholar] [CrossRef]
- Takase, Y.; Lévesque, M.-H.; Luu-The, V.; El-Alfy, M.; Labrie, F.; Pelletier, G. Expression of Enzymes Involved in Estrogen Metabolism in Human Prostate. J. Histochem. Cytochem. 2006, 54, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Risbridger, G.P. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J. Steroid Biochem. Mol. Biol. 2010, 118, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Machioka, K.; Mizokami, A.; Yamaguchi, Y.; Izumi, K.; Hayashi, S.; Namiki, M. Active estrogen synthesis and its function in prostate cancer-derived stromal cells. Anticancer Res. 2015, 35, 221–227. [Google Scholar] [PubMed]
- Prossnitz, E.R.; Barton, M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009, 89, 89–97. [Google Scholar] [CrossRef]
- Takizawa, I.; Lawrence, M.G.; Balanathan, P.; Rebello, R.; Pearson, H.B.; Garg, E.; Pedersen, J.; Pouliot, N.; Nadon, R.; Watt, M.J.; et al. Estrogen receptor alpha drives proliferation in PTEN-deficient prostate carcinoma by stimulating survival signaling, MYC expression and altering glucose sensitivity. Oncotarget 2015, 6, 604–616. [Google Scholar] [CrossRef]
- Morais-Santos, M.; Werneck-Gomes, H.; Campolina-Silva, G.H.; Santos, L.C.; Mahecha, G.A.B.; Hess, R.A.; Oliveira, C.A. Basal Cells Show Increased Expression of Aromatase and Estrogen Receptor alpha in Prostate Epithelial Lesions of Male Aging Rats. Endocrinology 2018, 159, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Cheboub, A.; Regouat, N.; Djidjik, R.; Slimani, A.; Hadj-Bekkouche, F. Short-term aromatase inhibition induces prostatic alterations in adult wistar rat: A biochemical, histopathological and immunohistochemical study. Acta Histochem. 2019, 121, 151441. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Byar, D.P.; Corle, D.K. Hormone therapy for prostate cancer: Results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. A Publ. Natl. Cancer Inst. 1988, 7, 165–170. [Google Scholar] [CrossRef]
- Lyrenäs, S.; Carlström, K.; Bäckström, T.; Von Schoultz, B. A comparison of serum oestrogen levels after percutaneous and oral administration of oestradiol-17β. BJOG Int. J. Obstet. Gynaecol. 1981, 88, 181–187. [Google Scholar] [CrossRef]
- de Liguieres, B.; Basdevant, A. Differential metabolic tolerance between oral and percutaneous administration of estradiol in postmenopausal women. Osteoporosis 1987, 2, 1120–1131. [Google Scholar]
- Bosset, P.-O.; Albiges, L.; Seisen, T.; de la Motte Rouge, T.; Phé, V.; Bitker, M.-O.; Rouprêt, M. Current role of diethylstilbestrol in the management of advanced prostate cancer. BJU Int. 2012, 110, E826–E829. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, Y.; Nakata, S.; Kobayashi, M.; Kosaku, N.; Fukabori, Y.; Yamanaka, H. Moderate dose diethylstilbestrol diphosphate therapy in hormone refractory prostate cancer. Scand. J. Urol. Nephrol. 2001, 35, 283–287. [Google Scholar] [PubMed]
- de Voogt, H.J.; Smith, P.H.; Pavone-Macaluso, M.; de Pauw, M.; Suciu, S.; Members of the European Organization for Research on Treatment of Cancer Urological Group. Cardiovascular side effects of diethylstilbestrol, cyproterone acetate, medroxyprogesterone acetate and estramustine phosphate used for the treatment of advanced prostatic cancer: Results from European Organization for Research on Treatment of Cancer trials 30761 and 30762. J. Urol. 1986, 135, 303–307. [Google Scholar]
- von Schoultz, B.; Carlström, K.; Collste, L.; Eriksson, A.; Henriksson, P.; Pousette, Å.; Stege, R. Estrogen therapy and liver function—Metabolic effects of oral and parenteral administration. Prostate 1989, 14, 389–395. [Google Scholar] [CrossRef]
- Henriksson, P.; Blombäck, M.; Eriksson, A.; Stege, R.; Carlström, K. Effect of parenteral oestrogen on the coagulation system in patients with prostatic carcinoma. Br. J. Urol. 1990, 65, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P.O.; Henriksson, P. Parenteral estrogen versus total androgen ablation in the treatment of advanced prostate carcinoma: Effects on overall survival and cardiovascular mortality. Urology 2000, 55, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P.O.; Ala-Opas, M.; Brekkan, E.; Damber, J.E.; Damber, L.; Hagerman, I.; Haukaas, S.; Henriksson, P.; Iversen, P.; Pousette, Å. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer-Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand. J. Urol. Nephrol. 2002, 36, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Olov Hedlund, P.; Damber, J.-E.; Hagerman, I.; Haukaas, S.; Henriksson, P.; Iversen, P.; Johansson, R.; Klarskov, P.; Lundbeck, F.; Rasmussen, F. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer: Part 2. Final evaluation of the Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand. J. Urol. Nephrol. 2008, 42, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.E.; Cafferty, F.H.; Alhasso, A.A.; Rosen, S.D.; Sundaram, S.K.; Freeman, S.C.; Pollock, P.; Jinks, R.C.; Godsland, I.F.; Kockelbergh, R. Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: The randomised, phase 2 MRC PATCH trial (PR09). Lancet Oncol. 2013, 14, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.E.; Godsland, I.F.; Kynaston, H.; Clarke, N.W.; Rosen, S.D.; Morgan, R.C.; Pollock, P.; Kockelbergh, R.; Lalani, E.N.; Dearnaley, D. Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer. BJU Int. 2008, 102, 442–445. [Google Scholar] [CrossRef]
- Stein, M.; Goodin, S.; Doyle-Lindrud, S.; Silberberg, J.; Kane, M.; Metzger, D.; Eddy, S.; Shih, W.; DiPaola, R.S. Transdermal estradiol in castrate and chemotherapy resistant prostate cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, CR260. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Shah, S.I.; Duong, T.; Abel, P.; Langley, R.E. Androgen deprivation therapy and the re-emergence of parenteral estrogen in prostate cancer. Oncol. Hematol. Rev. 2014, 10, 42. [Google Scholar] [CrossRef]
- Silva, É.D.; Ferreira, U.; Matheus, W.; Faria, E.F.; Silva, G.D.; Saito, M.; De Souza, A.A.; Laranjo, A.; Clark, O.; Magna, L.A. Goserelin versus leuprolide in the chemical castration of patients with prostate cancer. Int. Urol. Nephrol. 2012, 44, 1039–1044. [Google Scholar] [CrossRef]
- Otto, C.; Rohde-Schulz, B.; Schwarz, G.; Fuchs, I.; Klewer, M.; Brittain, D.; Langer, G.; Bader, B.; Prelle, K.; Nubbemeyer, R.; et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 2008, 149, 4846–4856. [Google Scholar] [CrossRef]
- Thomas, J.A.; Keenan, E.J. Principles of Endocrine Pharmacology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Oettel, M. Estrogens and antiestrogens in the male. In Estrogens and Antiestrogens II; Springer: Berlin/Heidelberg, Germany, 1999; pp. 505–571. [Google Scholar]
- Höfling, G.; Heynemann, H. Die orale Östrogentherapie des fortgeschrittenen Prostatakarzinoms—Anlaß für eine Neubewertung? Der. Urol. B 1998, 38, 165–170. [Google Scholar] [CrossRef]
- Hinkelbein, W.; Miller, K.; Wiegel, T. Prostatakarzinom Urologische und Strahlentherapeutische Aspekte; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Lupelescu, A. (Ed.) Hormones and hormonotherapy. In Hormones and Vitamins in Cancer Treatment; CRC Press: Boca Raton, FL, USA, 1990; pp. 33–90. [Google Scholar]
- Weinstein, R.S. Prostate Cancer|International Perspectives in Urology; Jacobi, G.H., Hohenfellner, R., Eds.; Williams & Wilkins Co.: Baltimore, MD, USA, 1983; Volume 3. [Google Scholar]
- Sweetman, S.C. Sex hormones and their modulators. In Martindale: The Complete Drug Reference; The Pharmaceutical Press: London, UK, 2009; p. 2097. [Google Scholar]
- Hager, H. Hagers Handbuch der Pharmazeutischen Praxis Band 8 Stoffe EO; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Dao, T.L. Pharmacology and Clinical utility of Hormones in Hormone related Neoplasms. In Antineoplastic and Immunosuppressive Agents; Springer: Berlin/Heidelberg, Germany, 1975; pp. 170–192. [Google Scholar]
- Perry, C.M.; McTavish, D. Estramustine phosphate sodium. Drugs Aging 1995, 7, 49–74. [Google Scholar] [CrossRef]
- Ockrim, J.; Lalani, E.-N.; Abel, P. Therapy insight: Parenteral estrogen treatment for prostate cancer—A new dawn for an old therapy. Nat. Rev. Clin. Oncol. 2006, 3, 552. [Google Scholar] [CrossRef] [PubMed]
- Ockrim, J.; Lalani, E.-N.; Laniado, M.; Carter, S.S.C.; Abel, P. Transdermal estradiol therapy for advanced prostate cancer—Forward to the past? J. Urol. 2003, 169, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Alikhan, M.; Spencer, H.; Carter, G. Phase I trial of intramuscular estradiol valerate (I/ME) in hormone refractory prostate cancer. J. Clin. Oncol. 2004, 22, 4726. [Google Scholar] [CrossRef]
- Norman, G.; Dean, M.; Langley, R.; Hodges, Z.; Ritchie, G.; Parmar, M.; Sydes, M.; Abel, P.; Eastwood, A. Parenteral oestrogen in the treatment of prostate cancer: A systematic review. Br. J. Cancer 2008, 98, 697. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.L.; Crawford, E.D. Estrogens in the treatment of prostate cancer. J. Urol. 1995, 154, 1991–1998. [Google Scholar] [CrossRef]
- Carlström, K.; Stege, R.; Henriksson, P.; Grande, M.; Gunnarsson, P.O.; Pousette, A. Possible bone-preserving capacity of high-dose intramuscular depot estrogen as compared to orchidectomy in the treatment of patients with prostatic carcinoma. Prostate 1997, 31, 193–197. [Google Scholar] [CrossRef]
- Bergenheim, A.T.; Henriksson, R. Pharmacokinetics and pharmacodynamics of estramustine phosphate. Clin. Pharmacokinet. 1998, 34, 163–172. [Google Scholar] [CrossRef]
- Radovick, S. Estrogenic regulation of the GnRH neuron. Front. Endocrinol. 2012, 3, 52. [Google Scholar] [CrossRef]
- Warde, P.; Mason, M.; Ding, K.; Kirkbride, P.; Brundage, M.; Cowan, R.; Gospodarowicz, M.; Sanders, K.; Kostashuk, E.; Swanson, G. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet 2011, 378, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Ehara, H.; Koji, T.; Deguchi, T.; Yoshii, A.; Nakano, M.; Nakane, P.K.; Kawada, Y. Expression of estrogen receptor in diseased human prostate assessed by non-radioactive in situ hybridization and immunohistochemistry. Prostate 1995, 27, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Domińska, K.; Kowalski, A.; Ochędalski, T.; Rębas, E. Effects of testosterone and 17β-estradiol on angiotensin-induced changes in tyrosine kinase activity in the androgen-independent human prostate cancer cell line, DU145. Int. J. Mol. Med. 2017, 40, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.; Nelson, P.S.; Vessella, R.; Kalhorn, T.; Hess, D.; Corey, E. Estradiol suppresses tissue androgens and prostate cancer growth in castration resistant prostate cancer. BMC Cancer 2010, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Green, S.; Greene, G.; Krust, A.; Bornert, J.-M.; Jeltsch, J.-M.; Staub, A.; Jensen, E.; Scrace, G.; Waterfield, M. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. USA 1985, 82, 7889–7893. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H.; Fixemer, T.; Hunsicker, I.; Remberger, K. Estrogen Receptor Expression in Prostate Cancer and Premalignant Prostatic Lesions. Am. J. Pathol. 1999, 155, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.Y.; Leav, I.; Lau, K.M.; Ho, S.M.; Pflueger, S.M. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate 2002, 52, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Makela, S.; Strauss, L.; Kuiper, G.; Valve, E.; Salmi, S.; Santti, R.; Gustafsson, J.A. Differential expression of estrogen receptors alpha and beta in adult rat accessory sex glands and lower urinary tract. Mol. Cell. Endocrinol. 2000, 164, 109–116. [Google Scholar] [CrossRef]
- Schulze, H.; Claus, S. Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate 1990, 16, 331–343. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Maehara, I.; Orikasa, S.; Sasano, H. Immunolocalization of oestrogen and progesterone receptors in prostatic hyperplasia and carcinoma. Histopathology 1996, 28, 163–168. [Google Scholar] [CrossRef]
- Bonkhoff, H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Linja, M.J.; Savinainen, K.J.; Tammela, T.L.J.; Isola, J.J.; Visakorpi, T. Expression of ERα and ERβ in prostate cancer. Prostate 2003, 55, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Recchia, A.G.; Carpino, A.; Vivacqua, A.; Fasanella, G.; Rago, V.; Pezzi, V.; Briand, P.-A.; Picard, D.; Ando, S. Oestrogen receptor beta is required for androgen-stimulated proliferation of LNCaP prostate cancer cells. J. Mol. Endocrinol. 2004, 32, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Härkönen, P.L.; Mäkelä, S.I. Role of estrogens in development of prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ricke, W.A.; McPherson, S.J.; Bianco, J.J.; Cunha, G.R.; Wang, Y.; Risbridger, G.P. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.J.; McNeil, C.M.; Musgrove, E.A.; Sutherland, R.L. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: The potential roles of c-Myc, cyclin D1 and cyclin E. Endocr.-Relat. Cancer 2005, 12, S47–S59. [Google Scholar] [CrossRef] [PubMed]
- Pugeat, M.M.; Dunn, J.F.; Nisula, B.C. Transport of steroid hormones: Interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J. Clin. Endocrinol. Metab. 1981, 53, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Koong, L.Y.; Watson, C.S. Direct estradiol and diethylstilbestrol actions on early- versus late-stage prostate cancer cells. Prostate 2014, 74, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, L.; Queiroz, J.F.; Magalhães, L.M.; Silva, A.M.; Segundo, M.A. Analysis of 17-β-estradiol and 17-α-ethinylestradiol in biological and environmental matrices—A review. Microchem. J. 2016, 126, 243–262. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, D.; Wang, B.; Han, Y.-H.; Balimane, P.; Yang, Z.; Sinz, M.; Rodrigues, A.D. Pharmacokinetic drug interactions involving 17α-ethinylestradiol. Clin. Pharmacokinet. 2007, 46, 133–157. [Google Scholar] [CrossRef]
- Ho, C.K.; Nanda, J.; Chapman, K.E.; Habib, F.K. Oestrogen and benign prostatic hyperplasia: Effects on stromal cell proliferation and local formation from androgen. J. Endocrinol. 2008, 197, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Mosli, H.A.; Tolba, M.F.; Al-Abd, A.M.; Abdel-Naim, A.B. Catechol estrogens induce proliferation and malignant transformation in prostate epithelial cells. Toxicol. Lett. 2013, 220, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ricke, W.A.; Ishii, K.; Ricke, E.A.; Simko, J.; Wang, Y.; Hayward, S.W.; Cunha, G.R. Steroid hormones stimulate human prostate cancer progression and metastasis. Int. J. Cancer 2006, 118, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, P.; Kisslinger, A.; Sinisi, A.A.; Abbondanza, C.; Tramontano, D. 17beta-estradiol-induced activation of ERK1/2 through endogenous androgen receptor-estradiol receptor alpha-Src complex in human prostate cells. Int. J. Oncol. 2003, 23, 797–801. [Google Scholar] [PubMed]
- Arnold, J.T.; Le, H.; McFann, K.K.; Blackman, M.R. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E573–E584. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, J.; Wang, L.; Shen, C.; Su, B.; Qi, M.; Hu, J.; Gao, W.; Tan, W.; Han, B. Estrogen induces androgen-repressed SOX4 expression to promote progression of prostate cancer cells. Prostate 2015, 75, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Saffarini, C.M.; McDonnell-Clark, E.V.; Amin, A.; Huse, S.M.; Boekelheide, K. Developmental exposure to estrogen alters differentiation and epigenetic programming in a human fetal prostate xenograft model. PLoS ONE 2015, 10, e0122290. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Timms, B.G.; Montano, M.M.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Dhar, M.D.; Ganjam, V.; Parmigiani, S.; Welshons, W.V. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc. Natl. Acad. Sci. USA 1997, 94, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Merk, F.B.; Kwan, P.W.-L.; Ho, S.-M. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact noble rats. Prostate 1989, 15, 23–40. [Google Scholar] [CrossRef]
- Bosland, M.C.; Ford, H.; Horton, L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17 beta or diethylstilbestrol. Carcinogenesis 1995, 16, 1311–1317. [Google Scholar] [CrossRef]
- Ozten, N.; Vega, K.; Liehr, J.; Huang, X.; Horton, L.; Cavalieri, E.L.; Rogan, E.G.; Bosland, M.C. Role of Estrogen in Androgen-Induced Prostate Carcinogenesis in NBL Rats. Horm. Cancer 2019, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Tisell, L.E. Morphology of rat prostatic lobes and seminal vesicles after long-term estrogen treatment. Acta Pathol. Microbiol. Et Immunol. Scandinavica. Sect. A Pathol. 1982, 90, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Aumuller, G.; Funke, P.J.; Hahn, A.; Hoffbauer, G.; Tunn, U.; Neumann, F. Phenotypic modulation of the canine prostate after long-term treatment with androgens and estrogens. Prostate 1982, 3, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Cunha, G.R.; Yonemura, C.U.; Kawamura, J. Temporal and spatial factors in diethylstilbestrol-induced squamous metaplasia of the developing human prostate. Hum. Pathol. 1988, 19, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.P.; Wang, H.; Frydenberg, M.; Cunha, G. The metaplastic effects of estrogen on mouse prostate epithelium: Proliferation of cells with basal cell phenotype. Endocrinology 2001, 142, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Bongiovanni, A.M. Effect of prenatal estrogen exposure on male genitalia. Pediatrics 1978, 62, 1160–1165. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Devanesan, P.; Bosland, M.C.; Badawi, A.F.; Rogan, E.G. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: Implications for estrogen-induced initiation of prostate cancer. Carcinogenesis 2002, 23, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, M.; Shiina, H.; Tokizane, T.; Deguchi, M.; Hirata, H.; Hinoda, Y.; Okayama, N.; Suehiro, Y.; Urakami, S.; et al. Catechol-O-methyltransferase gene polymorphisms in benign prostatic hyperplasia and sporadic prostate cancer. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2006, 15, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Gao, Y.; Cao, Y.; Gao, F.; Jian, L. An updating meta-analysis of the GSTM1, GSTT1, and GSTP1 polymorphisms and prostate cancer: A HuGE Review. Prostate 2009, 69, 662–688. [Google Scholar] [CrossRef]
- Nock, N.L.; Bock, C.; Neslund-Dudas, C.; Beebe-Dimmer, J.; Rundle, A.; Tang, D.; Jankowski, M.; Rybicki, B.A. Polymorphisms in glutathione S-transferase genes increase risk of prostate cancer biochemical recurrence differentially by ethnicity and disease severity. Cancer Causes Control 2009, 20, 1915. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, M.; Qi, X.-L.; Liu, F.; Mao, Z.-J.; Zhang, D.-H. Effect of NQO1 C609T polymorphism on prostate cancer risk: A meta-analysis. OncoTargets Ther. 2014, 7, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, É.; Laverdière, I.; Audet-Walsh, É.; Caron, P.; Rouleau, M.; Fradet, Y.; Lacombe, L.; Guillemette, C. Steroidogenic Germline Polymorphism Predictors of Prostate Cancer Progression in the Estradiol Pathway. Clin. Cancer Res. 2014, 20, 2971–2983. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.; Rogan, E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: Keynote lecture. Ann. N. Y. Acad. Sci. 2006, 1089, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Mosli, H.A.; Al-Abd, A.M.; El-Shaer, M.A.; Khedr, A.; Khedr, A.; Gazzaz, F.S.; Abdel-Naim, A.B. Local inflammation influences oestrogen metabolism in prostatic tissue. BJU Int. 2012, 110, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.P.; Hofland, J.; Foster, P.A. In touch with your feminine side: How oestrogen metabolism impacts prostate cancer. Endocr.-Relat. Cancer 2016, 23, R249–R266. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Barone, M.V.; Ametrano, D.; Zannini, M.S.; Abbondanza, C.; et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000, 19, 5406–5417. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, L.; Cai, L.Q.; Tan, C.; Imperato-McGinley, J.; Zhu, Y.S. Inhibition of aberrant androgen receptor induction of prostate specific antigen gene expression, cell proliferation and tumor growth by 17α-estradiol in prostate cancer. J. Urol. 2011, 185, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Erzurumlu, Y.; Dogan, H.K.; Catakli, D.; Aydogdu, E.; Muhammed, M.T. Estrogens drive the endoplasmic reticulum-associated degradation and promote proto-oncogene c-Myc expression in prostate cancer cells by androgen receptor/estrogen receptor signaling. J. Cell Commun. Signal. 2023, 17, 793–811. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Limonta, P.; Gagliano, N. Epithelial-To-Mesenchymal Transition Markers and CD44 Isoforms Are Differently Expressed in 2D and 3D Cell Cultures of Prostate Cancer Cells. Cells 2019, 8, 143. [Google Scholar] [CrossRef]
- Shi, X.; Peng, Y.; Du, X.; Liu, H.; Klocker, H.; Lin, Q.; Shi, J.; Zhang, J. Estradiol promotes epithelial-to-mesenchymal transition in human benign prostatic epithelial cells. Prostate 2017, 77, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Thobe, M.N.; Clark, R.J.; Bainer, R.O.; Prasad, S.M.; Rinker-Schaeffer, C.W. From prostate to bone: Key players in prostate cancer bone metastasis. Cancers 2011, 3, 478–493. [Google Scholar] [CrossRef]
- Megas, G.; Chrisofos, M.; Anastasiou, I.; Tsitlidou, A.; Choreftaki, T.; Deliveliotis, C. Estrogen receptor (α and β) but not androgen receptor expression is correlated with recurrence, progression and survival in post prostatectomy T3N0M0 locally advanced prostate cancer in an urban Greek population. Asian J. Androl. 2015, 17, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shi, J.; Cheng, S.; Zhu, Y.; Zhao, X.; Yang, K.; Du, X.; Klocker, H.; Yang, X.; Zhang, J. Estrogen promotes prostate cancer cell migration via paracrine release of ENO1 from stromal cells. Mol. Endocrinol. 2012, 26, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Altenberg, B.; Greulich, K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 2004, 84, 1014–1020. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Jaiswal, R.K.; Varshney, A.K.; Yadava, P.K. Diversity and functional evolution of the plasminogen activator system. Biomed. Pharmacother. 2018, 98, 886–898. [Google Scholar] [CrossRef]
- Vervoort, S.J.; van Boxtel, R.; Coffer, P.J. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene 2013, 32, 3397–3409. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Yang, X.; Chang, Y.W.Y.; Qi, M.; Zhou, Z.; Zhang, J.; Han, B. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013, 16, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cao, J.; Tian, L.; Shen, Y.; Yang, X.; Lin, Q.; Zhang, R.; Liu, H.; Du, X.; Shi, J.; et al. Aromatase-induced endogenous estrogen promotes tumour metastasis through estrogen receptor-alpha/matrix metalloproteinase 12 axis activation in castration-resistant prostate cancer. Cancer Lett. 2019, 467, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Nuttall, R.K.; Liu, S.; Edwards, D.R.; Yong, V.W. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 2006, 66, 11771–11780. [Google Scholar] [CrossRef]
- Chung, I.C.; Chen, L.C.; Chung, A.K.; Chao, M.; Huang, H.Y.; Hsueh, C.; Tsang, N.M.; Chang, K.P.; Liang, Y.; Li, H.P.; et al. Matrix metalloproteinase 12 is induced by heterogeneous nuclear ribonucleoprotein K and promotes migration and invasion in nasopharyngeal carcinoma. BMC Cancer 2014, 14, 348. [Google Scholar] [CrossRef]
- Nabha, S.M.; dos Santos, E.B.; Yamamoto, H.A.; Belizi, A.; Dong, Z.; Meng, H.; Saliganan, A.; Sabbota, A.; Bonfil, R.D.; Cher, M.L. Bone marrow stromal cells enhance prostate cancer cell invasion through type I collagen in an MMP-12 dependent manner. Int. J. Cancer 2008, 122, 2482–2490. [Google Scholar] [CrossRef]
- Lombardi, A.P.G.; Cavalheiro, R.P.; Porto, C.S.; Vicente, C.M. Estrogen Receptor Signaling Pathways Involved in Invasion and Colony Formation of Androgen-Independent Prostate Cancer Cells PC-3. Int. J. Mol. Sci. 2021, 22, 1153. [Google Scholar] [CrossRef]
- Lombardi, A.P.G.; Vicente, C.M.; Porto, C.S. Estrogen Receptors Promote Migration, Invasion and Colony Formation of the Androgen-Independent Prostate Cancer Cells PC-3 Through β-Catenin Pathway. Front. Endocrinol. 2020, 11, 184. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Vijayababu, M.R.; Ilangovan, R.; Senthilkumar, K.; Venkataraman, P.; Aruldhas, M.M.; Arunakaran, J. Effect of 17β-estradiol on apoptosis, IGF system components and gelatinases A and B in prostate cancer cells (PC-3). Clin. Chim. Acta 2007, 377, 70–78. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.K.; Cai, L.Q.; Tan, C.; Imperato-McGinley, J.L.; Zhu, Y.S. 17alpha-estradiol inhibits LAPC-4 prostatic tumor cell proliferation in cell cultures and tumor growth in xenograft animals. Prostate 2007, 67, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.D.; Yang, K.; Mao, Q.Q.; Kong, D.B.; Zheng, X.Y.; Xie, L.P. Estrogen in combination with 5-azacitidine up-regulates p75NTR expression and induces apoptosis in 22Rv1 prostate cancer cells. Mol. Med. Rep. 2009, 2, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.I.; Correia, S.; Vaz, C.V.; Cardoso, H.J.; Gomes, I.M.; Marques, R.; Maia, C.J.; Socorro, S. Estrogens down-regulate the stem cell factor (SCF)/c-KIT system in prostate cells: Evidence of antiproliferative and proapoptotic effects. Biochem. Pharmacol. 2016, 99, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.; Quinn, J.E.; Emond, M.J.; Buhler, K.R.; Brown, L.G.; Vessella, R.L. Inhibition of androgen-independent growth of prostate cancer xenografts by 17beta-estradiol. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 1003–1007. [Google Scholar]
- Landström, M.; Bergh, A.; Tomic, R.; Damber, J.E. Estrogen treatment combined with castration inhibits tumor growth more effectively than castration alone in the Dunning R3327 rat prostatic adenocarcinoma. Prostate 1990, 17, 57–68. [Google Scholar] [CrossRef]
- Landström, M.; Eklöv, S.; Colosetti, P.; Nilsson, S.; Damber, J.E.; Bergh, A.; Funa, K. Estrogen induces apoptosis in a rat prostatic adenocarcinoma: Association with an increased expression of TGF-beta 1 and its type-I and type-II receptors. Int. J. Cancer 1996, 67, 573–579. [Google Scholar] [CrossRef]
- Westin, P.; Brändström, A.; Damber, J.E.; Bergh, A. Castration plus oestrogen treatment induces but castration alone suppresses epithelial cell apoptosis in an androgen-sensitive rat prostatic adenocarcinoma. Br. J. Cancer 1995, 72, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Garcia, G.E.; Slaga, T.J. 2-methoxyestradiol blocks cell-cycle progression at G2/M phase and inhibits growth of human prostate cancer cells. Mol. Carcinog. 2001, 31, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Qadan, L.R.; Perez-Stable, C.M.; Anderson, C.; D’Ippolito, G.; Herron, A.; Howard, G.A.; Roos, B.A. 2-Methoxyestradiol induces G2/M arrest and apoptosis in prostate cancer. Biochem. Biophys. Res. Commun. 2001, 285, 1259–1266. [Google Scholar] [CrossRef]
- Ray, G.; Dhar, G.; Van Veldhuizen, P.J.; Banerjee, S.; Saxena, N.K.; Sengupta, K.; Banerjee, S.K. Modulation of cell-cycle regulatory signaling network by 2-methoxyestradiol in prostate cancer cells is mediated through multiple signal transduction pathways. Biochemistry 2006, 45, 3703–3713. [Google Scholar] [CrossRef]
- Horny, C.; Balasubashini, M.S.; Komanduri, K.; Ganapathy, M.; Yeh, I.T.; Ghosh, R.; Kumar, A.P. 2-methoxyestradiol Prevents LNCaP Tumor Development in Nude Mice: Potential Role of G2/M Regulatory Proteins. J. Cell Death 2009, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reiner, T.; de las Pozas, A.; Gomez, L.A.; Perez-Stable, C. Low dose combinations of 2-methoxyestradiol and docetaxel block prostate cancer cells in mitosis and increase apoptosis. Cancer Lett. 2009, 276, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Ganapathy, M.; Alworth, W.L.; Chan, D.C.; Kumar, A.P. Combination of 2-methoxyestradiol (2-ME2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J Steroid Biochem. Mol. Biol. 2009, 113, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Blaukat, A.; Fu, X.; Heldin, N.E.; Landström, M. Mechanisms for 2-methoxyestradiol-induced apoptosis of prostate cancer cells. FEBS Lett. 2002, 531, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Xie, J.; Olumi, A.F.; Ghosh, R.; Kumar, A.P. Activation of AKR1C1/ERβ induces apoptosis by downregulation of c-FLIP in prostate cancer cells: A prospective therapeutic opportunity. Oncotarget 2015, 6, 11600–11613. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Nakamura, M.; Ishida, E.; Kishi, M.; Matsuyoshi, S.; Konishi, N. The molecular mechanism of sensitization to Fas-mediated apoptosis by 2-methoxyestradiol in PC3 prostate cancer cells. Mol. Carcinog. 2004, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davoodpour, P.; Bergström, M.; Landström, M. Effects of 2-methoxyestradiol on proliferation, apoptosis and PET-tracer uptake in human prostate cancer cell aggregates. Nucl. Med. Biol. 2004, 31, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Davoodpour, P.; Landström, M. 2-Methoxyestradiol-induced apoptosis in prostate cancer cells requires Smad7. J. Biol. Chem. 2005, 280, 14773–14779. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, M.; Ghosh, R.; Jianping, X.; Zhang, X.; Bedolla, R.; Schoolfield, J.; Yeh, I.T.; Troyer, D.A.; Olumi, A.F.; Kumar, A.P. Involvement of FLIP in 2-methoxyestradiol-induced tumor regression in transgenic adenocarcinoma of mouse prostate model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 1601–1611. [Google Scholar] [CrossRef]
- Sato, F.; Fukuhara, H.; Basilion, J.P. Effects of hormone deprivation and 2-methoxyestradiol combination therapy on hormone-dependent prostate cancer in vivo. Neoplasia 2005, 7, 838–846. [Google Scholar] [CrossRef]
- Yang, F.; Song, L.; Wang, H.; Wang, J.; Xu, Z.; Xing, N. Combination of Quercetin and 2-Methoxyestradiol Enhances Inhibition of Human Prostate Cancer LNCaP and PC-3 Cells Xenograft Tumor Growth. PLoS ONE 2015, 10, e0128277. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.E.; Wisniewski, H.G.; Lucia, M.S.; Arevalo, N.; Slaga, T.J.; Kraft, S.L.; Strange, R.; Kumar, A.P. 2-Methoxyestradiol inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate: Role of tumor necrosis factor-alpha-stimulated gene 6. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Shughrue, P.J.; Merchenthaler, I.; Gustafsson, J.A. The estrogen receptor beta subtype: A novel mediator of estrogen action in neuroendocrine systems. Front. Neuroendocr. 1998, 19, 253–286. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Ström, A.; Gustafsson, J. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 2014, 33, 4213–4225. [Google Scholar] [CrossRef] [PubMed]
- Dondi, D.; Piccolella, M.; Biserni, A.; Della Torre, S.; Ramachandran, B.; Locatelli, A.; Rusmini, P.; Sau, D.; Caruso, D.; Maggi, A.; et al. Estrogen receptor beta and the progression of prostate cancer: Role of 5alpha-androstane-3beta,17beta-diol. Endocr. -Relat. Cancer 2010, 17, 731–742. [Google Scholar] [CrossRef]
- Guerini, V.; Sau, D.; Scaccianoce, E.; Rusmini, P.; Ciana, P.; Maggi, A.; Martini, P.G.; Katzenellenbogen, B.S.; Martini, L.; Motta, M.; et al. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits prostate cancer cell migration through activation of the estrogen receptor beta subtype. Cancer Res. 2005, 65, 5445–5453. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, P.; Christopoulos, P.F.; Koutsilieris, M. The Role of Estrogen Receptor β in Prostate Cancer. Mol. Med. 2014, 20, 427–434. [Google Scholar] [CrossRef]
- Kowalska, K.; Piastowska-Ciesielska, A.W. Oestrogens and oestrogen receptors in prostate cancer. SpringerPlus 2016, 5, 522. [Google Scholar] [CrossRef]
- Lennartsson, J.; Rönnstrand, L. Stem Cell Factor Receptor/c-Kit: From Basic Science to Clinical Implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, P.; Zoli, W.; Medri, L.; Amadori, D.; Saragoni, L.; Barbanti, F.; Calistri, D.; Silvestrini, R. c-kit and SCF expression in normal and tumor breast tissue. Breast Cancer Res. Treat. 2004, 83, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Autorino, R.; D’Armiento, F.P.; Mignogna, C.; De Laurentiis, M.; De Sio, M.; D’Armiento, M.; Damiano, R.; Vecchio, G.; De Placido, S. Expression of proto-oncogene c-kit in high risk prostate cancer. Eur. J. Surg. Oncol. (EJSO) 2004, 30, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Nurse, P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 1989, 342, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Nurse, P. Universal control mechanism regulating onset of M-phase. Nature 1990, 344, 503–508. [Google Scholar] [CrossRef]
- Perez-Stable, C. 2-Methoxyestradiol and paclitaxel have similar effects on the cell cycle and induction of apoptosis in prostate cancer cells. Cancer Lett. 2006, 231, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; O’Rourke, K.; Tewari, M.; Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995, 81, 505–512. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis by death factor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef]
- Krueger, A.; Baumann, S.; Krammer, P.H.; Kirchhoff, S. FLICE-inhibitory proteins: Regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 2001, 21, 8247–8254. [Google Scholar] [CrossRef]
- Irmler, M.; Thome, M.; Hahne, M.; Schneider, P.; Hofmann, K.; Steiner, V.; Bodmer, J.L.; Schröter, M.; Burns, K.; Mattmann, C.; et al. Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Souchelnytskyi, S.; Heldin, C.H. Smad regulation in TGF-beta signal transduction. J. Cell Sci. 2001, 114, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Edlund, S.; Bu, S.; Schuster, N.; Aspenström, P.; Heuchel, R.; Heldin, N.-E.; Dijke, P.T.; Heldin, C.-H.; Landström, M. Transforming Growth Factor-β1 (TGF-β)–induced Apoptosis of Prostate Cancer Cells Involves Smad7-dependent Activation of p38 by TGF-β-activated Kinase 1 and Mitogen-activated Protein Kinase Kinase 3. Mol. Biol. Cell 2003, 14, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Mazars, A.; Lallemand, F.; Prunier, C.; Marais, J.; Ferrand, N.; Pessah, M.; Cherqui, G.; Atfi, A. Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J. Biol. Chem. 2001, 276, 36797–36803. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Majid, S.; Saini, S.; Zaman, M.S.; Yamamura, S.; Chiyomaru, T.; Shahryari, V.; Fukuhara, S.; Deng, G.; Dahiya, R.; et al. Hrk mediates 2-methoxyestradiol-induced mitochondrial apoptotic signaling in prostate cancer cells. Mol. Cancer Ther. 2013, 12, 1049–1059. [Google Scholar] [CrossRef]
- Van Veldhuizen, P.J.; Ray, G.; Banerjee, S.; Dhar, G.; Kambhampati, S.; Dhar, A.; Banerjee, S.K. 2-Methoxyestradiol modulates beta-catenin in prostate cancer cells: A possible mediator of 2-methoxyestradiol-induced inhibition of cell growth. Int. J. Cancer 2008, 122, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am J. Clin. Exp. Urol. 2018, 6, 62–77. [Google Scholar] [PubMed]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, J.; Kaulfuß, S.; Jarry, H.; Bremmer, F.; Stettner, M.; Burfeind, P.; Thelen, P. Prospects of estrogen receptor β activation in the treatment of castration-resistant prostate cancer. Oncotarget 2017, 8, 34971–34979. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.J.; Hussain, S.; Balanathan, P.; Hedwards, S.L.; Niranjan, B.; Grant, M.; Chandrasiri, U.P.; Toivanen, R.; Wang, Y.; Taylor, R.A.; et al. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc. Natl. Acad. Sci. USA 2010, 107, 3123–3128. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Lawrence, M.G.; Taylor, R.A.; Lo, C.Y.; Frydenberg, M.; Ellem, S.J.; Furic, L.; Risbridger, G.P. Estrogen receptor β activation impairs prostatic regeneration by inducing apoptosis in murine and human stem/progenitor enriched cell populations. PLoS ONE 2012, 7, e40732. [Google Scholar] [CrossRef] [PubMed]
- Mobley, J.A.; L’Esperance, J.O.; Wu, M.; Friel, C.J.; Hanson, R.H.; Ho, S.M. The novel estrogen 17alpha-20Z-21-[(4-amino)phenyl]-19-norpregna-1,3,5(10),20-tetraene-3,17beta-diol induces apoptosis in prostate cancer cell lines at nanomolar concentrations in vitro. Mol. Cancer Ther. 2004, 3, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.Z.; Williams, R.L.; Elliott, M.S.; Beebe, S.J. Resveratrol induces apoptosis in LNCaP cells and requires hydroxyl groups to decrease viability in LNCaP and DU 145 cells. Prostate 2002, 52, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.N.; Roberson, K.M.; Padilla, G.M.; O’Brien, E.T.; Cook, J.M.; Kim, C.S.; Fine, R.L. Induction of apoptosis by diethylstilbestrol in hormone-insensitive prostate cancer cells. J. Natl. Cancer Inst. 1996, 88, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Ito, K.; Takezawa, Y.; Oyama, T.; Yamanaka, H.; Suzuki, K. Insulin-like growth factor binding protein-6 inhibits prostate cancer cell proliferation: Implication for anticancer effect of diethylstilbestrol in hormone refractory prostate cancer. Br. J. Cancer 2005, 92, 1538–1544. [Google Scholar] [CrossRef]
- Montgomery, R.B.; Bonham, M.; Nelson, P.S.; Grim, J.; Makary, E.; Vessella, R.; Stahl, W.L. Estrogen effects on tubulin expression and taxane mediated cytotoxicity in prostate cancer cells. Prostate 2005, 65, 141–150. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Wang, Z.X.; Keith, T.J.; Knull, H.R.; Srivastava, D.K. Binding constants and stoichiometries of glyceraldehyde 3-phosphate dehydrogenase-tubulin complexes. Arch. Biochem. Biophys. 1994, 313, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Tanaka, T.; Cheng, J.S.; Sugita, S.; Ezaki, K.; Kurisu, T.; Nakatani, T. Effect of anti-estrogens on the androgen receptor activity and cell proliferation in prostate cancer cells. Urol. Res. 2004, 32, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Armiñán, A.; Mendes, L.; Carrola, J.; Movellan, J.; Vicent, M.J.; Duarte, I.F. HIF-1α inhibition by diethylstilbestrol and its polyacetal conjugate in hypoxic prostate tumour cells: Insights from NMR metabolomics. J. Drug Target. 2017, 25, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Trauger, R.; Corey, E.; Bell, D.; White, S.; Garsd, A.; Stickney, D.; Reading, C.; Frincke, J. Inhibition of androstenediol-dependent LNCaP tumour growth by 17α-ethynyl-5α-androstane-3α, 17β-diol (HE3235). Br. J. Cancer 2009, 100, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Koreckij, T.D.; Trauger, R.J.; Montgomery, R.B.; Pitts, T.E.; Coleman, I.; Nguyen, H.; Reading, C.L.; Nelson, P.S.; Vessella, R.L.; Corey, E. HE3235 inhibits growth of castration-resistant prostate cancer. Neoplasia 2009, 11, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Moriyama-Gonda, N.; Shiina, H.; Terashima, M.; Satoh, K.; Igawa, M. Rationale and clinical implication of combined chemotherapy with cisplatin and oestrogen in prostate cancer: Primary evidence based on methylation analysis of oestrogen receptor-alpha. BJU Int. 2008, 101, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Arvelo, F.; Sojo, F.; Cotte, C. Tumour progression and metastasis. Ecancermedicalscience 2016, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.P.; Pisolato, R.; Vicente, C.M.; Lazari, M.F.; Lucas, T.F.; Porto, C.S. Estrogen receptor beta (ERβ) mediates expression of β-catenin and proliferation in prostate cancer cell line PC-3. Mol. Cell. Endocrinol. 2016, 430, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Aberle, H.; Schwartz, H.; Kemler, R. Cadherin-catenin complex: Protein interactions and their implications for cadherin function. J. Cell. Biochem. 1996, 61, 514–523. [Google Scholar] [CrossRef]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef]
- Silva, R.S.; Lombardi, A.P.G.; de Souza, D.S.; Vicente, C.M.; Porto, C.S. Activation of estrogen receptor beta (ERβ) regulates the expression of N-cadherin, E-cadherin and β-catenin in androgen-independent prostate cancer cells. Int. J. Biochem. Cell Biol. 2018, 96, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Significance of MMP-2 Expression in Prostate Cancer. an Immunohistochem. Study 2003, 63, 8511–8515. [Google Scholar]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Prostate cancer: MMP2, MMP9, MMP14, TIMP2 and disease-free survival. Cancer Res. 2004, 64, 264. [Google Scholar]
- van Moorselaar, R.J.; Voest, E.E. Angiogenesis in prostate cancer: Its role in disease progression and possible therapeutic approaches. Mol. Cell. Endocrinol. 2002, 197, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cozzi, P.J. Angiogenesis as a strategic target for prostate cancer therapy. Med. Res. Rev. 2010, 30, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.N.; Xia, S.J. Stroma-epithelium crosstalk in prostate cancer. Asian J. Androl. 2009, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. = Rev. Roum. De Morphol. Et Embryol. 2018, 59, 455–467. [Google Scholar]
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F.Q.; Dermime, S.; Mohammad, R.M.; Uddin, S. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr. Vasc. Pharmacol. 2017, 15, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhao, Y.; Li, J.; Weng, C.; Cai, J.; Yang, K.; Yuan, H.; Imperato-McGinley, J.; Zhu, Y.S. Suppression of DHT-induced paracrine stimulation of endothelial cell growth by estrogens via prostate cancer cells. Prostate 2013, 73, 1069–1081. [Google Scholar] [CrossRef]
- Yu, J.; Moon, A.; Kim, H.-R.C. Both Platelet-Derived Growth Factor Receptor (PDGFR)-α and PDGFR-β Promote Murine Fibroblast Cell Migration. Biochem. Biophys. Res. Commun. 2001, 282, 697–700. [Google Scholar] [CrossRef]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. CCS 2013, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Phytoestrogens and breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 83, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Epidemiology of phytoestrogens. Bailliere’S Clin. Endocrinol. Metab. 1998, 12, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. Vitr. 2006, 20, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Tombal, B. Chemoprevention of prostate cancer with nutrients and supplements. Cancer Manag. Res. 2011, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Millstine, D.; Ruddy, B.; Wallace, M.; Fields, H. Effect of Plant- and Animal-Based Foods on Prostate Cancer Risk. J. Osteopath. Med. 2019, 119, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Arnoldi, A.; Johnson, S.K. Phytoestrogens: End of a tale? Ann. Med. 2005, 37, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, M.J. Effects of phytoestrogen on sexual development. Korean J. Pediatr. 2012, 55, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef]
- Lephart, E.D. Modulation of Aromatase by Phytoestrogens. Enzym. Res. 2015, 2015, 594656. [Google Scholar] [CrossRef]
- Michel, T.; Halabalaki, M.; Skaltsounis, A.-L. New concepts, experimental approaches, and dereplication strategies for the discovery of novel phytoestrogens from natural sources. Planta Medica 2013, 79, 514–532. [Google Scholar] [PubMed]
- Hajirahimkhan, A.; Dietz, B.M.; Bolton, J.L. Botanical modulation of menopausal symptoms: Mechanisms of action? Planta Medica 2013, 79, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Cassidy. Potential risks and benefits of phytoestrogen-rich diets. Int. J. Vitam. Nutr. Res. 2003, 73, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Branca, F.; Lorenzetti, S. Health effects of phytoestrogens. In Diet Diversification and Health Promotion; Karger Publishers: Basel, Switzerland, 2005; Volume 57, pp. 100–111. [Google Scholar]
- Lichtenstein, A.H. Soy protein, isoflavones and cardiovascular disease risk. J. Nutr. 1998, 128, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-S.; Pan, T.-M. Beneficial effects of phytoestrogens and their metabolites produced by intestinal microflora on bone health. Appl. Microbiol. Biotechnol. 2013, 97, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Saczko, J.; Michel, O.; Chwiłkowska, A.; Sawicka, E.; Mączyńska, J.; Kulbacka, J. Estrogen receptors in cell membranes: Regulation and signaling. In Transport Across Natural and Modified Biological Membranes and Its Implications in Physiology and Therapy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 93–105. [Google Scholar]
- Toyohira, Y.; Ueno, S.; Tsutsui, M.; Itoh, H.; Sakai, N.; Saito, N.; Takahashi, K.; Yanagihara, N. Stimulatory effects of the soy phytoestrogen genistein on noradrenaline transporter and serotonin transporter activity. Mol. Nutr. Food Res. 2010, 54, 516–524. [Google Scholar] [CrossRef]
- Woodside, J.V.; Campbell, M.J.; Denholm, E.E.; Newton, L.; Honour, J.W.; Morton, M.S.; Young, I.S.; Leathem, A.J. Short-term phytoestrogen supplementation alters insulin-like growth factor profile but not lipid or antioxidant status. J. Nutr. Biochem. 2006, 17, 211–215. [Google Scholar] [CrossRef]
- Teas, J.; Irhimeh, M.R.; Druker, S.; Hurley, T.G.; Hébert, J.R.; Savarese, T.M.; Kurzer, M.S. Serum IGF-1 concentrations change with soy and seaweed supplements in healthy postmenopausal American women. Nutr. Cancer 2011, 63, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Signaling pathways mediating the neuroprotective effects of sex steroids and SERMs in Parkinson’s disease. Front. Neuroendocr. 2012, 33, 169–178. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity of South African herbal teas: Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lim, U.; Song, M.-A. Dietary and lifestyle factors of DNA methylation. In Cancer Epigenetics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 359–376. [Google Scholar]
- Ming, L.G.; Chen, K.M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Bustamante, F.A.; Bhoola, K.D.; Figueroa, C.D.; Ehrenfeld, P. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin. Sci. 2018, 132, 2583–2598. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.C.S.; Ponte, L.G.S.; Silva, C.H.R.; Fagundes, I.; Pavan, I.C.B.; Romeiro, S.A.; da Silva, L.G.S.; Morelli, A.P.; Rostagno, M.A.; Simabuco, F.M.; et al. Beetroot and leaf extracts present protective effects against prostate cancer cells, inhibiting cell proliferation, migration, and growth signaling pathways. Phytother. Res. PTR 2021, 35, 5241–5258. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Wang, X.; Xu, C.; Wang, L.; Jian, J.; Wu, D.; Wu, G. CDK6 is upregulated and may be a potential therapeutic target in enzalutamide-resistant castration-resistant prostate cancer. Eur. J. Med. Res. 2022, 27, 105. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Commun. 2001, 287, 914–920. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakata, T.; Kuzumaki, T. Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer Lett. 2002, 176, 17–23. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Phytochemical, antioxidant, anti-proliferative and antimicrobial properties of Catharanthus roseus root extract, saponin-enriched and aqueous fractions. Mol. Biol. Rep. 2019, 46, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Hnit, S.S.T.; Yao, M.; Xie, C.; Bi, L.; Wong, M.; Liu, T.; De Souza, P.; Li, Z.; Dong, Q. Apigenin impedes cell cycle progression at G(2) phase in prostate cancer cells. Discover. Oncol. 2022, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2004, 39, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol. Cancer Ther. 2006, 5, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, B.S.; Chun, S.Y.; Park, Y.K.; Kang, K.S.; Kwon, T.G. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011, 26, 1489–1494. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Suppression of constitutive and tumor necrosis factor-induced nuclear factor (NF)-B activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: Correlation with down-regulation of NF-B-responsive genes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 31693178. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008, 44, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle 2007, 6, 1102–1114. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Gupta, S. Apigenin attenuates insulin-like growth factor-I signaling in an autochthonous mouse prostate cancer model. Pharm. Res. 2012, 29, 1506–1517. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin suppresses insulin-like growth factor I receptor signaling in human prostate cancer: An in vitro and in vivo study. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2009, 48, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Babcook, M.A.; Gupta, S. Apigenin modulates insulin-like growth factor axis: Implications for prevention and therapy of prostate cancer. Curr. Drug Targets 2012. epub ahead of print. [Google Scholar]
- Palmieri, D.; Perego, P.; Palombo, D. Apigenin inhibits the TNFα-induced expression of eNOS and MMP-9 via modulating Akt signalling through oestrogen receptor engagement. Mol. Cell. Biochem. 2012, 371, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.T.; Gupta, S. Suppression of NF-κB and NF-κB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, Z.B.; Doganlar, O.; Bilir, A. Midkine silencing enhances the anti-prostate cancer stem cell activity of the flavone apigenin: Cooperation on signaling pathways regulated by ERK, p38, PTEN, PARP, and NF-κB. Investig. New Drugs 2020, 38, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef]
- Kaur, P.; Shukla, S.; Gupta, S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: An in vitro and in vivo study. Carcinogenesis 2008, 29, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.; O’Neill, A.; Spengler, B.; Christoffel, V.; Fitzpatrick, J.M.; Watson, R.W.G. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate 2005, 63, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor–related apoptosis-inducing ligand. Mol. Cancer Ther. 2006, 5, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Iizumi, Y.; Taniguchi, T.; Goi, W.; Miki, T.; Sakai, T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 2013, 8, e55922. [Google Scholar] [CrossRef]
- Chien, M.-H.; Lin, Y.-W.; Wen, Y.-C.; Yang, Y.-C.; Hsiao, M.; Chang, J.-L.; Huang, H.-C.; Lee, W.-J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef]
- Franzen, C.A.; Amargo, E.; Todorović, V.; Desai, B.V.; Huda, S.; Mirzoeva, S.; Chiu, K.; Grzybowski, B.A.; Chew, T.-L.; Green, K.J. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev. Res. 2009, 2, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; MacLennan, G.T.; Flask, C.A.; Fu, P.; Mishra, A.; Resnick, M.I.; Gupta, S. Blockade of β-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007, 67, 6925–6935. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Li, S.; Wang, X.; Liang, Z.; Xu, X.; Xu, X.; Hu, Z.; Lin, Y.; Chen, H. Apigenin inhibits migration and invasion via modulation of epithelial mesenchymal transition in prostate cancer. Mol. Med. Rep. 2015, 11, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, S.; Kim, N.D.; Chiu, K.; Franzen, C.A.; Bergan, R.C.; Pelling, J.C. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2008, 47, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, S.; Franzen, C.A.; Pelling, J.C. Apigenin inhibits TGF-β-induced VEGF expression in human prostate carcinoma cells via a Smad2/3-and Src-dependent mechanism. Mol. Carcinog. 2014, 53, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhou, Q.; Liu, L.-Z.; Xia, C.; Hu, X.; Shi, X.; Jiang, B.-H. Apigenin inhibits tumor angiogenesis through decreasing HIF-1α and VEGF expression. Carcinogenesis 2007, 28, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Menendez, P.; Hevia, D.; Rodriguez-Garcia, A.; Mayo, J.C.; Sainz, R.M. Regulation of GLUT transporters by flavonoids in androgen-sensitive and-insensitive prostate cancer cells. Endocrinology 2014, 155, 3238–3250. [Google Scholar] [CrossRef]
- Melstrom, L.G.; Salabat, M.R.; Ding, X.Z.; Strouch, M.J.; Grippo, P.J.; Mirzoeva, S.; Pelling, J.C.; Bentrem, D.J. Apigenin down-regulates the hypoxia response genes: HIF-1α, GLUT-1, and VEGF in human pancreatic cancer cells. J. Surg. Res. 2011, 167, 173–181. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Van de Sande, T.; Roskams, T.; Lerut, E.; Joniau, S.; Van Poppel, H.; Verhoeven, G.; Swinnen, J.V. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J. Pathol. 2005, 206, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Lin, F.-M.; Yang, Y.-C.; Lee, M.-T.; Cha, T.-L.; Wu, G.-J.; Hsieh, S.-C.; Hsiao, P.-W. Herbal extract of Wedelia chinensis attenuates androgen receptor activity and orthotopic growth of prostate cancer in nude mice. Clin. Cancer Res. 2009, 15, 5435–5444. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Tzeng, S.-F.; Hsieh, S.-C.; Yang, Y.-C.; Hsiao, Y.-W.; Tsai, M.-H.; Hsiao, P.-W. A standardized herbal extract mitigates tumor inflammation and augments chemotherapy effect of docetaxel in prostate cancer. Sci. Rep. 2017, 7, 15624. [Google Scholar] [CrossRef] [PubMed]
- Genc, F.; Atabey, U.S.; Serttas, R.; Erdogan, S. Abiraterone Acetate, in Combination with Apigenin, Attenuates the Survival of Human Castration-Sensitive Prostate Cancer Cells. Anti-Cancer Agents Med. Chem. 2022, 22, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Ayyildiz, A.; Koc, H.; Turkekul, K.; Erdogan, S. Co-administration of apigenin with doxorubicin enhances anti-migration and antiproliferative effects via PI3K/PTEN/AKT pathway in prostate cancer cells. Exp. Oncol. 2021, 43, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Taghavi Bahreghani, M.; Geraily, G.H.; Alizadeh, S.H.; Najafi, M.; Shirazi, A. Apigenin Enhanced Radiation-Induced Apoptosis/Necrosis by Sensitization of LNCaP Prostate Cancer Cells to 6 MV Photon Beams. Cell J. 2021, 23, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro antitumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Medica 2009, 75, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Xue, J.; Li, Z.; Shi, X.; Jiang, B.-H.; Fang, J. Chrysin inhibits expression of hypoxia-inducible factor-1α through reducing hypoxia-inducible factor-1α stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007, 6, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gresa-Arribas, N.; Serratosa, J.; Saura, J.; Solà, C. Inhibition of CCAAT/enhancer binding protein δ expression by chrysin in microglial cells results in anti-inflammatory and neuroprotective effects. J. Neurochem. 2010, 115, 526–536. [Google Scholar] [CrossRef]
- Torres-Piedra, M.; Ortiz-Andrade, R.; Villalobos-Molina, R.; Singh, N.; Medina-Franco, J.L.; Webster, S.P.; Binnie, M.; Navarrete-Vázquez, G.; Estrada-Soto, S. A comparative study of flavonoid analogues on streptozotocin–nicotinamide induced diabetic rats: Quercetin as a potential antidiabetic agent acting via 11β-hydroxysteroid dehydrogenase type 1 inhibition. Eur. J. Med. Chem. 2010, 45, 2606–2612. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J.; Dong, J.; Li, H.; Luo, M.; Dai, X.; Zhang, Y.; Leng, B.; Niu, X.; Zhao, S. Chrysin protects mice from Staphylococcus aureus pneumonia. J. Appl. Microbiol. 2011, 111, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Gu, X.; Cai, J.; Huang, M.; Su, M. Chrysin attenuates allergic airway inflammation by modulating the transcription factors T-bet and GATA-3 in mice. Mol. Med. Rep. 2012, 6, 100–104. [Google Scholar] [PubMed]
- Anand, K.V.; Mohamed Jaabir, M.S.; Thomas, P.A.; Geraldine, P. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatr. Gerontol. Int. 2012, 12, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Gano, C.A.; Fatima, S.; Failes, T.W.; Arndt, G.M.; Sajinovic, M.; Mahns, D.; Saedisomeolia, A.; Coorssen, J.R.; Bucci, J.; de Souza, P.; et al. Anti-cancer potential of synergistic phytochemical combinations is influenced by the genetic profile of prostate cancer cell lines. Front. Nutr. 2023, 10, 1119274. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Denison, M.S.; Tachibana, H.; Yamada, K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2002, 66, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jung, J. Upregulation of G Protein-Coupled Estrogen Receptor by Chrysin-Nanoparticles Inhibits Tumor Proliferation and Metastasis in Triple Negative Breast Cancer Xenograft Model. Front. Endocrinol. 2020, 11, 560605. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Kwon, H.J.; Lee, G.S.; Moon, J.H.; Jung, J. Chrysin-Induced G Protein-Coupled Estrogen Receptor Activation Suppresses Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 9673. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Han, H.; Lee, S.O.; Xu, Y.; Kim, J.E.; Lee, H.J. SPHK/HIF-1α Signaling Pathway Has a Critical Role in Chrysin-Induced Anticancer Activity in Hypoxia-Induced PC-3 Cells. Cells 2022, 11, 2787. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-K.; Kim, K.M.; Jeong, S.-Y.; Choi, E.K.; Jung, J. Chrysin increases the therapeutic efficacy of docetaxel and mitigates docetaxel-induced edema. Integr. Cancer Ther. 2017, 16, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Jungbauer, A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 2008, 108, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, V.; Jain, A.; Jain, R.K.; Maikhuri, J.P.; Gupta, G. Synergistic chemoprotective mechanisms of dietary phytoestrogens in a select combination against prostate cancer. J. Nutr. Biochem. 2011, 22, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.B.; Vago, J.P.; Fernandes, D.O.; Martins, D.G.; Moreira, I.Z.; Gonçalves, W.A.; Costa, W.C.; Araújo, J.M.D.; Queiroz-Junior, C.M.; Campolina-Silva, G.H.; et al. Biochanin A Regulates Key Steps of Inflammation Resolution in a Model of Antigen-Induced Arthritis via GPR30/PKA-Dependent Mechanism. Front. Pharmacol. 2021, 12, 662308. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.; Samedi, V.G.; Medrano, T.A.; Sweeney, C.A.; Baker, H.V.; Stenstrom, A.; Furman, J.; Shiverick, K.T. Mechanisms of the growth inhibitory effects of the isoflavonoid biochanin A on LNCaP cells and xenografts. Prostate 2002, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mertas, A.; Paradysz, A.; Krol, W. The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol Oncol 2013, 31, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Zand, R.S.R.; Jenkins, D.J.; Brown, T.J.; Diamandis, E.P. Flavonoids can block PSA production by breast and prostate cancer cell lines. Clin. Chim. Acta 2002, 317, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. Biofactors 2018, 44, 407–417. [Google Scholar] [CrossRef]
- Handayani, R.; Rice, L.; Cui, Y.; Medrano, T.A.; Samedi, V.G.; Baker, H.V.; Szabo, N.J.; Shiverick, K.T. Soy isoflavones alter expression of genes associated with cancer progression, including interleukin-8, in androgen-independent PC-3 human prostate cancer cells. J. Nutr. 2006, 136, 75–82. [Google Scholar] [CrossRef]
- Hsu, A.; Bray, T.M.; Helferich, W.G.; Doerge, D.R.; Ho, E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp. Biol. Med. 2010, 235, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, M.; Fukuda, K.; Ohtani, M.; Akaza, H.; Sugimura, T.; Wakabayashi, K. Effects of soybean isoflavones on cell growth and apoptosis of the human prostatic cancer cell line LNCaP. Jpn. J. Clin. Oncol. 1998, 28, 360–363. [Google Scholar] [CrossRef]
- Szliszka, E.; Krol, W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncol. Rep. 2011, 26, 533–541. [Google Scholar] [PubMed]
- Hempstock, J.; Kavanagh, J.; George, N. Growth inhibition of prostate cell lines in vitro by phyto-oestrogens. Br. J. Urol. 1998, 82, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, W.; Sikes, R.A.; Wu, C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chem. 2013, 141, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.; Pérez-Martínez, F.; Calahorra, F.; Esbrit, P. Phytoestrogen modulation of bone-related cytokines and its impact on cell viability in human prostate cancer cells. Life Sci. 2009, 85, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; DeGroff, V.L.; Clinton, S.K. Tomato and soy polyphenols reduce insulin-like growth factor-I–stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J. Nutr. 2003, 133, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.F.; Wang, J.S.; Lu, J.F.; Kao, T.H.; Chen, B.H. Antiproliferation effect and mechanism of prostate cancer cell lines as affected by isoflavones from soybean cake. J. Agric. Food Chem. 2009, 57, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Ajdžanović, V.; Mojić, M.; Maksimović-Ivanić, D.; Bulatović, M.; Mijatović, S.; Milošević, V.; Spasojević, I. Membrane fluidity, invasiveness and dynamic phenotype of metastatic prostate cancer cells after treatment with soy isoflavones. J. Membr. Biol. 2013, 246, 307–314. [Google Scholar] [CrossRef]
- Rabiau, N.; Kossaï, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.-J.; Bernard-Gallon, D.J. Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol. 2010, 34, 200–206. [Google Scholar] [CrossRef]
- Singh-Gupta, V.; Zhang, H.; Banerjee, S.; Kong, D.; Raffoul, J.J.; Sarkar, F.H.; Hillman, G.G. Radiation-induced HIF-1α cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int. J. Cancer 2009, 124, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mao, Z.; Brinton, R.D. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology 2009, 150, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Ranjithkumar, R.; Saravanan, K.; Balaji, B.; Hima, S.; Sreeja, S.; Timane, S.R.; Ram Pravin Kumar, M.; Kabilan, S.; Ramanathan, M. Novel daidzein molecules exhibited anti-prostate cancer activity through nuclear receptor ERβ modulation, in vitro and in vivo studies. J. Chemother. 2021, 33, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Rzemieniec, J.; Litwa, E.; Lason, W.; Lenartowicz, M.; Krzeptowski, W.; Wojtowicz, A.K. The key involvement of estrogen receptor β and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience 2013, 238, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, P.; Han, M.; Gao, X. Daidzein stimulates fatty acid-induced fat deposition in C2C12 myoblast cells via the G protein-coupled receptor 30 pathway. Anim. Biotechnol. 2022, 33, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Dalais, F.S.; Meliala, A.; Wattanapenpaiboon, N.; Frydenberg, M.; Suter, D.A.; Thomson, W.K.; Wahlqvist, M.L. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology 2004, 64, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Landström, M.; Zhang, J.X.; Hallmans, G.; Äman, P.; Bergh, A.; Damber, J.E.; Mazur, W.; Wähälä, K.; Adlercreutz, H. Inhibitory effects of soy and rye diets on the development of Dunning R3327 prostate adenocarcinoma in rats. Prostate 1998, 36, 151–161. [Google Scholar] [CrossRef]
- McCormick, D.L.; Johnson, W.D.; Bosland, M.C.; Lubet, R.A.; Steele, V.E. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr. Cancer 2007, 57, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Takahashi, S.; Cui, L.; Toda, T.; Suzuki, S.; Futakuchi, M.; Sugiura, S.; Shirai, T. Suppressive effects of dietary genistin and daidzin on rat prostate carcinogenesis. Jpn. J. Cancer Res. 2000, 91, 786–791. [Google Scholar] [CrossRef]
- Jarred, R.A.; Keikha, M.; Dowling, C.; McPherson, S.J.; Clare, A.M.; Husband, A.J.; Pedersen, J.S.; Frydenberg, M.; Risbridger, G.P. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol. Prev. Biomark. 2002, 11, 1689–1696. [Google Scholar]
- Onozawa, M.; Kawamori, T.; Baba, M.; Fukuda, K.; Toda, T.; Sato, H.; Ohtani, M.; Akaza, H.; Sugimura, T.; Wakabayashi, K. Effects of a soybean isoflavone mixture on carcinogenesis in prostate and seminal vesicles of F344 rats. Jpn. J. Cancer Res. 1999, 90, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Singh-Gupta, V.; Knoll, Z.E.; Fite, A.; Zhang, H.; Abrams, J.; Sarkar, F.H.; Hillman, G.G. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007, 67, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Che, M.; Knoll, Z.E.; Doerge, D.R.; Abrams, J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer 2007, 120, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Luckert, P. Influence of Isoflavones in Soy Protein Isolates on Development of Induced Prostate-Related Cancers in L-W Rats; Taylor & Francis: Abingdon, UK, 1997. [Google Scholar]
- DeVere White, R.W.; Tsodikov, A.; Stapp, E.C.; Soares, S.E.; Fujii, H.; Hackman, R.M. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr. Cancer 2010, 62, 1036–1043. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Gugger, E.T.; Tanaka, T.; Guo, Y.; Blackburn, G.L.; Clinton, S.K. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J. Nutr. 1999, 129, 1628–1635. [Google Scholar] [CrossRef]
- Singh-Gupta, V.; Zhang, H.; Yunker, C.K.; Ahmad, Z.; Zwier, D.; Sarkar, F.H.; Hillman, G.G. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: Potentiation of radiotherapy. Pharm. Res. 2010, 27, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.K.; Zollinger, T.W.; Liu, Z.; Jones, J.F.; Zhang, J. Dietary intake of isoflavones and coumestrol and the risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2018, 142, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Miltyk, W.; Craciunescu, C.N.; Fischer, L.; Jeffcoat, R.A.; Koch, M.A.; Lopaczynski, W.; Mahoney, C.; Crowell, J.; Paglieri, J.; Zeisel, S.H. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am. J. Clin. Nutr. 2003, 77, 875–882. [Google Scholar] [CrossRef]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R-and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorganic Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef]
- Moriyama, M.; Hashimoto, A.; Satoh, H.; Kawabe, K.; Ogawa, M.; Takano, K.; Nakamura, Y. S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflamm. 2018, 2018, 8496973. [Google Scholar] [CrossRef]
- Rowlands, D.J.; Chapple, S.; Siow, R.C.; Mann, G.E. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: Roles for F-actin and GPR30. Hypertension 2011, 57, 833–840. [Google Scholar] [CrossRef]
- Uehara, M.; Ishimi, Y.; Katsumata, S.-I.; Suzuki, K. Transformation of Daidzein to Equol and Its Bioactivity; ACS Publications: Washington, DC, USA, 2008. [Google Scholar]
- Hedlund, T.E.; Johannes, W.U.; Miller, G.J. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate 2003, 54, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, T.E.; Bokhoven, A.v.; Johannes, W.U.; Nordeen, S.K.; Ogden, L.G. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate 2006, 66, 557–566. [Google Scholar] [CrossRef]
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M. Equol inhibits prostate cancer growth through degradation of androgen receptor by S-phase kinase-associated protein 2. Cancer Sci. 2016, 107, 1022–1028. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Duthie, S.J.; Collins, A.R. Effects of phytoestrogens on growth and DNA integrity in human prostate tumor cell lines: PC-3 and LNCaP. Nutr. Cancer 2000, 38, 223–228. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Y.; Ma, D.; Shi, Y.; Liu, C.; Wang, P. (±) Equol inhibits invasion in prostate cancer DU145 cells possibly via down-regulation of matrix metalloproteinase-9, matrix metalloproteinase-2 and urokinase-type plasminogen activator by antioxidant activity. J. Clin. Biochem. Nutr. 2011, 51, 1203270148. [Google Scholar] [CrossRef]
- Kurahashi, N.; Iwasaki, M.; Inoue, M.; Sasazuki, S.; Tsugane, S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: The Japan Public Health Center. J. Clin. Oncol. 2008, 26, 5923–5929. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Masumori, N.; Fukuta, F.; Yoneta, A.; Hida, T.; Yamashita, T.; Minatoya, M.; Nagata, Y.; Mori, M.; Tsuji, H. Influence of isoflavone intake and equol-producing intestinal flora on prostate cancer risk. Asian Pac. J. Cancer Prev. 2013, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.D.; Blake, C.; Bu, L.; Hamaker, A.N.; Lephart, E.D. Equol an isoflavonoid: Potential for improved prostate health, in vitro and in vivoevidence. Reprod. Biol. Endocrinol. 2011, 9, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Jiang, H. High dietary fat intake lowers serum equol concentration and promotes prostate carcinogenesis in a transgenic mouse prostate model. Nutr. Metab. 2019, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, N.; Akaza, H.; Hinotsu, S.; Fujioka, T.; Naito, S.; Namiki, M.; Takahashi, S.; Hirao, Y.; Horie, S.; Tsukamoto, T. Prostate cancer chemoprevention study: An investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012, 103, 125–130. [Google Scholar] [CrossRef]
- Battaglia, A.; Devos, G.; Boeckx, G.; Goeman, L.; Tosco, L.; de Meerleer, G.; Joniau, S. Prostate-Specific Antigen Modulatory Effect of a Fermented Soy Supplement for Patients with an Elevated Risk of Prostate Cancer: A Non-Randomized, Retrospective Observational Registration. Curr. Urol. 2020, 14, 142–149. [Google Scholar] [CrossRef]
- Mu, H.; Bai, Y.-H.; Wang, S.-T.; Zhu, Z.-M.; Zhang, Y.-W. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine 2009, 16, 314–319. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Mo, Z.; Huang, W.; Yan, H.; Ling, Z.; Ye, Y. Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell. Physiol. Biochem. 2014, 34, 1351–1358. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, L.; Ye, Y.; Chen, J. Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr. Cancer 2014, 66, 656–661. [Google Scholar] [CrossRef]
- Ye, Y.; Hou, R.; Chen, J.; Mo, L.; Zhang, J.; Huang, Y.; Mo, Z. Formononetin-induced apoptosis of human prostate cancer cells through ERK1/2 mitogen-activated protein kinase inactivation. Horm. Metab. Res. 2012, 44, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Bi, L.-Y.; Li, Z.-Z.; Zhang, X.; Ye, Y. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm. Biol. 2014, 52, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Li, Y.-Q.; Chen, Q.-Y.; Xiao, S.-J.; Zeng, S.-E. Up-regulating of RASD1 and apoptosis of DU-145 human prostate cancer cells induced by formononetin in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 2835–2839. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, Y.; Guo, J.; Tian, C.; Pan, W.; Wang, H.; Yan, J. Prostate Cancer Therapy Using Docetaxel and Formononetin Combination: Hyaluronic Acid and Epidermal Growth Factor Receptor Targeted Peptide Dual Ligands Modified Binary Nanoparticles to Facilitate the in vivo Anti-Tumor Activity. Drug Des. Dev. Ther. 2022, 16, 2683–2693. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, Y.; Zhou, Y.; Bao, K.; Yu, X.; Xu, Y.; Zhang, Y.; Zheng, J.; Jiang, G.; Hong, M. Formononetin attenuates atopic dermatitis by upregulating A20 expression via activation of G protein-coupled estrogen receptor. J. Ethnopharmacol. 2021, 266, 113397. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Ye, Y.; Wang, Y.; Tian, J. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 32, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Perabo, F.; Von Löw, E.; Ellinger, J.; Von Rücker, A.; Müller, S.; Bastian, P. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008, 11, 6–12. [Google Scholar] [CrossRef]
- Morito, K.; Aomori, T.; Hirose, T.; Kinjo, J.; Hasegawa, J.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors alpha and beta (II). Biol. Pharm. Bull. 2002, 25, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef]
- Maggiolini, M.; Vivacqua, A.; Fasanella, G.; Recchia, A.G.; Sisci, D.; Pezzi, V.; Montanaro, D.; Musti, A.M.; Picard, D.; Andò, S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004, 279, 27008–27016. [Google Scholar] [CrossRef]
- Vivacqua, A.; Bonofiglio, D.; Albanito, L.; Madeo, A.; Rago, V.; Carpino, A.; Musti, A.M.; Picard, D.; Andò, S.; Maggiolini, M. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol. Pharmacol. 2006, 70, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Wang, Y.; Kucuk, O.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G. Genistein inhibits radiation-induced activation of NF-κB in prostate cancer cells promoting apoptosis and G 2/M cell cycle arrest. BMC Cancer 2006, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Lee, W.H.; Park, K.Y.; Zhang, L. p53-independent induction of p21 (WAF1/CIP1), reduction of cyclin B1 and G2/M arrest by the isoflavone genistein in human prostate carcinoma cells. Jpn. J. Cancer Res. 2000, 91, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K. Synergistic effects of thearubigin and genistein on human prostate tumor cell (PC-3) growth via cell cycle arrest. Cancer Lett. 2000, 151, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xiang, N.; Domann, F.E.; Zhong, W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr. Cancer 2009, 61, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, H.; Ishiguro, H.; Ikeda, N.; Hori, M.; Kubota, Y.; Uemura, H. Genistein induces cell growth inhibition in prostate cancer through the suppression of telomerase activity. Int. J. Urol. 2005, 12, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Jiao, M.; Wu, D.; Wu, K.; Li, X.; Zhu, G.; Yang, L.; Wang, X.; Hsieh, J.-T. Genistein inhibits the stemness properties of prostate cancer cells through targeting Hedgehog–Gli1 pathway. Cancer Lett. 2012, 323, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Dahiya, R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer 2014, 110, 1645–1654. [Google Scholar] [CrossRef]
- Davis, J.N.; Singh, B.; Bhuiyan, M.; Sarkar, F.H. Genistein-induced upregulation of p21 WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr. Cancer 1998, 32, 123–131. [Google Scholar] [CrossRef]
- Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules 2023, 28, 2925. [Google Scholar] [CrossRef]
- Khamesi, S.M.; Salehi Barough, M.; Zargan, J.; Shayesteh, M.; Banaee, N.; Haji Noormohammadi, A.; Keshavarz Alikhani, H.; Mousavi, M. Evaluation of Anticancer and Cytotoxic Effects of Genistein on PC3 Prostate Cell Line under Three-Dimensional Culture Medium. Iran. Biomed. J. 2022, 26, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Saidijam, M.; Tayebinia, H.; Khodadadi, I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2022, 128, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Guo, F.; Chen, Y.; Li, Y.; Yu, B.; Li, Y. Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Lett. 2019, 448, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yan, J.; Li, Y.; Cheng, J.; Zheng, L.; Fu, T.; Zhu, Y. Inhibition of castration-resistant prostate cancer growth by genistein through suppression of AKR1C3. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Hill, D.L.; Chen, X.; Wang, H.; Zhang, R. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005, 65, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sarkar, F.H. Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar] [PubMed]
- Zhang, Z.; Wang, C.-Z.; Du, G.-J.; Qi, L.-W.; Calway, T.; He, T.-C.; Du, W.; Yuan, C.-S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Miękus, K.; Madeja, Z. Genistein inhibits the contact-stimulated migration of prostate cancer cells. Cell. Mol. Biol. Lett. 2007, 12, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Xu, L.; Liu, Y.; Deb, D.K.; Platanias, L.C.; Bergan, R.C. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005, 65, 3470–3478. [Google Scholar] [CrossRef]
- Kumi-Diaka, J.; Hassanhi, M.; Merchant, K.; Horman, V. Influence of genistein isoflavone on matrix metalloproteinase-2 expression in prostate cancer cells. J. Med. Food 2006, 9, 491–497. [Google Scholar] [CrossRef]
- Li, Y.; Che, M.; Bhagat, S.; Ellis, K.-L.; Kucuk, O.; Doerge, D.R.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Regulation of gene expression and inhibition of experimental prostate cancer bone metastasis by dietary genistein. Neoplasia 2004, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gordon, R.; Li, W.; Yang, X.; Pattanayak, A.; Fowler, G.; Zhang, L.; Catalona, W.J.; Ding, Y.; Xu, L. Genistein treatment duration effects biomarkers of cell motility in human prostate. PLoS ONE 2019, 14, e0214078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Li, L.; Wu, D.P.; Fan, J.H.; Li, X.; Wu, K.J.; Wang, X.Y.; He, D.L. A novel anti-cancer effect of genistein: Reversal of epithelial mesenchymal transition in prostate cancer cells 1. Acta Pharmacol. Sin. 2008, 29, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ding, Y.; Catalona, W.J.; Yang, X.J.; Anderson, W.F.; Jovanovic, B.; Wellman, K.; Killmer, J.; Huang, X.; Scheidt, K.A. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. JNCI J. Natl. Cancer Inst. 2009, 101, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Kyle, E.; Neckers, L.; Takimoto, C.; Curt, G.; Bergan, R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol. Pharmacol. 1997, 51, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.; Khandrika, L.; Kumar, B.; Venezia, S.; Koul, S.; Chandhoke, R.; Maroni, P.; Donohue, R.; Meacham, R.B.; Koul, H.K. Focal Adhesion Kinase Controls Aggressive Phenotype of Androgen-Independent Prostate Cancer. Mol. Cancer Res. 2008, 6, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, S.; Hoot, D.R.; Clinton, S.K. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J. Nutr. Biochem. 2007, 18, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sarkar, F.H. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002, 186, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Kucuk, O.; Sarkar, F.H. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2002, 34, 91–101. [Google Scholar] [CrossRef]
- Davis, J.; Muqim, N.; Bhuiyan, M.; Kucuk, O.; Pienta, K.; Sarkar, F. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int. J. Oncol. 2000, 16, 1091–1098. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernández de la Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al-alem, U.; Ali, M.M.; Bosland, M.C. Genistein increases estrogen receptor beta expression in prostate cancer via reducing its promoter methylation. J. Steroid Biochem. Mol. Biol. 2015, 152, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Z.; Chen, D.; Sun, Y.; Jin, Z.; Christman, J.K.; Yang, C.S. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 7033–7041. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.P.; Fontana, L.; Bignon, Y.J.; Guy, L.; et al. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo 2010, 24, 393–400. [Google Scholar] [PubMed]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Zaman, M.S.; Majid, S.; Deng, G.; Shahryari, V.; Saini, S.; Hirata, H.; Ueno, K.; Chang, I.; et al. Genistein Suppresses Prostate Cancer Growth through Inhibition of Oncogenic MicroRNA-151. PLoS ONE 2012, 7, e43812. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Hidaka, H.; Majid, S.; Saini, S.; Arora, S.; Deng, G.; Shahryari, V.; Chang, I.; et al. Genistein Up-Regulates Tumor Suppressor MicroRNA-574-3p in Prostate Cancer. PLoS ONE 2013, 8, e58929. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Q.; Cai, J.; Wu, W.; Zhu, Y.S. 17α-Estradiol and genistein inhibit high fat diet induced prostate gene expression and prostate growth in the rat. J. Urol. 2011, 186, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Eltoum, I.E.; Carpenter, M.; Lamartiniere, C.A. Genistein mechanisms and timing of prostate cancer chemoprevention in lobund-wistar rats. Asian Pac. J. Cancer Prev. 2009, 10, 143–150. [Google Scholar]
- Harper, C.E.; Cook, L.M.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Arabshahi, A.; Shirai, T.; Lamartiniere, C.A. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. Prostate 2009, 69, 1668–1682. [Google Scholar] [CrossRef]
- Wang, J.; Eltoum, I.-E.; Lamartiniere, C.A. Dietary genistein suppresses chemically induced prostate cancer in Lobund–Wistar rats. Cancer Lett. 2002, 186, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Aronson, W.J.; Tymchuk, C.N.; Elashoff, R.M.; McBride, W.H.; McLean, C.; Wang, H.; Heber, D. Decreased growth of human prostate LNCaP tumors in SCID mice fed a low-fat, soy protein diet with isoflavones. Nutr. Cancer 1999, 35, 130–136. [Google Scholar] [CrossRef] [PubMed]
- El Touny, L.H.; Banerjee, P.P. Akt–GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis 2007, 28, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.A.; Greenberg, N.M.; Elgavish, A. Dietary genistein improves survival and reduces expression of osteopontin in the prostate of transgenic mice with prostatic adenocarcinoma (TRAMP). J. Nutr. 2005, 135, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Eltoum, I.-E.; Lamartiniere, C.A. Genistein chemoprevention of prostate cancer in TRAMP mice. J. Carcinog. 2007, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.-E.; Greenberg, N.M.; Elgavish, A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. 2001, 61, 6777–6782. [Google Scholar] [PubMed]
- Fritz, W.A.; Wang, J.; Eltoum, I.-E.; Lamartiniere, C.A. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Mol. Cell. Endocrinol. 2002, 186, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Bektic, J.; Berger, A.P.; Pfeil, K.; Dobler, G.; Bartsch, G.; Klocker, H. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor β. Eur. Urol. 2004, 45, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.; Zhou, S.; Liao, L.; Tien, J.C.; Greenberg, N.M.; Xu, J. Genetic ablation of the amplified-in-breast cancer 1 inhibits spontaneous prostate cancer progression in mice. Cancer Res. 2007, 67, 5965–5975. [Google Scholar] [CrossRef]
- Wolk, A.; Mantzoros, C.S.; Andersson, S.O.; Bergström, R.; Signorello, L.B.; Lagiou, P.; Adami, H.O.; Trichopoulos, D. Insulin-like growth factor 1 and prostate cancer risk: A population-based, case-control study. J. Natl. Cancer Inst. 1998, 90, 911–915. [Google Scholar] [CrossRef]
- Wu, T.T.; Sikes, R.A.; Cui, Q.; Thalmann, G.N.; Kao, C.; Murphy, C.F.; Yang, H.; Zhau, H.E.; Balian, G.; Chung, L.W. Establishing human prostate cancer cell xenografts in bone: Induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int. J. Cancer 1998, 77, 887–894. [Google Scholar] [CrossRef]
- Thalmann, G.N.; Sikes, R.A.; Devoll, R.E.; Kiefer, J.A.; Markwalder, R.; Klima, I.; Farach-Carson, C.M.; Studer, U.E.; Chung, L.W.K. Osteopontin: Possible Role in Prostate Cancer Progression. Clin. Cancer Res. 1999, 5, 2271–2277. [Google Scholar] [PubMed]
- Tuck, A.B.; O’Malley, F.P.; Singhal, H.; Tonkin, K.S.; Harris, J.F.; Bautista, D.; Chambers, A.F. Osteopontin and p53 expression are associated with tumor progression in a case of synchronous, bilateral, invasive mammary carcinomas. Arch. Pathol. Lab. Med. 1997, 121, 578–584. [Google Scholar] [PubMed]
- Oates, A.J.; Barraclough, R.; Rudland, P.S. The identification of osteopontin as a metastasis-related gene product in a rodent mammary tumour model. Oncogene 1996, 13, 97–104. [Google Scholar] [PubMed]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef]
- Geller, J.; Sionit, L.; Partido, C.; Li, L.; Tan, X.; Youngkin, T.; Nachtsheim, D.; Hoffman, R.M. Genistein inhibits the growth of human-patient BPH and prostate cancer in histoculture. Prostate 1998, 34, 75–79. [Google Scholar] [CrossRef]
- Takimoto, C.H.; Glover, K.; Huang, X.; Hayes, S.A.; Gallot, L.; Quinn, M.; Jovanovic, B.D.; Shapiro, A.; Hernandez, L.; Goetz, A. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol. Prev. Biomark. 2003, 12, 1213–1221. [Google Scholar]
- Jarrard, D.; Konety, B.; Huang, W.; Downs, T.; Kolesar, J.; Kim, K.M.; Havighurst, T.; Slaton, J.; House, M.G.; Parnes, H.L. Phase IIa, randomized placebo-controlled trial of single high dose cholecalciferol (vitamin D3) and daily Genistein (G-2535) versus double placebo in men with early stage prostate cancer undergoing prostatectomy. Am. J. Clin. Exp. Urol. 2016, 4, 17. [Google Scholar] [PubMed]
- White, R.W.D.; Hackman, R.M.; Soares, S.E.; Beckett, L.A.; Li, Y.; Sun, B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology 2004, 63, 259–263. [Google Scholar] [CrossRef]
- Bosland, M.C.; Huang, J.; Schlicht, M.J.; Enk, E.; Xie, H.; Kato, I. Impact of 18-Month Soy Protein Supplementation on Steroid Hormones and Serum Biomarkers of Angiogenesis, Apoptosis, and the Growth Hormone/IGF-1 Axis: Results of a Randomized, Placebo-Controlled Trial in Males Following Prostatectomy. Nutr. Cancer 2022, 74, 110–121. [Google Scholar] [CrossRef]
- Bosland, M.C.; Schmoll, J.; Watanabe, H.; Randolph, C.; Kato, I. Randomized, Placebo-Controlled Six-Month Intervention Study of Soy Protein Isolate in Men with Biochemical Recurrence after Radical Prostatectomy: A Pilot Study. Nutr. Cancer 2022, 74, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Burich, R.A.; Holland, W.S.; Vinall, R.L.; Tepper, C.; DeVere White, R.W.; Mack, P.C. Genistein combined polysaccharide enhances activity of docetaxel, bicalutamide and Src kinase inhibition in androgen-dependent and independent prostate cancer cell lines. BJU Int. 2008, 102, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Chen, Z.; Kim, S.; Iqbal, S.; Chi, A.; Ritenour, C.; Wang, Y.A.; Kucuk, O.; Wu, D. Genistein enhances the efficacy of cabazitaxel chemotherapy in metastatic castration-resistant prostate cancer cells. Prostate 2013, 73, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.B.; Woo, S.U.; Yim, H. Cotargeting Plk1 and androgen receptor enhances the therapeutic sensitivity of paclitaxel-resistant prostate cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846375. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.M.; Wolff, H.A.; Jarry, H.; Thelen, P.; Gruendker, C.; Rave-Fraenk, M.; Schmidberger, H.; Christiansen, H. In vitro studies on the modification of low-dose hyper-radiosensitivity in prostate cancer cells by incubation with genistein and estradiol. Radiat. Oncol. 2008, 3, 19. [Google Scholar] [CrossRef]
- Hillman, G.G.; Wang, Y.; Kucuk, O.; Che, M.; Doerge, D.R.; Yudelev, M.; Joiner, M.C.; Marples, B.; Forman, J.D.; Sarkar, F.H. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol. Cancer Ther. 2004, 3, 1271–1279. [Google Scholar] [CrossRef]
- Hillman, G.G.; Forman, J.D.; Kucuk, O.; Yudelev, M.; Maughan, R.L.; Rubio, J.; Layer, A.; Tekyi-Mensah, S.; Abrams, J.; Sarkar, F.H. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin. Cancer Res. 2001, 7, 382–390. [Google Scholar]
- Tang, Q.; Ma, J.; Sun, J.; Yang, L.; Yang, F.; Zhang, W.; Li, R.; Wang, L.; Wang, Y.; Wang, H. Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol. Rep. 2018, 40, 579–588. [Google Scholar] [PubMed]
- Wang, Y.; Raffoul, J.J.; Che, M.; Doerge, D.R.; Joiner, M.C.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Prostate cancer treatment is enhanced by genistein in vitro and in vivo in a syngeneic orthotopic tumor model. Radiat. Res. 2006, 166, 73–80. [Google Scholar] [CrossRef]
- Jackson, I.L.; Pavlovic, R.; Alexander, A.A.; Connors, C.Q.; Newman, D.; Mahmood, J.; Eley, J.; Harvey, A.J.; Kaytor, M.D.; Vujaskovic, Z. BIO 300, a nanosuspension of genistein, mitigates radiation-induced erectile dysfunction and sensitizes human prostate cancer xenografts to radiation therapy. Int. J. Radiat. Oncol. * Biol. * Phys. 2019, 105, 400–409. [Google Scholar] [CrossRef]
- Davis, D.A.; Sarkar, S.H.; Hussain, M.; Li, Y.; Sarkar, F.H. Increased therapeutic potential of an experimental anti-mitotic inhibitor SB715992 by genistein in PC-3 human prostate cancer cell line. BMC Cancer 2006, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.; Shim, M.; Lee, I.; Lee, Y.; Im, M.-H.; Ko, S. Curcumin and genistein coloaded nanostructured lipid carriers: In vitro digestion and antiprostate cancer activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Yuan, Y.; Shi, X.; Che, Y.Y. Improved drug delivery and anti-tumor efficacy of combinatorial liposomal formulation of genistein and plumbagin by targeting Glut1 and Akt3 proteins in mice bearing prostate tumor. Colloids Surf. B Biointerfaces 2020, 190, 110966. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Y.; Chi, C.L.; Bian, G.; Xing, D.; Guo, F.J.; Wang, X.Q. PSMA conjugated combinatorial liposomal formulation encapsulating genistein and plumbagin to induce apoptosis in prostate cancer cells. Colloids Surf. B Biointerfaces 2021, 203, 111723. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Vodnik, V.V.; Mojić, M.; Stamenović, U.; Otoničar, M.; Ajdžanović, V.; Maksimović-Ivanić, D.; Mijatović, S.; Marković, M.M.; Barudžija, T.; Filipović, B.; et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112078. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Wang, Y.; Kurita, T.; Adomat, H.; Cunha, G.R.; Wang, Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS ONE 2011, 6, e20034. [Google Scholar] [CrossRef] [PubMed]
- El Touny, L.H.; Banerjee, P.P. Genistein induces the metastasis suppressor kangai-1 which mediates its anti-invasive effects in TRAMP cancer cells. Biochem. Biophys. Res. Commun. 2007, 361, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Terzioglu-Usak, S.; Yildiz, M.T.; Goncu, B.; Ozten-Kandas, N. Achieving the balance: Biphasic effects of genistein on PC-3 cells. J. Food Biochem. 2019, 43, e12951. [Google Scholar] [CrossRef]
- El Touny, L.H.; Banerjee, P.P. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009, 69, 3695–3703. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Zhu, T.; Parray, A.; Siddique, H.R.; Yang, W.; Saleem, M.; Bosland, M.C. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS ONE 2013, 8, e78479. [Google Scholar] [CrossRef] [PubMed]
- Totta, P.; Acconcia, F.; Leone, S.; Cardillo, I.; Marino, M. Mechanisms of Naringenin-induced Apoptotic Cascade in Cancer Cells: Involvement of Estrogen Receptor a and ß Signalling. IUBMB Life 2004, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ruh, M.F.; Zacharewski, T.; Connor, K.; Howell, J.; Chen, I.; Safe, S. Naringenin: A weakly estrogenic bioflavonoid that exhibits antiestrogenic activity. Biochem. Pharmacol. 1995, 50, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Naringenin-Induced Apoptotic Cell Death in Prostate Cancer Cells Is Mediated via the PI3K/AKT and MAPK Signaling Pathways. J. Cell. Biochem. 2017, 118, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Elia, A.C.; Magara, G.; Feriotto, G.; Forni, C.; Borromeo, I.; De Martino, A.; Tabolacci, C.; Mischiati, C.; Beninati, S. Reduction of oxidative stress and ornithine decarboxylase expression in a human prostate cancer cell line PC-3 by a combined treatment with α-tocopherol and naringenin. Amino Acids 2021, 53, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.G.; Akgun, T. Naringenin inhibits prostate cancer metastasis by blocking voltage-gated sodium channels. Biomed. Pharmacother. 2018, 106, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-Y.; Chen, P.-N.; Hong, M.-C.; Hseu, Y.-C.; Chen, K.-M.; Hsu, L.-S.; Chen, W.-J. Naringenin Attenuated Prostate Cancer Invasion via Reversal of Epithelial–to–Mesenchymal Transition and Inhibited uPA Activity. Anticancer Res. 2018, 38, 6753–6758. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Henning, S.M.; Niu, Y.; Youssefian, A.A.; Seeram, N.P.; Xu, A.; Heber, D. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. J. Nutr. Biochem. 2006, 17, 89–95. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, B.; Liu, J.; Wu, X.; Luo, N.; Wang, X.; Zheng, X.; Pan, X. Combinatorial anti-proliferative effects of tamoxifen and naringenin: The role of four estrogen receptor subtypes. Toxicology 2018, 410, 231–246. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Geybels, M.S.; Verhage, B.A.J.; Arts, I.C.W.; van Schooten, F.J.; Goldbohm, R.A.; van den Brandt, P.A. Dietary Flavonoid Intake, Black Tea Consumption, and Risk of Overall and Advanced Stage Prostate Cancer. Am. J. Epidemiol. 2013, 177, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Boam, T. Anti-androgenic effects of flavonols in prostate cancer. Ecancermedicalscience 2015, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal. Cell. Pathol. 2019, 2019, 1907698. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.P.; Ho, T.M.; Yang, C.W.; Chang, Y.J.; Chen, J.F.; Shaw, N.S.; Horng, J.C.; Hsu, S.L.; Liao, M.Y.; Wu, L.C.; et al. Chemopreventive Potential of Ethanolic Extracts of Luobuma Leaves (Apocynum venetum L.) in Androgen Insensitive Prostate Cancer. Nutrients 2017, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Mamouni, K.; Zhang, S.; Li, X.; Chen, Y.; Yang, Y.; Kim, J.; Bartlett, M.G.; Coleman, I.M.; Nelson, P.S.; Kucuk, O. A novel flavonoid composition targets androgen receptor signaling and inhibits prostate cancer growth in preclinical models. Neoplasia 2018, 20, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, J.; Sanderson, J.T. Growth inhibitory, antiandrogenic, and pro-apoptotic effects of punicic acid in LNCaP human prostate cancer cells. J. Agric. Food Chem. 2010, 58, 12149–12156. [Google Scholar] [CrossRef] [PubMed]
- Knowles, L.M.; Zigrossi, D.A.; Tauber, R.A.; Hightower, C.; Milner, J.A. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr. Cancer 2000, 38, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, J.; Yan, H.; Shi, M.; Zheng, Q.; Wang, Y.; Zhu, Y.; Miao, L.; Gao, X. Kaempferol inhibits benign prostatic hyperplasia by resisting the action of androgen. Eur. J. Pharmacol. 2021, 907, 174251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Fang, W.; Liang, K.; Li, X.; Zhang, F.; Pang, Y.; Fang, G.; Wang, X. Kaempferol suppresses androgen-dependent and androgen-independent prostate cancer by regulating Ki67 expression. Mol. Biol. Rep. 2022, 49, 4607–4617. [Google Scholar] [CrossRef]
- Szliszka, E.; Zydowicz, G.; Janoszka, B.; Dobosz, C.; Kowalczyk-Ziomek, G.; Krol, W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2011, 38, 941–953. [Google Scholar]
- Halimah, E.; Diantini, A.; Destiani, D.P.; Pradipta, I.S.; Sastramihardja, H.S.; Lestari, K.; Subarnas, A.; Abdulah, R.; Koyama, H. Induction of caspase cascade pathway by kaempferol-3-O-rhamnoside in LNCaP prostate cancer cell lines. Biomed. Rep. 2015, 3, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, M.; Wang, J. Kaempferol inhibits cancer cell growth by antagonizing estrogen-related receptor α and γ activities. Cell Biol. Int. 2013, 37, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fu, W.; Wang, J.; Kang, D.; Xu, L.; Zhao, Y.; Liu, A.L.; Du, G.H. Identification of Estrogen Receptor α Antagonists from Natural Products via In Vitro and In Silico Approaches. Oxid. Med. Cell. Longev. 2018, 2018, 6040149. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.-M.; Chibane, M.; Monti, J.-P.; Richard, T. Wine polyphenols: Potential agents in neuroprotection. Oxidative Med. Cell. Longev. 2012, 2012, 805762. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Khoo, H.-E. Biological effects of myricetin. Gen. Pharmacol. Vasc. Syst. 1997, 29, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, Y.; Ye, X.; Xue, S.; Shi, J.; Pan, J.; Chen, Q. Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem. 2013, 138, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, C.; Huang, H.; Yang, B.; Xiao, G.; Kong, D.; Tian, Q.; Song, Q.; Song, Y.; Tan, H. The natural compound myricetin effectively represses the malignant progression of prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4 interaction. Cell. Physiol. Biochem. 2018, 48, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Nawijn, M.C.; Alendar, A.; Berns, A. For better or for worse: The role of Pim oncogenes in tumorigenesis. Nat. Rev. Cancer 2011, 11, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.M.; Barrette, T.R.; Ghosh, D.; Shah, R.; Varambally, S.; Kurachi, K.; Pienta, K.J.; Rubin, M.A.; Chinnaiyan, A.M. Delineation of prognostic biomarkers in prostate cancer. Nature 2001, 412, 822–826. [Google Scholar] [CrossRef]
- Santio, N.M.; Eerola, S.K.; Paatero, I.; Yli-Kauhaluoma, J.; Anizon, F.; Moreau, P.; Tuomela, J.; Härkönen, P.; Koskinen, P.J. Pim Kinases Promote Migration and Metastatic Growth of Prostate Cancer Xenografts. PLoS ONE 2015, 10, e0130340. [Google Scholar] [CrossRef] [PubMed]
- Linn, D.E.; Yang, X.; Xie, Y.; Alfano, A.; Deshmukh, D.; Wang, X.; Shimelis, H.; Chen, H.; Li, W.; Xu, K.; et al. Differential regulation of androgen receptor by PIM-1 kinases via phosphorylation-dependent recruitment of distinct ubiquitin E3 ligases. J. Biol. Chem. 2012, 287, 22959–22968. [Google Scholar] [CrossRef] [PubMed]
- Santio, N.M.; Salmela, M.; Arola, H.; Eerola, S.K.; Heino, J.; Rainio, E.M.; Koskinen, P.J. The PIM1 kinase promotes prostate cancer cell migration and adhesion via multiple signalling pathways. Exp. Cell Res. 2016, 342, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Grundler, R.; Brault, L.; Gasser, C.; Bullock, A.N.; Dechow, T.; Woetzel, S.; Pogacic, V.; Villa, A.; Ehret, S.; Berridge, G.; et al. Dissection of PIM serine/threonine kinases in FLT3-ITD-induced leukemogenesis reveals PIM1 as regulator of CXCL12-CXCR4-mediated homing and migration. J. Exp. Med. 2009, 206, 1957–1970. [Google Scholar] [CrossRef]
- Liu, J.S.; Fang, W.K.; Yang, S.M.; Wu, M.C.; Chen, T.J.; Chen, C.M.; Lin, T.Y.; Liu, K.L.; Wu, C.M.; Chen, Y.C.; et al. Natural product myricetin is a pan-KDM4 inhibitor which with poly lactic-co-glycolic acid formulation effectively targets castration-resistant prostate cancer. J. Biomed. Sci. 2022, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Maruthanila, V.L.; Elancheran, R.; Roy, N.K.; Bhattacharya, A.; Kunnumakkara, A.B.; Kabilan, S.; Kotoky, J. In silico Molecular Modelling of Selected Natural Ligands and their Binding Features with Estrogen Receptor Alpha. Curr. Comput. -Aided Drug Des. 2019, 15, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Recchia, A.G.; Bonofiglio, D.; Catalano, S.; Vivacqua, A.; Carpino, A.; Rago, V.; Rossi, R.; Andò, S. The red wine phenolics piceatannol and myricetin act as agonists for estrogen receptor alpha in human breast cancer cells. J. Mol. Endocrinol. 2005, 35, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Lamson, D.W.; Brignall, M. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. A J. Clin. Ther. 2000, 5, 196–208. [Google Scholar]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Choi, J.H.; Kim, E.B.; Yin, J.; Seonu, S.Y.; Jin, S.Y.; Oh, J.Y.; Lee, M.W. Chemopreventive Activity of Ellagitannins from Acer pseudosieboldianum (Pax) Komarov Leaves on Prostate Cancer Cells. Plants 2023, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Yen, C.Y.; Wu, R.S.C.; Yang, J.S.; Lu, H.F.; Lu, K.W.; Lo, C.; Chen, H.Y.; Tang, N.Y.; Wu, C.C. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014, 29, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Xu, C.-J.; Nair, S.S.; Chen, C.; Hebbar, V.; Kong, A.-N.T. Modulation of activator protein-1 (AP-1) and MAPK pathway by flavonoids in human prostate cancer PC3 cells. Arch. Pharmacal Res. 2006, 29, 633. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.D.; Pramanik, R.; Zhang, X.; Carey, A.-M.; Ragavan, N.; Martin, F.L.; Muir, G.H. Selenium-or quercetin-induced retardation of DNA synthesis in primary prostate cells occurs in the presence of a concomitant reduction in androgen-receptor activity. Cancer Lett. 2006, 239, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Phan, T.; Gordon, D.; Chung, S.; Henning, S.M.; Vadgama, J.V. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol. Nutr. Food Res. 2015, 59, 250–261. [Google Scholar] [CrossRef]
- Kampa, M.; Hatzoglou, A.; Notas, G.; Damianaki, A.; Bakogeorgou, E.; Gemetzi, C.; Kouroumalis, E.; Martin, P.-M.; Castanas, E. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr. Cancer 2000, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr. Cancer 2012, 64, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Nguyen, T.T.T.; Chan, E.; Tran, E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-α)-and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int. J. Oncol. 2003, 23, 821–829. [Google Scholar] [CrossRef]
- Firdous, A.; Sharmila, G.; Balakrishnan, S.; RajaSingh, P.; Suganya, S.; Srinivasan, N.; Arunakaran, J. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the EGFR signaling pathway. Food Funct. 2014, 5, 2632–2645. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Arunakaran, J. Effects of quercetin on insulin-like growth factors (IGFs) and their binding protein-3 (IGFBP-3) secretion and induction of apoptosis in human prostate cancer cells. J. Carcinog. 2006, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Vijayababu, M.; Kanagaraj, P.; Arunkumar, A.; Ilangovan, R.; Aruldhas, M.; Arunakaran, J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J. Cancer Res. Clin. Oncol. 2005, 131, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bai, J.; Wang, S.; Liu, L.; Zhao, Z. Effects of Quercetin on Autophagy and Phosphatidylinositol 3-kinase/Protein Kinase B/Mammalian Target of Rapamycin Signaling Pathway in Human Prostate Cancer PC-3 Cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Acad. Med. Sin. 2020, 42, 578–584. [Google Scholar] [CrossRef]
- Yuan, H.; Young, C.Y.; Tian, Y.; Liu, Z.; Zhang, M.; Lou, H. Suppression of the androgen receptor function by quercetin through protein–protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol. Cell. Biochem. 2010, 339, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.G.; Cong, Y.; Bao, N.R.; Li, Y.G.; Zhao, J.N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. BioMed Res. Int. 2018, 2018, 4178021. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-H.; Heo, J.; Lee, Y.J.; Kwon, T.K.; Kim, Y.-H. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci. 2010, 86, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Lee, D.-H.; Jeong, J.-H.; Guo, Z.S.; Lee, Y.J. Quercetin augments TRAIL-induced apoptotic death: Involvement of the ERK signal transduction pathway. Biochem. Pharmacol. 2008, 75, 1946–1958. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Bindukumar, B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate 2008, 68, 1773–1789. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Banudevi, S.; Gunadharini, N.D.; Sharmila, G.; Selvakumar, K.; Arunakaran, J. Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3). Mol. Cell. Biochem. 2010, 344, 173–184. [Google Scholar] [CrossRef]
- Vijayababu, M.R.; Kanagaraj, P.; Arunkumar, A.; Ilangovan, R.; Dharmarajan, A.; Arunakaran, J. Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: A possible mediation by IGFBP-3. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2006, 16, 67–74. [Google Scholar] [CrossRef]
- Wang, G.; Song, L.; Wang, H.; Xing, N. Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth and inducing apoptosis in human prostate cancer cells. Oncol. Rep. 2013, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Szczepanski, M.; Lee, Y.J. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem. Pharmacol. 2008, 75, 2345–2355. [Google Scholar] [CrossRef]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Arunkumar, R.; Elumalai, P.; Sharmila, G.; Gunadharini, D.N.; Banudevi, S.; Krishnamoorthy, G.; Benson, C.S.; Arunakaran, J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 2011, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, D.; Yang, F.; Xing, N. Quercetin Inhibits Epithelial-to-Mesenchymal Transition (EMT) Process and Promotes Apoptosis in Prostate Cancer via Downregulating lncRNA MALAT1. Cancer Manag. Res. 2020, 12, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-H.; Cheng, C.-H.; Lin, C.-Y.; Huang, Y.-T.; Lee, L.-T.; Kandaswami, C.C.; Lin, Y.-C.; Lee, K.P.-H.; Hung, C.-C.; Hwang, J.-J. Dietary flavonoids luteolin and quercetin suppressed cancer stem cell properties and metastatic potential of isolated prostate cancer cells. Anticancer Res. 2016, 36, 6367–6380. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Qiu, H.; Wang, Y.; Wang, J.; Xu, H.; Zhang, T.; Liu, L.; Tao, Y.; Ren, Z. Expression of prostate stem cell antigen is downregulated during flavonoid-induced cytotoxicity in prostate cancer cells. Exp. Ther. Med. 2017, 14, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Baruah, M.M.; Khandwekar, A.P.; Sharma, N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biol. 2016, 37, 14025–14034. [Google Scholar] [CrossRef]
- Bhat, F.A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Singh, P.R.; Srinivasan, N.; Arunakaran, J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J. Nutr. Biochem. 2014, 25, 1132–1139. [Google Scholar] [CrossRef]
- Vijayababu, M.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell. Biochem. 2006, 287, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.-O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Xu, M. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Romero, J.R.; Chattopadhyay, N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol. Cell. Endocrinol. 2008, 287, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin Enhanced Paclitaxel Therapeutic Effects Towards PC-3 Prostate Cancer Through ER Stress Induction and ROS Production. OncoTargets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cwiklinski, K.; Mahajan, S.D.; Schwartz, S.A.; Aalinkeel, R. Combination Modality Using Quercetin to Enhance the Efficacy of Docetaxel in Prostate Cancer Cells. Cancers 2023, 15, 902. [Google Scholar] [CrossRef] [PubMed]
- Omoboyede, V.; Ibrahim, O.; Umar, H.I.; Oke, G.A.; Onile, O.S.; Chukwuemeka, P.O. Computer-aided analysis of quercetin mechanism of overcoming docetaxel resistance in docetaxel-resistant prostate cancer. J. Genet. Eng. Biotechnol. 2023, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Prajapati, K.S.; Kushwaha, P.P.; Shuaib, M.; Kumar Singh, A.; Kumar, S.; Gupta, S. Resistance to second generation antiandrogens in prostate cancer: Pathways and mechanisms. Cancer Drug Resist. 2020, 3, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.I. Splicing Factors Have an Essential Role in Prostate Cancer Progression and Androgen Receptor Signaling. Biomolecules 2019, 9, 131. [Google Scholar] [CrossRef]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef]
- Chen, D.; Chou, F.J.; Chen, Y.; Huang, C.P.; Tian, H.; Wang, Y.; Niu, Y.; You, B.; Yeh, S.; Xing, N.; et al. Targeting the radiation-induced ARv7-mediated circNHS/miR-512-5p/XRCC5 signaling with Quercetin increases prostate cancer radiosensitivity. J. Exp. Clin. Cancer Res. CR 2022, 41, 235. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hung Nguyen, T.; Hoa Huynh, T.; Tien Do, P.; Huynh, H. Reduction of rat prostate weight by combined quercetin-finasteride treatment is associated with cell cycle deregulation. J. Endocrinol. 2004, 181, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Hemati, M.; Haghiralsadat, F.; Yazdian, F.; Jafari, F.; Moradi, A.; Malekpour-Dehkordi, Z. Development and characterization of a novel cationic PEGylated niosome-encapsulated forms of doxorubicin, quercetin and siRNA for the treatment of cancer by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Wei, T.; Ma, X.; Cheng, Q.; Huo, S.; Zhang, C.; Zhang, Y.; Duan, X.; Liang, X.-J. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale 2016, 8, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-conjugated, PEGylated, poly-lactide-co-glycolide nanocapsules for targeted delivery of combinational chemotherapeutic drugs Docetaxel and Quercetin for prostate cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111035. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, F.; Hosseini Motlagh, N.S.; Moradi, A.; Haghiralsadat, F. Carboxylated Graphene Oxide as a Nanocarrier for Drug Delivery of Quercetin as an Effective Anticancer Agent. Iran. Biomed. J. 2022, 26, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells. Biomedicines 2023, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, N.; Rahimi, S.; Emami, H.; Kazemi, A.H.; Mohammad Taghi Kashi, R.; Heidarian, R. The Effect of Quercetin Nanosuspension on Prostate Cancer Cell Line LNCaP via Hedgehog Signaling Pathway. Rep. Biochem. Mol. Biol. 2021, 10, 69–75. [Google Scholar] [CrossRef]
- Castro, C.C.; Pagnussat, A.S.; Orlandi, L.; Worm, P.; Moura, N.; Etgen, A.M.; Netto, C.A. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012, 1474, 82–90. [Google Scholar] [CrossRef]
- Wu, X.T.; Wang, B.; Wei, J.N. Coumestrol promotes proliferation and osteoblastic differentiation in rat bone marrow stromal cells. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hsieh, D.; Yang, Y.-L.; Xu, Z.; Peto, C.; Jablons, D.M.; You, L. Coumestrol from the national cancer Institute’s natural product library is a novel inhibitor of protein kinase CK2. BMC Pharmacol. Toxicol. 2013, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Mu, Q. Phytoestrogen biological actions on mammalian reproductive system and cancer growth. Sci. Pharm. 2011, 79, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Jeong, M.; Bazer, F.W.; Song, G. Coumestrol inhibits proliferation and migration of prostate cancer cells by regulating AKT, ERK1/2, and JNK MAPK cell signaling cascades. J. Cell. Physiol. 2017, 232, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Cho, S.; Park, E.; Kim, B.; Sohn, E.J.; Oh, B.; Lee, E.-O.; Lee, H.-J.; Kim, S.-H. Coumestrol suppresses hypoxia inducible factor 1α by inhibiting ROS mediated sphingosine kinase 1 in hypoxic PC-3 prostate cancer cells. Bioorganic Med. Chem. Lett. 2014, 24, 2560–2564. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Lee, M.J.; Ancellin, N.; Liu, C.H.; Thangada, S.; Thompson, B.D.; Kluk, M. Sphingosine-1-phosphate: Extracellular mediator or intracellular second messenger. Biochem. Pharmacol. 1999, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Cuvillier, O.; Edsall, L.C.; Kohama, T.; Menzeleev, R.; Olah, Z.; Olivera, A.; Pirianov, G.; Thomas, D.M.; Tu, Z.; et al. Sphingosine-1-Phosphate in Cell Growth and Cell Deatha. Ann. N. Y. Acad. Sci. 1998, 845, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Oskouian, B.; Saba, J. Sphingosine-1-Phosphate Metabolism and Intestinal Tumorigenesis: Lipid Signaling Strikes Again. Cell Cycle 2007, 6, 522–527. [Google Scholar] [CrossRef]
- Strom, S.S.; Yamamura, Y.; Duphorne, C.M.; Spitz, M.R.; Babaian, R.J.; Pillow, P.C.; Hursting, S.D. Phytoestrogen intake and prostate cancer: A case-control study using a new database. Nutr. Cancer 1999, 33, 20–25. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Hedelin, M.; Bälter, K.A.; Chang, E.T.; Bellocco, R.; Klint, A.; Johansson, J.E.; Wiklund, F.; Thellenberg-Karlsson, C.; Adami, H.O.; Grönberg, H. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate 2006, 66, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Levenson, A.S.; Biswas, P.K. Structural insights into Resveratrol’s antagonist and partial agonist actions on estrogen receptor alpha. BMC Struct. Biol. 2013, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.H.; Chen, J.C.; He, Y.L.; Xu, J.J.; Mei, Y.A. Resveratrol inhibits K(v)2.2 currents through the estrogen receptor GPR30-mediated PKC pathway. Am. J. Physiol. Cell Physiol. 2013, 305, C547–C557. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Chen, J.; Yang, S.; Liu, B.; Zhang, L.; Zhang, Q.; Cheng, J.C.; Fang, L. Resveratrol stimulates StAR expression and progesterone production by GPER-mediated downregulation of Snail expression in human granulosa cells. J. Food Drug Anal. 2023, 31, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Moradi, F.; Maddalena, L.A.; Ferreira-Tollstadius, B.; Selim, S.; Stuart, J.A. Resveratrol integrates metabolic and growth effects in PC3 prostate cancer cells-involvement of prolyl hydroxylase and hypoxia inducible factor-1. Oncol. Lett. 2019, 17, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.G.; Go, R.E.; Hwang, K.A.; Choi, K.C. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J Steroid Biochem. Mol. Biol. 2019, 192, 105406. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, H.; Cao, J.; Nan, N.; Zhai, X.; Liu, Y.; Chong, T. Resveratrol inhibits TRAF6/PTCH/SMO signal and regulates prostate cancer progression. Cytotechnology 2022, 74, 549–558. [Google Scholar] [CrossRef]
- Khusbu, F.Y.; Zhou, X.; Roy, M.; Chen, F.-Z.; Cao, Q.; Chen, H.-C. Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int. J. Biochem. Cell Biol. 2020, 118, 105644. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-M.; Chin, Y.-T.; Ho, Y.; Chen, Y.-R.; Yang, Y.-N.; Yang, Y.-C.; Shih, Y.-J.; Lin, T.-I.; Lin, H.-Y.; Davis, P.J. Resveratrol induces sumoylated COX-2-dependent anti-proliferation in human prostate cancer LNCaP cells. Food Chem. Toxicol. 2018, 112, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C. Antiproliferative effects of resveratrol and the mediating role of resveratrol targeting protein NQO2 in androgen receptor-positive, hormone-non-responsive CWR22Rv1 cells. Anticancer Res. 2009, 29, 3011–3017. [Google Scholar] [PubMed]
- Hsieh, T.-C.; Wu, J.M. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp. Cell Res. 1999, 249, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol enhances prostate cancer cell response to ionizing radiation. Modulation of the AMPK, Akt and mTOR pathways. Radiat. Oncol. 2011, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Empl, M.T.; Albers, M.; Wang, S.; Steinberg, P. The Resveratrol Tetramer r-Viniferin Induces a Cell Cycle Arrest Followed by Apoptosis in the Prostate Cancer Cell Line LNCaP. Phytother. Res. 2015, 29, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, S.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21 WAF1/CIP1 and p27 KIP1 pathway. Oncotarget 2017, 8, 17216. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.; Pozo-Guisado, E.; Clementi, M.; Castellón, E.; Fernandez-Salguero, P. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: Modulation of the phosphoinositide 3-kinase pathway. Br. J. Cancer 2007, 96, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.E.; Patel, B.B.; Wang, J.; Arabshahi, A.; Eltoum, I.A.; Lamartiniere, C.A. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007, 28, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Hermoso, M.A.; Pozo-Guisado, E.; Fernández-Salguero, P.M.; Castellón, E.A. Regulation of cell survival by resveratrol involves inhibition of NFκB-regulated gene expression in prostate cancer cells. Prostate 2009, 69, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Iizumi, Y.; Watanabe, M.; Masuda, M.; Morita, M.; Aono, Y.; Toriyama, S.; Oishi, M.; Goi, W.; Sakai, T. Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death Dis. 2016, 7, e2211. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Eroglu, E.; Stokes, J.A., III; Scissum-Gunn, K.; Saldanha, S.N.; Singh, U.P.; Manne, U.; Ponnazhagan, S.; Mishra, M.K. Resveratrol induces mitochondria-mediated, caspase-independent apoptosis in murine prostate cancer cells. Oncotarget 2017, 8, 20895. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Z.; Zhang, X. Resveratrol induces apoptosis in murine prostate cancer cells via hypoxia-inducible factor 1-alpha (HIF-1α)/reactive oxygen species (ROS)/P53 signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8970. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Nihal, M.; Fu, V.X.; Jarrard, D.F.; Ahmad, N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006, 5, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Pozo-Guisado, E.; Alvarez-Barrientos, A.; Fernandez-Salguero, P.M.; Castellón, E.A. Mechanisms Involved in Resveratrol-Induced Apoptosis and Cell Cycle Arrest in Prostate Cancer—Derived Cell Lines. J. Androl. 2007, 28, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Ferruelo, A.; Romero, I.; Cabrera, P.; Arance, I.; Andrés, G.; Angulo, J. Effects of resveratrol and other wine polyphenols on the proliferation, apoptosis and androgen receptor expression in LNCaP cells. Actas Urológicas Españolas (Engl. Ed.) 2014, 38, 397–404. [Google Scholar] [CrossRef]
- Ganapathy, S.; Chen, Q.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS ONE 2010, 5, e15627. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Chen, Q.; Siddiqui, I.; Sarva, K.; Srivastava, R.K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4′, 5 tri-hydroxystilbene): Molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2016, 55, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef]
- Fraser, S.P.; Peters, A.; Fleming-Jones, S.; Mukhey, D.; Djamgoz, M.B.A. Resveratrol: Inhibitory effects on metastatic cell behaviors and voltage-gated Na+ channel activity in rat prostate cancer in vitro. Nutr. Cancer 2014, 66, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, Z.; Shi, J.; Feng, Y.; Yu, M.; Sun, Y.; Zhuang, Q.; Liang, B.; Luo, G.; Xu, X.; et al. Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells. Prostate 2020, 80, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Resveratrol Suppresses Prostate Cancer Epithelial Cell Scatter/Invasion by Targeting Inhibition of Hepatocyte Growth Factor (HGF) Secretion by Prostate Stromal Cells and Upregulation of E-cadherin by Prostate Cancer Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 1760. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Harada, N.; Tanimori, S.; NAkANO, Y.; Inui, H.; Yamaji, R. Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J. Nutr. Sci. Vitaminol. 2014, 60, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lodi, A.; Saha, A.; Lu, X.; Wang, B.; Sentandreu, E.; Collins, M.; Kolonin, M.G.; DiGiovanni, J.; Tiziani, S. Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ Precis. Oncol. 2017, 1, 30. [Google Scholar] [CrossRef] [PubMed]
- Saralkar, P.; Dash, A.K. Alginate nanoparticles containing curcumin and resveratrol: Preparation, characterization, and in vitro evaluation against DU145 prostate cancer cell line. AAPS PharmSciTech 2017, 18, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-Loaded Nanoparticles Based on Poly (epsilon-caprolactone) and Poly (d, l-lactic-co-glycolic acid)–Poly (ethylene glycol) Blend for Prostate Cancer Treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Lillard, J.W., Jr.; Singh, R. Reversal of drug resistance by planetary ball milled (PBM) nanoparticle loaded with resveratrol and docetaxel in prostate cancer. Cancer Lett. 2018, 427, 49–62. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef]
- Thipe, V.C.; Amiri, K.P.; Bloebaum, P.; Karikachery, A.R.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int. J. Nanomed. 2019, 14, 4413. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E. A Resveratrol-Loaded Poly(2-hydroxyethyl methacrylate)-Chitosan Based Nanotherapeutic: Characterization and In Vitro Cytotoxicity Against Prostate Cancer. J. Nanosci. Nanotechnol. 2021, 21, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Z.; Chen, Y.; Gao, D.; Wang, P.; Lin, Y.; Wang, Y.; Wang, F.; Han, Y.; Yuan, H. Co-delivery of Docetaxel and Resveratrol by liposomes synergistically boosts antitumor efficiency against prostate cancer. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, evaluation, pharmacokinetic and biodistribution estimation of resveratrol-loaded solid lipid nanoparticles for prostate cancer targeting. J. Microencapsul. 2022, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, D.; Soto, A.; Gil-Araujo, B.; Gallego, B.; Chiloeches, A.; Lasa, M. Resveratrol promotes apoptosis through the induction of dual specificity phosphatase 1 and sensitizes prostate cancer cells to cisplatin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 124, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, Y.J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Q.; Li, J.; Xiao, M.; Gao, T.; Zhang, X.; Lu, G.; Wang, J.; Guo, Y.; Wen, P.; et al. Co-Treatment With Resveratrol and FGF1 Protects Against Acute Liver Toxicity After Doxorubicin Treatment via the AMPK/NRF2 Pathway. Front. Pharmacol. 2022, 13, 940406. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; DeMarco, V.G.; Nicholl, M.B. Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci. 2012, 103, 1090–1098. [Google Scholar] [CrossRef]
- Fang, Y.; Herrick, E.J.; Nicholl, M.B. A Possible Role for Perforin and Granzyme B in Resveratrol-Enhanced Radiosensitivity of Prostate Cancer. J. Androl. 2012, 33, 752–760. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lien, H.-M.; Kao, M.-C.; Lo, U.-G.; Lin, L.-C.; Lin, C.-J.; Chang, S.-J.; Chen, C.-C.; Hsieh, J.-T.; Lin, H. Sensitization of radioresistant prostate cancer cells by resveratrol isolated from arachis hypogaea stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef] [PubMed]
- El-Benhawy, S.A.; Morsi, M.I.; Fahmy, E.I.; Soula, M.A.; Khalil, F.; Arab, A.R. Role of Resveratrol as Radiosensitizer by Targeting Cancer Stem Cells in Radioresistant Prostate Cancer Cells (PC-3). Asian Pac. J. Cancer Prev. 2021, 22, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Heath, E.I.; Taplin, M.-E.; Stein, M.N.; Bubley, G.J.; Pili, R.; Mayer, T.M.; Zhou, X.C.; Hudson, T.; Abbas, M.; et al. A phase II study of muscadine grape skin extract in men with biochemically recurrent prostate cancer. J. Clin. Oncol. 2017, 35, 248. [Google Scholar] [CrossRef]
- Sautour, M.; Mitaine-Offer, A.-C.; Lacaille-Dubois, M.-A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Djerassi, C.; Rosenkranz, G.; Pataki, J.; Kaufmann, S. Steroids, XXVII. Synthesis of allopregnane-3beta, 11beta, 17alpha-, 20beta, 21-pentol from cortisone and diosgenin. J. Biol. Chem. 1952, 194, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.J.L.S. Extraction of Diosgenin, a Bioactive Compound from Natural Source Dioscorea alata Var purpurae. J. Anal. Bioanal. Tech. 2012, 3, 141. [Google Scholar] [CrossRef]
- Król-Kogus, B.; Lamine, K.M.; Migas, P.; Boudjeniba, M.; Krauze-Baranowska, M. HPTLC determination of diosgenin in fenugreek seeds. Acta Pharm. 2018, 68, 97–107. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Chaudhary, P.S.; Syed, B.A.; Misra, R.; Bagali, P.G.; Vitalini, S.; Iriti, M. Validation of a method for diosgenin extraction from fenugreek (Trigonella foenum-graecum L.). Acta Sci. Pol. Technol. Aliment. 2018, 17, 377–385. [Google Scholar] [CrossRef]
- Selim, S.; Al Jaouni, S. Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Complement. Altern. Med. 2015, 15, 301. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)-An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Liagre, B.; Bertrand, J.; Leger, D.Y.; Beneytout, J.L. Diosgenin, a plant steroid, induces apoptosis in COX-2 deficient K562 cells with activation of the p38 MAP kinase signalling and inhibition of NF-kappaB binding. Int. J. Mol. Med. 2005, 16, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Leger, D.Y.; Liagre, B.; Corbière, C.; Cook-Moreau, J.; Beneytout, J.L. Diosgenin induces cell cycle arrest and apoptosis in HEL cells with increase in intracellular calcium level, activation of cPLA2 and COX-2 overexpression. Int. J. Oncol. 2004, 25, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Wang, Z.; Ju, Y.; Wong, R.N.; Wu, Q.Y. Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother. Pharmacol. 2005, 55, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fan, J.; Wang, Q.; Ju, D.; Feng, M.; Li, J.; Guan, Z.B.; An, D.; Wang, X.; Ye, L. Diosgenin induces ROS-dependent autophagy and cytotoxicity via mTOR signaling pathway in chronic myeloid leukemia cells. Phytomedicine 2016, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Xu, L.; Yin, L.; Qi, Y.; Li, H.; Xu, Y.; Han, X.; Peng, J.; Wan, X. Cytotoxicity of dioscin in human gastric carcinoma cells through death receptor and mitochondrial pathways. J. Appl. Toxicol. JAT 2013, 33, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, L.; Zheng, L.; Yin, L.; Qi, Y.; Han, X.; Xu, Y.; Peng, J. Potent effects of dioscin against gastric cancer in vitro and in vivo. Phytomedicine 2016, 23, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Rahmati-Yamchi, M.; Ghareghomi, S.; Haddadchi, G.; Milani, M.; Aghazadeh, M.; Daroushnejad, H. Fenugreek extract diosgenin and pure diosgenin inhibit the hTERT gene expression in A549 lung cancer cell line. Mol. Biol. Rep. 2014, 41, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, D.; Li, Z.; Gao, Y.; Chen, H. Synthesis and potent cytotoxic activity of a novel diosgenin derivative and its phytosomes against lung cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1933–1942. [Google Scholar] [CrossRef]
- Mohammad, R.Y.; Somayyeh, G.; Gholamreza, H.; Majid, M.; Yousef, R. Diosgenin inhibits hTERT gene expression in the A549 lung cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 6945–6948. [Google Scholar] [CrossRef]
- Srinivasan, S.; Koduru, S.; Kumar, R.; Venguswamy, G.; Kyprianou, N.; Damodaran, C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int. J. Cancer 2009, 125, 961–967. [Google Scholar] [CrossRef]
- He, Z.; Chen, H.; Li, G.; Zhu, H.; Gao, Y.; Zhang, L.; Sun, J. Diosgenin inhibits the migration of human breast cancer MDA-MB-231 cells by suppressing Vav2 activity. Phytomedicine 2014, 21, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fernandez, P.P.; Rajendran, P.; Hui, K.M.; Sethi, G. Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett. 2010, 292, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, J.; Wu, Y.; Lei, S.; Liu, H.; Meng, Q.; Xia, Z. Diosgenin inhibited the expression of TAZ in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, Y.; Niu, C.; Cheng, Y. Diosgenin increased DDX3 expression in hepatocellular carcinoma. Am. J. Transl. Res. 2018, 10, 3590–3599. [Google Scholar] [PubMed]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Malisetty, V.S.; Patlolla, J.M.R.; Raju, J.; Marcus, L.A.; Choi, C.-I.; Rao, C.V. Chemoprevention of colon cancer by diosgenin, a steroidal saponin constituent of fenugreek. Cancer Res. 2005, 65, 580. [Google Scholar]
- Lepage, C.; Léger, D.Y.; Bertrand, J.; Martin, F.; Beneytout, J.L.; Liagre, B. Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett. 2011, 301, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and Treatment of Colorectal Cancer by Natural Agents From Mother Nature. Curr. Color. Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, Y.; Gao, J.; Zhong, K.; Wei, H.; Li, K.; Tang, M.; Zhao, X.; Liu, X.; Nie, C.; et al. Diosgenin exhibits tumor suppressive function via down-regulation of EZH2 in pancreatic cancer cells. Cell Cycle 2019, 18, 1745–1758. [Google Scholar] [CrossRef]
- Long, C.; Chen, J.; Zhou, H.; Jiang, T.; Fang, X.; Hou, D.; Liu, P.; Duan, H. Diosgenin exerts its tumor suppressive function via inhibition of Cdc20 in osteosarcoma cells. Cell Cycle 2019, 18, 346–358. [Google Scholar] [CrossRef]
- Hernández-Vázquez, J.M.V.; López-Muñoz, H.; Escobar-Sánchez, M.L.; Flores-Guzmán, F.; Weiss-Steider, B.; Hilario-Martínez, J.C.; Sandoval-Ramírez, J.; Fernández-Herrera, M.A.; Sánchez Sánchez, L. Apoptotic, necrotic, and antiproliferative activity of diosgenin and diosgenin glycosides on cervical cancer cells. Eur. J. Pharmacol. 2020, 871, 172942. [Google Scholar] [CrossRef] [PubMed]
- Corbiere, C.; Liagre, B.; Terro, F.; Beneytout, J.L. Induction of antiproliferative effect by diosgenin through activation of p53, release of apoptosis-inducing factor (AIF) and modulation of caspase-3 activity in different human cancer cells. Cell Res. 2004, 14, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, K.K.; Dey, G.; Pal, I.; Majumder, A.; MaitiChoudhury, S.; Kundu, S.C.; Mandal, M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS ONE 2012, 7, e46641. [Google Scholar] [CrossRef] [PubMed]
- Pons-Fuster López, E.; Wang, Q.T.; Wei, W.; López Jornet, P. Potential chemotherapeutic effects of diosgenin, zoledronic acid and epigallocatechin-3-gallate on PE/CA-PJ15 oral squamous cancer cell line. Arch. Oral Biol. 2017, 82, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Shih, Y.W.; Huang, H.C.; Cheng, H.W. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS ONE 2011, 6, e20164. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhou, J.; Qin, X.; Shi, X.; Zeng, Q.; Liu, J.; Yan, S.; Zhang, L. Diosgenin-induced autophagy and apoptosis in a human prostate cancer cell line. Mol. Med. Rep. 2016, 14, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.C.; Jan, C.R.; Liang, W.Z. Exploring the impact of a naturally occurring sapogenin diosgenin on underlying mechanisms of Ca(2+) movement and cytotoxicity in human prostate cancer cells. Environ. Toxicol. 2020, 35, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, J.J.; Zhou, S.J.; Chen, J.; Hu, Q.; Pu, J.X.; Lu, J.L. Diosgenin inhibits the expression of NEDD4 in prostate cancer cells. Am. J. Transl. Res. 2019, 11, 3461–3471. [Google Scholar] [PubMed]
- Shabbeer, S.; Sobolewski, M.; Anchoori, R.K.; Kachhap, S.; Hidalgo, M.; Jimeno, A.; Davidson, N.; Carducci, M.A.; Khan, S.R. Fenugreek: A naturally occurring edible spice as an anticancer agent. Cancer Biol. Ther. 2009, 8, 272–278. [Google Scholar] [CrossRef]
- Lorin, S.; Hamaï, A.; Mehrpour, M.; Codogno, P. Autophagy regulation and its role in cancer. Semin. Cancer Biol. 2013, 23, 361–379. [Google Scholar] [CrossRef]
- Thorburn, A.; Thamm, D.H.; Gustafson, D.L. Autophagy and cancer therapy. Mol. Pharmacol. 2014, 85, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Polewska, J. Autophagy--molecular mechanism, apoptosis and cancer. Postep. Hig. I Med. Dosw. 2012, 66, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Kao, M.C.; Way, T.D.; Ho, C.T.; Fu, E. Diosgenin suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition by down-regulation of Mdm2 and vimentin. J. Agric. Food Chem. 2011, 59, 5357–5363. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.F.; Xiong, C.M.; Ruan, J.L. Inhibitory effect of diosgenin on experimentally induced benign prostatic hyperplasia in rats. J. Huazhong Univ. Sci. Technology. Med. Sci. = Hua Zhong Ke Ji Da Xue Xue Bao. Yi Xue Ying De Wen Ban = Huazhong Keji Daxue Xuebao. Yixue Yingdewen Ban 2016, 36, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tang, R.; Ding, L.; Zheng, R.; Liu, Y.; Yin, L.; Fu, Y.; Deng, T.; Li, X. Diosgenin inhibits prostate cancer progression by inducing UHRF1 protein degradation. Eur. J. Pharmacol. 2023, 942, 175522. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.; Shanmugam, M.K.; Siveen, K.S.; Ong, T.H.; Yang, M.H.; Lee, J.H.; Rajendran, P.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; et al. Diosgenin attenuates tumor growth and metastasis in transgenic prostate cancer mouse model by negatively regulating both NF-κB/STAT3 signaling cascades. Eur. J. Pharmacol. 2021, 906, 174274. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, F.; Zhao, G.D.; Wang, D.F.; Lou, H.X.; Liu, Z.P. Synthetic studies towards 1α-hydroxysolasodine from diosgenin and the unexpected tetrahydrofuran ring opening in the Birch reduction process. Steroids 2015, 104, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Kaushal, T.; Ashraf, R.; Singh, A.; Chand Gupta, A.; Prakash, O.; Sarkar, J.; Chanda, D.; Bawankule, D.U.; Khan, F.; et al. (22β,25R)-3β-Hydroxy-spirost-5-en-7-iminoxy-heptanoic acid exhibits anti-prostate cancer activity through caspase pathway. Steroids 2017, 119, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mironov, M.E.; Oleshko, O.S.; Pokrovskii, M.A.; Rybalova, T.V.; Pechurov, V.K.; Pokrovskii, A.G.; Cheresis, S.V.; Mishinov, S.V.; Stupak, V.V.; Shults, E.E. 6-(4′-Aryl-1′,2′,3′-triazolyl)-spirostan-3,5-diols and 6-(4′-Aryl-1′,2′,3′-triazolyl)-7-hydroxyspirosta-1,4-dien-3-ones: Synthesis and analysis of their cytotoxicity. Steroids 2019, 151, 108460. [Google Scholar] [CrossRef]
- Michalak, O.; Krzeczyński, P.; Cieślak, M.; Cmoch, P.; Cybulski, M.; Królewska-Golińska, K.; Kaźmierczak-Barańska, J.; Trzaskowski, B.; Ostrowska, K. Synthesis and anti-tumour, immunomodulating activity of diosgenin and tigogenin conjugates. J Steroid Biochem. Mol. Biol. 2020, 198, 105573. [Google Scholar] [CrossRef]
- Parama, D.; Boruah, M.; Yachna, K.; Rana, V.; Banik, K.; Harsha, C.; Thakur, K.K.; Dutta, U.; Arya, A.; Mao, X.; et al. Diosgenin, a steroidal saponin, and its analogs: Effective therapies against different chronic diseases. Life Sci. 2020, 260, 118182. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Kaufman, D.; George, D.; Oh, W.K.; Kazanis, M.; Manola, J.; Kantoff, P.W. Selective aromatase inhibition for patients with androgen-independent prostate carcinoma. Cancer 2002, 95, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Petroni, G.R.; Fisch, M.J.; Myers, C.E.; Theodorescu, D.; Cohen, R.B. Use of the aromatase inhibitor anastrozole in the treatment of patients with advanced prostate carcinoma. Cancer 2001, 92, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.Z.; Hennenlotter, J.; Scharpf, M.; Todenhofer, T.; Heni, M.; Guirguis, A.; Peter, A.; Staiger, H.; Fritsche, A.; Stenzl, A.; et al. The classical nuclear estrogen receptors ERα and ERβ, and the G-protein-coupled receptor-30 are differently expressed in prostate cancer depending on diabetes status. In Proceedings of the EASD Virtual Meeting, Munich, Germany, 12–16 September 2016. [Google Scholar]
- Gross, M.; Ramirez, C.; Luthringer, D.; Nepomuceno, E.; Vollmer, R.; Burchette, J.; Freedland, S.J. Expression of androgen and estrogen related proteins in normal weight and obese prostate cancer patients. Prostate 2009, 69, 520–527. [Google Scholar] [CrossRef] [PubMed]
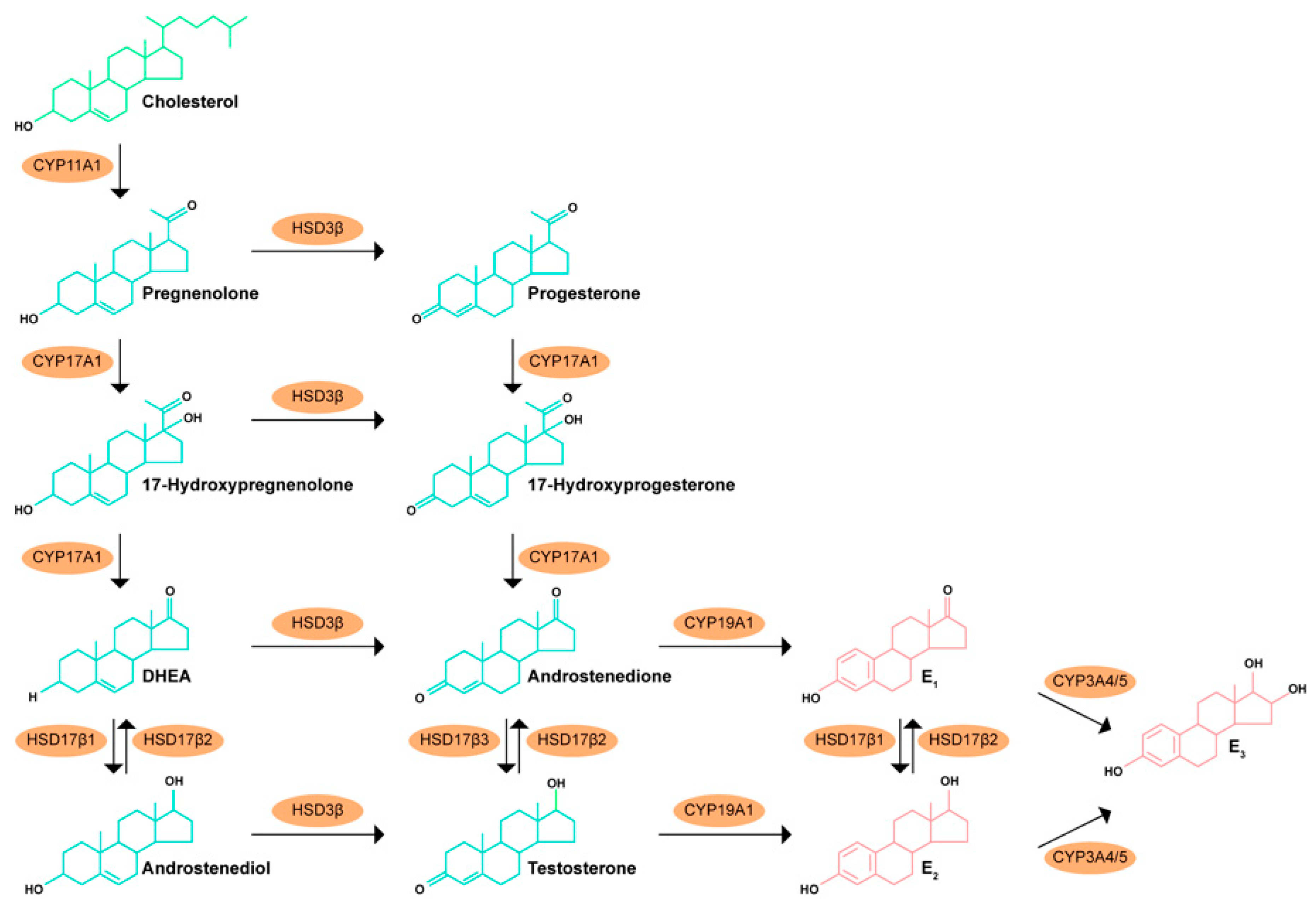

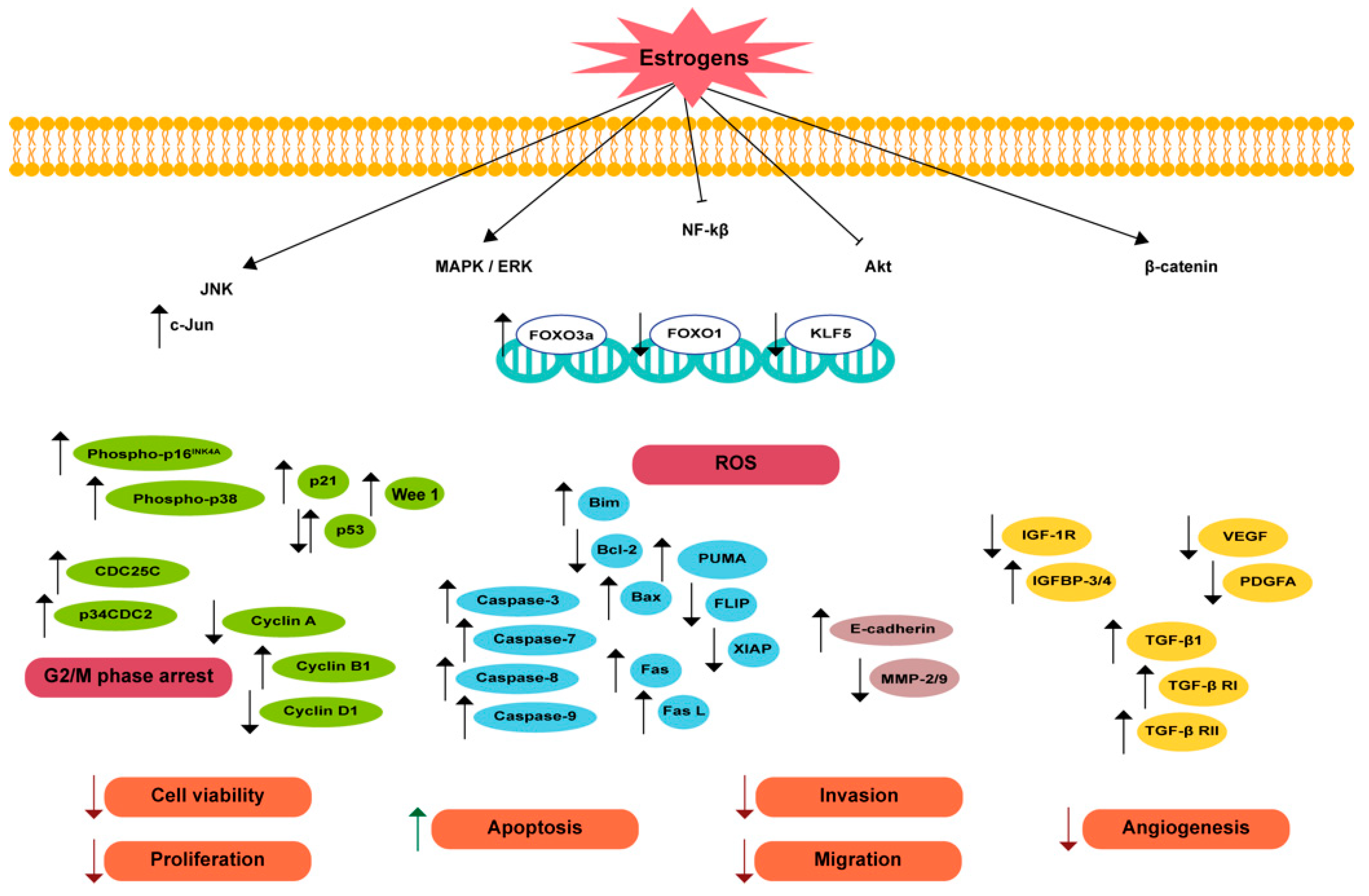

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, M.I.; Carvalho, T.M.A.; Macário-Monteiro, J.; Cardoso, H.J.; Correia, S.; Vaz, C.V.; Duarte, A.P.; Socorro, S. The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens. Biomedicines 2024, 12, 1636. https://doi.org/10.3390/biomedicines12081636
Figueira MI, Carvalho TMA, Macário-Monteiro J, Cardoso HJ, Correia S, Vaz CV, Duarte AP, Socorro S. The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens. Biomedicines. 2024; 12(8):1636. https://doi.org/10.3390/biomedicines12081636
Chicago/Turabian StyleFigueira, Marília I., Tiago M. A. Carvalho, Joana Macário-Monteiro, Henrique J. Cardoso, Sara Correia, Cátia V. Vaz, Ana P. Duarte, and Sílvia Socorro. 2024. "The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens" Biomedicines 12, no. 8: 1636. https://doi.org/10.3390/biomedicines12081636
APA StyleFigueira, M. I., Carvalho, T. M. A., Macário-Monteiro, J., Cardoso, H. J., Correia, S., Vaz, C. V., Duarte, A. P., & Socorro, S. (2024). The Pros and Cons of Estrogens in Prostate Cancer: An Update with a Focus on Phytoestrogens. Biomedicines, 12(8), 1636. https://doi.org/10.3390/biomedicines12081636








