Decreased FXR Agonism in the Bile Acid Pool Is Associated with Impaired FXR Signaling in a Pig Model of Pediatric NAFLD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Serum Biochemistry and Hormones
2.3. Histology and Immunohistochemistry
2.4. Metabolomics and Transcriptomics Analyses
2.5. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahota, A.K.; Shapiro, W.L.; Newton, K.P.; Kim, S.T.; Chung, J.; Schwimmer, J.B. Incidence of nonalcoholic fatty liver disease in children: 2009–2018. Pediatrics 2020, 146, e20200771. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.L.; Schwimmer, J.B. Epidemiology of pediatric nonalcoholic fatty liver disease. Clin. Liver Dis. 2021, 17, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.L.; Golshan, S.; Harlow, K.E.; Angeles, J.E.; Durelle, J.; Goyal, N.P.; Newton, K.P.; Sawh, M.C.; Hooker, J.; Sy, E.Z.; et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J. Pediatr. 2019, 207, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Conjeevaram Selvakumar, P.K.; Kabbany, M.N.; Lopez, R.; Rayas, M.S.; Lynch, J.L.; Alkhouri, N. Prevalence of suspected nonalcoholic fatty liver disease in lean adolescents in the United States. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 75–79. [Google Scholar] [CrossRef]
- Fang, Y.L.; Chen, H.; Wang, C.L.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Charatcharoenwitthaya, P.; Treeprasertsuk, S.; Benson, J.T.; Enders, F.B.; Angulo, P. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut 2009, 58, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Grimaldi, C.; Liccardo, D.; Francalanci, P.; Monti, L.; Castellano, A.; de Ville de Goyet, J. Non-alcoholic fatty liver disease and hepatocellular carcinoma in a 7-year-old obese boy: Coincidence or comorbidity? Pediatr. Obes. 2014, 9, e99–e102. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology; Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef]
- Fiorucci, S.; Rizzo, G.; Donini, A.; Distrutti, E.; Santucci, L. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol. Med. 2007, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Regulation of bile acid synthesis: Pathways; nuclear receptors; and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Kim, D.H.; Ryerson, D.; Kim, Y.C.; Sun, H.; Kong, B.; Yau, P.; Guo, G.; Xu, H.E.; Kemper, B.; et al. Postprandial FGF19-induced phosphorylation by Src is critical for FXR function in bile acid homeostasis. Nat. Commun. 2018, 9, 2590. [Google Scholar] [CrossRef] [PubMed]
- Massafra, V.; Milona, A.; Vos, H.R.; Ramos, R.J.J.; Gerrits, J.; Willemsen, E.C.L.; Ramos Pittol, J.M.; Ijssennagger, N.; Houweling, M.; Prinsen, H.C.M.T.; et al. Farnesoid X receptor activation promotes hepatic amino acid catabolism and ammonium clearance in mice. Gastroenterology 2017, 152, 1462–1476.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Chen, W.D.; Wang, M.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 2008, 48, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signaling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Janus, D.; Dolezal-Oltarzewska, K.; Kalicka-Kasperczyk, A.; Poplawska, K.; Drozdz, D.; Sztefko, K.; Starzyk, J.B. A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. J Pediatr. Endocrinol. Metab. 2012, 25, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Mosca, A.; Della Corte, C.; Veraldi, S.; De Vito, R.; De Stefanis, C.; D’Oria, V.; Jahnel, J.; Zohrer, E.; et al. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int. 2018, 38, 342–349. [Google Scholar] [CrossRef]
- Alisi, A.; Ceccarelli, S.; Panera, N.; Prono, F.; Petrini, S.; De Stefanis, C.; Pezzullo, M.; Tozzi, A.; Villani, A.; Bedogni, G.; et al. Association between serum atypical fibroblast growth factors 21 and 19 and pediatric nonalcoholic fatty liver disease. PLoS ONE 2013, 8, e67160. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.K.; et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Karpen, S.J.; Dawson, P.A.; Arrese, M.; Trauner, M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017, 65, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Chevre, R.; Trigueros-Motos, L.; Castaño, D.; Chua, T.; Corlianò, M.; Patankar, J.V.; Sng, L.; Sim, L.; Juin, T.L.; Carissimo, G.; et al. Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice. FASEB J. 2018, 32, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Patankar, J.V.; Wong, C.K.; Morampudi, V.; Gibson, W.T.; Vallance, B.; Ioannou, G.N.; Hayden, M.R. Genetic ablation of Cyp8b1 preserves host metabolic function by repressing steatohepatitis and altering gut microbiota composition. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E418–E432. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Chèvre, R.; Castaño Mayan, D.; Corlianò, M.; Cochran, B.J.; Sem, K.P.; van Dijk, T.H.; Peng, J.; Tan, L.J.; Hartimath, S.V.; et al. Haploinsufficiency of CYP8B1 associates with increased insulin sensitivity in humans. J. Clin. Investig. 2022, 132, e152961. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.V.; Smith, V.A.; Melnyk, M.; Burd, M.A.; Sprayberry, K.A.; Edwards, M.S.; Peterson, D.G.; Bennet, D.C.; Fanter, R.K.; Columbus, D.A.; et al. Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G582–G609. [Google Scholar] [CrossRef]
- Zeltser, N.; Meyer, I.; Hernandez, G.V.; Trahan, M.J.; Fanter, R.K.; Abo-Ismail, M.; Glanz, H.; Strand, C.R.; Burrin, D.G.; La Frano, M.R.; et al. Neurodegeneration in juvenile Iberian pigs with diet-induced nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E592–E606. [Google Scholar] [CrossRef]
- Manjarín, R.; Dillard, K.; Coffin, M.; Hernandez, G.V.; Smith, V.A.; Noland-Lidell, T.; Gehani, T.R.; Smart, H.J.; Wheeler, K.; Sprayberry, K.A.; et al. Dietary fat composition shapes bile acid metabolism and severity of liver injury in a pig model of pediatric NAFLD. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E187–E206. [Google Scholar] [CrossRef]
- Khairnar, R.; Islam, M.A.; Fleishman, J.; Kumar, S. Shedding light on non-alcoholic fatty liver disease: Pathogenesis, molecular mechanisms, models, and emerging therapeutics. Life Sci. 2023, 1, 121185. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Manjarin, R.; Maj, M.A.; La Frano, M.R.; Glanz, H. %polynova_2way: A SAS macro for implementation of mixed models for metabolomics data. PLoS ONE 2020, 15, e0244013. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.A.; Gehani, T.R.; Immoos, C.; Medrano, M.S.; Fanter, R.K.; Strand, C.R.; Glanz, H.; Piccolo, B.D.; Abo-Ismail, M.K.; La Frano, M.R.; et al. Olive- and coconut-oil-enriched diets decreased secondary bile acids and regulated metabolic and transcriptomic markers of brain injury in the frontal cortexes of NAFLD pigs. Brain Sci. 2022, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Björkhem, I.; Reihnér, E.; Einarsson, K. The plasma level of 7 alpha-hydroxy-4-cholesten-3-one reflects the activity of hepatic cholesterol 7 alpha-hydroxylase in man. FEBS Lett. 1991, 284, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, T.C.; Marsman, H.A.; Lenicek, M.; van Werven, J.R.; Nederveen, A.J.; Jansen, P.L.; Schaap, F.G. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest. Liv. Physiol. 2010, 298, G440–G445. [Google Scholar] [CrossRef]
- Grzych, G.; Chávez-Talavera, O.; Descat, A.; Thuillier, D.; Verrijken, A.; Kouach, M.; Legry, V.; Verkindt, H.; Raverdy, V.; Legendre, B.; et al. NASH-related increases in plasma bile acid levels depend on insulin resistance. JHEP Rep. 2020, 3, 100222. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574–582.e1. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Rauch, J.B.; Behling, C.; Newbury, R.; Lavine, J.E. Obesity; insulin resistance; and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J. Pediatr. 2003, 143, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Duran-Sandoval, D.; Mautino, G.; Martin, G.; Percevault, F.; Barbier, O.; Fruchart, J.C.; Kuipers, F.; Staels, B. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 2004, 53, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.; Hoekman, M.F.; Lehmann, E.M.; Meijer, P.; Mager, W.H.; Princen, H.M. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology 1995, 21, 501–510. [Google Scholar] [PubMed]
- Rinella, M.E.; Green, R.M. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 2004, 40, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Elias, M.S.; Smolak, R.R.; Fu, T.; Borensztajn, J.; Green, R.M. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 2008, 49, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef] [PubMed]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Eggink, H.M.; van Nierop, F.S.; Schooneman, M.G.; Boelen, A.; Kalsbeek, A.; Koehorst, M.; Ten Have, G.A.M.; de Brauw, L.M.; Groen, A.K.; Romijn, J.A.; et al. Transhepatic bile acid kinetics in pigs and humans. Clin. Nutr. 2018, 37, 1406–1414. [Google Scholar] [CrossRef]
- Luo, W.; Guo, S.; Zhou, Y.; Zhu, J.; Zhao, J.; Wang, M.; Sang, L.; Wang, B.; Chang, B. Hepatocellular carcinoma: Novel understandings and therapeutic strategies based on bile acids (Review). Int. J. Oncol. 2022, 61, 117. [Google Scholar] [CrossRef]
- Myronovych, A.; Kirby, M.; Ryan, K.K.; Zhang, W.; Jha, P.; Setchell, K.D.; Dexheimer, P.J.; Aronow, B.; Seeley, R.J.; Kohli, R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity 2014, 22, 390–400. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.K.; Yin, L.; Zhang, Y. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef]
- Tully, D.C.; Rucker, P.V.; Chianelli, D.; Williams, J.; Vidal, A.; Alper, P.B.; Mutnick, D.; Bursulaya, B.; Schmeits, J.; Wu, X.; et al. Discovery of Tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J. Med. Chem. 2017, 60, 9960–9973. [Google Scholar] [CrossRef]
- Hunt, M.C.; Yang, Y.Z.; Eggertsen, G.; Carneheim, C.M.; Gåfvels, M.; Einarsson, C.; Alexson, S.E. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J. Biol. Chem. 2000, 275, 28947–28953. [Google Scholar] [CrossRef]
- Bisschop, P.H.; Bandsma, R.H.; Stellaard, F.; ter Harmsel, A.; Meijer, A.J.; Sauerwein, H.P.; Kuipers, F.; Romijn, J.A. Low-fat; high-carbohydrate and high-fat; low-carbohydrate diets decrease primary bile acid synthesis in humans. Am. J. Clin. Nutr. 2004, 79, 570–576. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, F.; Yuan, W.; Wei, Y.; Wei, M.; Zhou, Y.; Yang, Y.; Chang, Y.; Wu, X. Hepatic cholesterol accumulation ascribed to the activation of ileum Fxr-Fgf15 pathway inhibiting hepatic Cyp7a1 in high-fat diet-induced obesity rats. Life Sci. 2019, 232, 116638. [Google Scholar] [CrossRef]
- Maj, M.; Harbottle, B.; Thomas, P.A.; Hernandez, G.V.; Smith, V.A.; Edwards, M.S.; Fanter, R.K.; Glanz, H.S.; Immoos, C.; Burrin, D.G.; et al. Consumption of high-fructose corn syrup compared with sucrose promotes adiposity and increased triglyceridemia but comparable NAFLD severity in juvenile iberian pigs. J. Nutr. 2021, 151, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
| Item | CON | HFF |
|---|---|---|
| Whey protein concentrate 1 | 8.50 | 8.90 |
| Fructose 2 | 0.00 | 12.0 |
| Dextrose 2 | 6.00 | 3.00 |
| Fat Pak 80 3 | 3.20 | 0.00 |
| Hydrogenated lard 4 | 0.00 | 3.70 |
| Hydrogenated coconut oil 2 | 0.00 | 5.00 |
| Corn oil 5 | 3.20 | 0.00 |
| Xanthan gum 6 | 0.40 | 0.40 |
| Vitamin premix 7,8 | 0.32 | 0.32 |
| Mineral premix 7,8 | 1.20 | 1.20 |
| Cholesterol 7 | 0.00 | 0.60 |
| Water | 77.2 | 64.4 |
| Item | CON | HFF |
|---|---|---|
| Feed amount, L/kg BW/day | 0.18 | 0.18 |
| Dry matter, g/kg BW/day | 40.8 | 62.6 |
| Crude protein, g/kg BW/day | 12.9 | 13.0 |
| Metabolizable energy, kcal/kg BW/day | 199.3 | 302.6 |
| Carbohydrates, g/kg BW/day | 12.8 | 29.1 |
| Ether extract, g/kg BW/day | 11.2 | 16.7 |
| Amino acids, g/kg BW/day | ||
| Arginine | 0.30 | 0.29 |
| Histidine | 0.27 | 0.26 |
| Isoleucine | 0.78 | 0.76 |
| Leucine | 1.44 | 1.40 |
| Lysine | 1.11 | 1.07 |
| Methionine | 0.26 | 0.26 |
| Cysteine | 0.32 | 0.31 |
| Phenylalanine | 0.41 | 0.39 |
| Tyrosine | 0.32 | 0.32 |
| Threonine | 0.76 | 0.74 |
| Tryptophan | 0.20 | 0.20 |
| Valine | 0.69 | 0.69 |
| Fatty acids, g/kg BW/day | ||
| Caprylic | 0.00 | 0.48 |
| Capric | 0.00 | 1.07 |
| Lauric | 0.00 | 3.39 |
| Myristic | 0.07 | 1.38 |
| Palmitic | 1.78 | 2.58 |
| Stearic | 0.67 | 1.28 |
| Arachidic/eicosanoic | 0.01 | 0.06 |
| Behenic | 0.00 | 0.00 |
| Palmitoleic | 0.14 | 0.23 |
| Oleic | 3.48 | 3.88 |
| Gadoleic | 0.06 | 0.08 |
| Linoleic | 3.86 | 0.96 |
| Linolenic | 0.04 | 0.06 |
| Arachidonic | 0.02 | 0.02 |
| Cholesterol, g/kg BW/day | 0.03 | 1.12 |
| Calcium, g/kg BW/day | 0.62 | 0.62 |
| Phosphorus, g/kg BW/day | 0.43 | 0.42 |
| Fructose, g/kg BW/day | 0.00 | 21.6 |
| Dextrose, g/kg BW/day | 10.8 | 5.40 |
| Item 1,2,3 | CON | HFF |
|---|---|---|
| N° pigs (pen) | 8 (4) | 10 (5) |
| Sex (M/F) | 6/2 | 6/4 |
| Serum hormones and glucose | ||
| Insulin, µIU/mL | 9.38 ± 4.07 | 7.20 ± 2.03 |
| Glucose, mg/dL | 133.3 c ± 13.1 | 113.8 d ± 13.4 |
| HOMA 4 | 2.67 ± 0.70 | 2.05 ± 0.69 |
| Leptin, ng/mL | 4.78 ± 1.75 | 4.52 ± 1.44 |
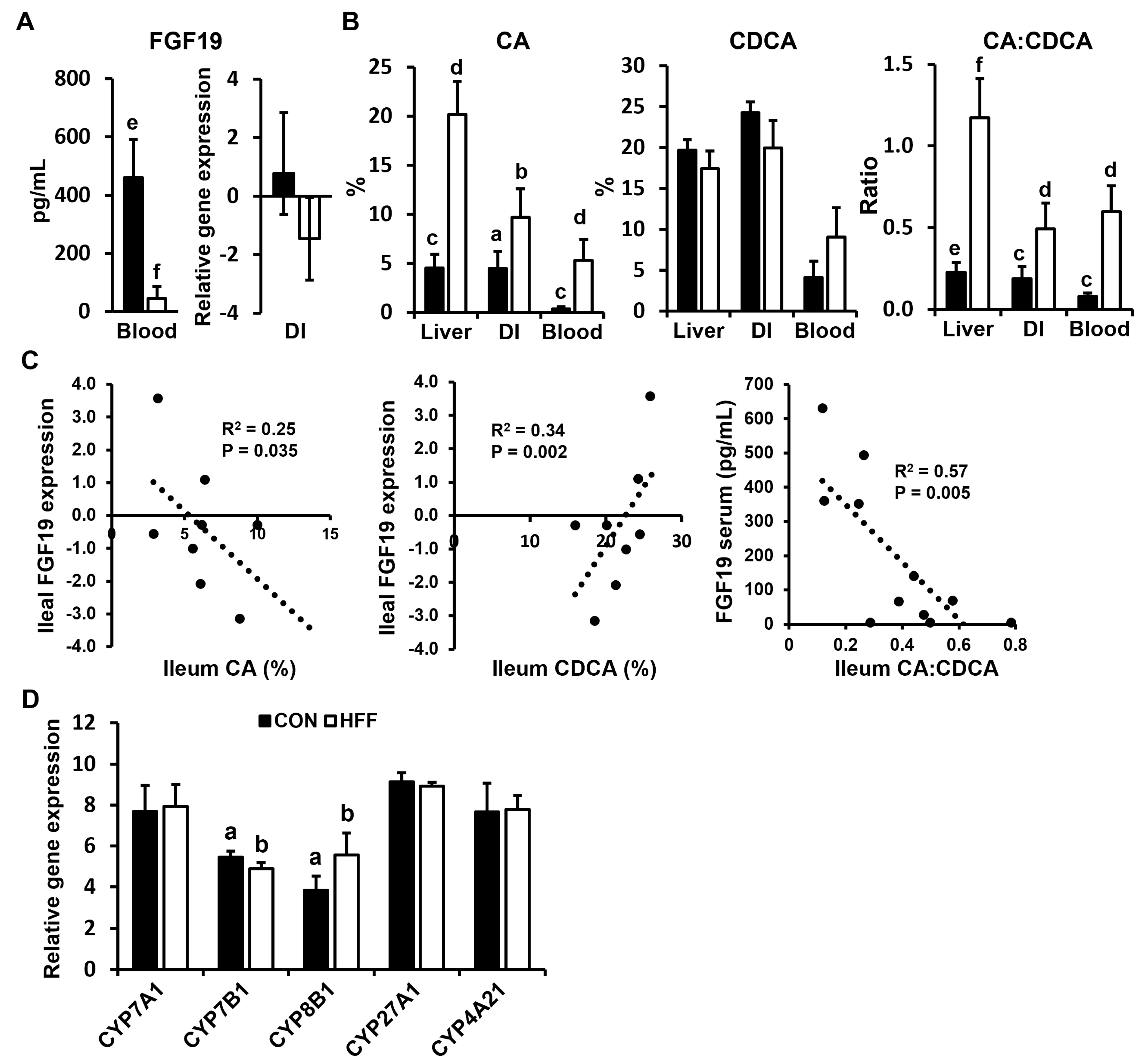
| FGF19, pg/mL | 405.6 e ± 143.6 | 74.7 f ± 74.5 |
| Liver biochemistry | ||
| ALT, U/L | 19.5 a ± 3.48 | 62.18 b ± 40.37 |
| AST, U/L | 26.9 c ± 12.8 | 199.6 d ± 149.2 |
| ALP, U/L | 332.9 ± 59.8 | 391.6 ± 149.2 |
| GGT, U/L | 29.1 a ± 5.91 | 56.8 b ± 29.4 |
| LDH, U/L | 959.3 c ± 205.8 | 3114.2 d ± 1492.7 |
| Total bilirubin, mg/dL | 0.02 a ± 0.01 | 0.05 b ± 0.03 |
| BUN, mg/dL | 29.9 e ± 4.14 | 21.9 f ± 3.83 |
| Albumin, g/dL | 5.41 ± 0.43 | 5.60 ± 0.50 |
| Total protein, g/dL | 6.50 ± 0.67 | 6.83 ± 0.72 |
| Creatinine, mg/dL | 0.77 a ± 0.08 | 0.61 b ± 0.16 |
| Lipid profile | ||
| HDL, mg/dL | 47.6 e ± 4.34 | 29.2 f ± 10.0 |
| LDL, mg/dL | 72.2 a ± 22.0 | 49.8 b ± 18.3 |
| NEFA, mEq/L | 0.25 ± 0.06 | 0.24 ± 0.14 |
| Cholesterol, mg/dL | 113.4 a ± 22.4 | 78.8 b ± 27.1 |
| TAG, mg/dL | 73.3 ± 16.0 | 72.3 ± 36.3 |
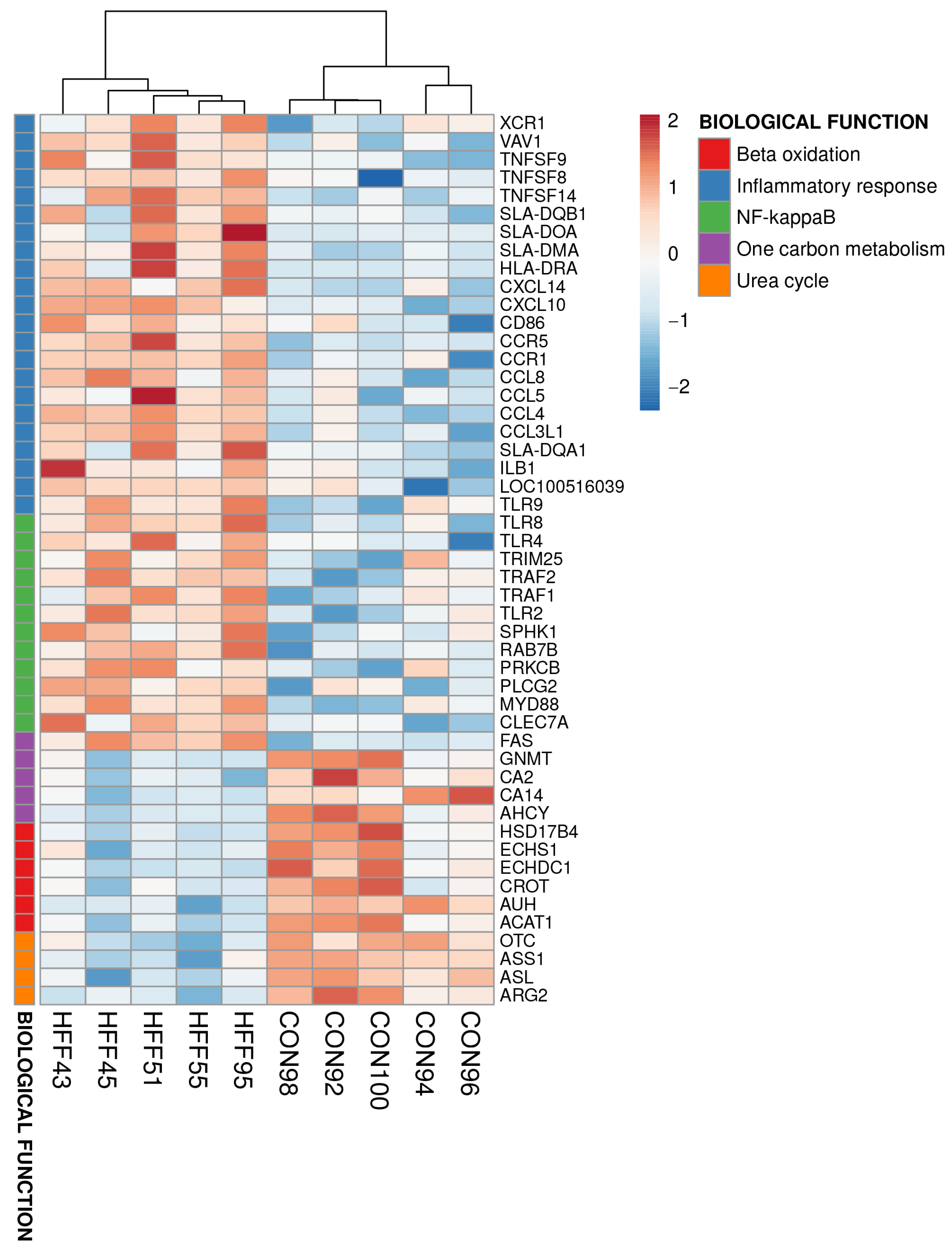
| Biological Process | p-Value |
|---|---|
| Upregulated HFF vs. CON | |
| Immune response | ≤0.001 |
| Inflammatory response | ≤0.001 |
| NF-kappa B transcription factor activity | ≤0.001 |
| Neutrophil chemotaxis | ≤0.001 |
| Interferon-beta production | ≤0.001 |
| Positive regulation of ERK1 and ERK2 cascade | ≤0.001 |
| Positive regulation of interleukin-6 production | ≤0.001 |
| Antigen processing and presentation | ≤0.001 |
| Interleukin-8 production | ≤0.001 |
| Tumor necrosis factor production | ≤0.001 |
| Chemokine-mediated signaling pathway | ≤0.001 |
| Phagocytosis | ≤0.001 |
| Antigen processing and presentation by MHC class II | ≤0.001 |
| Toll-like receptor signaling pathway | ≤0.001 |
| Cytokine-mediated signaling pathway | ≤0.001 |
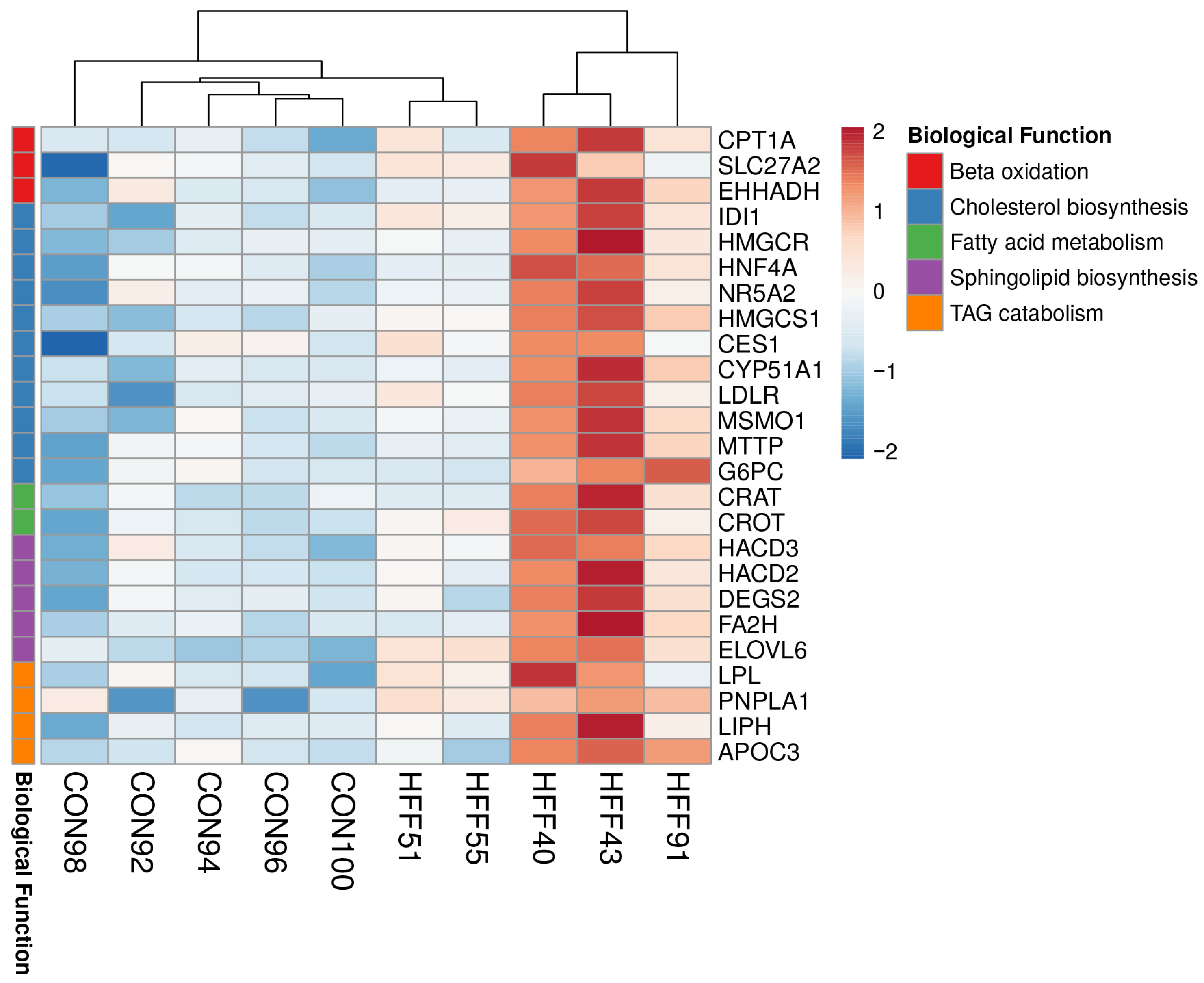
| Downregulated HFF vs. CON | |
| Fatty acid beta-oxidation | ≤0.001 |
| Urea cycle | ≤0.001 |
| One-carbon metabolism | ≤0.01 |
| Biological Process | p-Value |
|---|---|
| Upregulated HFF vs. CON | |
| Sphingolipid biosynthetic process | ≤0.001 |
| Cholesterol biosynthetic process | ≤0.001 |
| Medium-chain fatty acid metabolic process | ≤0.01 |
| Fatty acid beta-oxidation | ≤0.01 |
| Cholesterol homeostasis | ≤0.01 |
| Triglyceride catabolic process | ≤0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, M.A.; Burrin, D.G.; Manjarín, R. Decreased FXR Agonism in the Bile Acid Pool Is Associated with Impaired FXR Signaling in a Pig Model of Pediatric NAFLD. Biomedicines 2023, 11, 3303. https://doi.org/10.3390/biomedicines11123303
Maj MA, Burrin DG, Manjarín R. Decreased FXR Agonism in the Bile Acid Pool Is Associated with Impaired FXR Signaling in a Pig Model of Pediatric NAFLD. Biomedicines. 2023; 11(12):3303. https://doi.org/10.3390/biomedicines11123303
Chicago/Turabian StyleMaj, Magdalena A., Douglas G. Burrin, and Rodrigo Manjarín. 2023. "Decreased FXR Agonism in the Bile Acid Pool Is Associated with Impaired FXR Signaling in a Pig Model of Pediatric NAFLD" Biomedicines 11, no. 12: 3303. https://doi.org/10.3390/biomedicines11123303
APA StyleMaj, M. A., Burrin, D. G., & Manjarín, R. (2023). Decreased FXR Agonism in the Bile Acid Pool Is Associated with Impaired FXR Signaling in a Pig Model of Pediatric NAFLD. Biomedicines, 11(12), 3303. https://doi.org/10.3390/biomedicines11123303







