mRNA—From COVID-19 Treatment to Cancer Immunotherapy
Abstract
:1. Introduction
2. Lessons Learned from COVID-19
3. mRNA-Based Cancer Immunotherapy
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 1mΨ | N1-methyl-pseudouridine |
| ADAMDEC1 | ADAM like decysin 1 |
| ADAMTSL4 | ADAMTS Like 4 |
| ADAMTS18 | ADAM metallopeptidase with thrombospondin type 1 motif 18 |
| ALC-0159 | 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide |
| ALC-0315 | ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) |
| ALDH3A2 | aldehyde dehydrogenase 3 family member A2 |
| ALL | acute lymphocytic leukemia |
| AP2S1 | AP-2 complex subunit sigma |
| AUNIP | aurora kinase A and ninein interacting protein |
| BRAF | B-RAF, B rapidly accelerated fibrosarcoma |
| caTLR4 | constitutively activated TLR4 |
| CARD11 | caspase recruitment domain family member 11 |
| CEA | carcinoembryonic antigen |
| CLL | chronic lymphocytic leukemia |
| CMA | conditional marketing authorization |
| COL10A1 | collagen type X alpha 1 chain |
| COL6A1 | collagen type VI alpha 1 chain |
| COVID-19 | coronavirus disease 2019 |
| CpG 1018 | synthetic oligomer cytosine phospho-guanine |
| CPI | checkpoint inhibitor |
| CRC | colorectal cancer |
| CTSL | cathepsin L |
| CYTH4 | cytohesin 4 |
| DC | dendritic cell |
| DLin-MC3-DMA | dilinoleylmethyl-4-dimethylaminobutyrate |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| dsRNA | double-stranded RNA |
| DOPE | 1,2-dioleoyl-sn-glycerol-3phosphoethanolamine |
| eIF4E | eukaryotic translation initiation factor 4E |
| EGFLAM | EGF-like, fibronectin type III and laminin G domains |
| ESCC | esophageal squameous cell carcinoma |
| FAM134B | family with sequence similarity 134, member B |
| FANCI | FA complementation group I |
| FCRL4 | Fc receptor-like 4 |
| flLAMP | full-length (fl) lysosomal-associated membrane protein (LAMP) |
| FN1 | fibronectin 1 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| Her-2/neu | human epidermal growth factor receptor 2 |
| HLTF | helicase-like transcription factor |
| HBV | Hepatitis B |
| HOXB13 | homeobox B13 |
| HPLC | high-pressure liquid chromatography |
| HPV | human papillomavirus |
| IFN | interferon |
| ITGA10 | integrin subunit alpha 10 |
| IVT | in vitro transcription |
| KRAS | Kirsten rat sarcoma virus |
| LASP1 | LIM And SH3 protein 1 |
| LCP2 | lymphocyte cytosolic protein 2 |
| LILRB1 | leukocyte immunoglobulin-like receptor B1 |
| LSP1 | lymphocyte-specific protein 1 |
| MAGE-A1 | melanoma-associated antigen family A1 |
| MAGE-A3 | melanoma antigen family A3 |
| MAGE-C1 | melanoma antigen family C1 |
| MAGE-C2 | melanoma antigen family C2 |
| MERS | Middle East respiratory syndrome |
| MERS-CoV | Middle East respiratory syndrome-related coronavirus |
| MHC | major histocompatibility complex |
| MPZL2 | Myelin protein zero like 2 |
| mRNA | messenger ribonucleic acid |
| MDSC | myeloid-derived suppressor cell |
| MUC1 | mucin 1 |
| NHL | non-Hodgkin lymphoma |
| NLRC5 | NOD(nucleotide-binding oligomerization)-like receptor family CARD domain containing 5 |
| NRAS | neuroblastoma RAS |
| NSCLC | non-small cell lung cancer |
| NY-ESO-1 | New York esophageal squamous cell carcinoma 1 |
| ORF | open reading frame |
| OX40L | OX40 ligand |
| P3H4 | prolyl 3-hydroxylase family member 4 |
| PAP | phosphatidate phosphatase |
| PEG | polyethylene glycol |
| PEG2000-DMG | 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 |
| PKR | protein kinase R |
| PLCG1 | phospholipase C gamma 1 |
| Poly(A) | multiple adenosine units |
| PPEF1 | protein phosphatase with EF- protein phosphatase with EF-hand domain 1 |
| PPR | pentatricopeptide repeat |
| PSA | prostate-specific antigen |
| PSCA | prostate stem cell antigen |
| PSMA | prostate-specific membrane antigen |
| PSMD8 | proteasome 26S subunit, non-ATPase 8 |
| PTPRC | protein tyrosine phosphatase receptor type C |
| RAC3 | Rac family small GTPase 3 |
| RBD | receptor-binding domain |
| RIG-I | retinoic acid-inducible gene I |
| RORC | RAR-related orphan receptor C |
| SAA2 | serum amyloid A2 |
| saRNA | self-amplifying RNA |
| SARS | severe acute respiratory syndrome |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SAV1 | salvador family WW domain containing protein 1 |
| SCLC | small cell lung cancer |
| SIGLEC10 | sialic acid binding Ig like lectin 10 |
| SM-102 | (heptadecan-9-yl 8-{(2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino}octanoate) |
| STEAP1 | six transmembrane epithelial antigen of the prostate 1 |
| STRA6 | signaling receptor and transporter of retinol STRA6 |
| taRNA | trans-amplifying RNA |
| TAA | tumor-associated antigen |
| TPBG | trophoblast glycoprotein |
| TLR | toll-like receptor |
| TLR4 | toll like receptor 4 |
| TLR7 | toll like receptor 7 |
| TMEM229B | transmembrane protein 229B |
| TPTE | transmembrane phosphatase with tensin homology |
| TSA | tumor-specific antigen |
| TTC3 | tetratricopeptide repeat domain 3 |
| XPO5 | exportin 5 |
References
- Brenner, S.; Jacob, F.; Meselson, M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 1961, 190, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehwinkel, J.; Tan, C.P.; Goubau, D.; Schulz, O.; Pichlmair, A.; Bier, K.; Robb, N.; Vreede, F.; Barclay, W.; Fodor, E.; et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 2010, 140, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nallagatla, S.R.; Hwang, J.; Toroney, R.; Zheng, X.; Cameron, C.E.; Bevilacqua, P.C. 5’-Triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 2007, 318, 1455–1458. [Google Scholar] [CrossRef] [Green Version]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Yong, C.Y.; Ong, H.K.; Yeap, S.K.; Ho, K.L.; Tan, W.S. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front. Microbiol. 2019, 10, 1781. [Google Scholar] [CrossRef] [Green Version]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsoussi, W.B.; Turner, J.S.; Case, J.B.; Zhao, H.; Schmitz, A.J.; Zhou, J.Q.; Chen, R.E.; Lei, T.; Rizk, A.A.; McIntire, K.M.; et al. A potently neutralizing antibody protects mice against SARS-CoV-2 infection. J. Immunol. 2020, 205, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Patel, G.B.; Hu, S.; Chen, W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccines Immunother. 2016, 12, 1070–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuman, S. What messenger RNA capping tells us about eukaryotic evolution. Nat. Rev. Mol. Cell Biol. 2002, 3, 619–625. [Google Scholar] [CrossRef]
- Cao, J.; He, L.; Lin, G.; Hu, C.; Dong, R.; Zhang, J.; Zhu, H.; Hu, Y.; Wagner, C.R.; He, Q.; et al. Cap-dependent translation initiation factor, eIF4E, is the target for Ouabain-mediated inhibition of HIF-1α. Biochem. Pharmacol. 2014, 89, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Azizian, K.T.; Haque, A.A.; Henderson, J.M.; Hendel, A.; Shore, S.; Antony, J.S.; Hogrefe, R.I.; Kormann, M.S.; Porteus, M.H.; et al. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther.-Nucleic Acids 2018, 12, 530–542. [Google Scholar] [CrossRef]
- Pascolo, S. Synthetic messenger RNA-based vaccines: From scorn to hype. Viruses 2021, 13, 270. [Google Scholar] [CrossRef]
- Muttach, F.; Muthmann, N.; Rentmeister, A. Synthetic mRNA capping. Beilstein J. Org. Chem. 2017, 13, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Kocmik, I.; Piecyk, K.; Rudzinska, M.; Niedzwiecka, A.; Darzynkiewicz, E.; Grzela, R.; Jankowska-Anyszka, M. Modified ARCA analogs providing enhanced translational properties of capped mRNAs. Cell Cycle 2018, 17, 1624–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J. mRNA stability in mammalian cells. Microbiol. Rev. 1995, 59, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991, 5, 2108–2116. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef]
- Pickering, B.M.; Willis, A.E. The implications of structured 5’ untranslated regions on translation and disease. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2005; Volume 16, pp. 39–47. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Lacroix, C.; Exposito, J.-Y.; Verrier, B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 2019, 26, 311–323. [Google Scholar] [CrossRef]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef] [Green Version]
- Kudla, G.; Lipinski, L.; Caffin, F.; Helwak, A.; Zylicz, M. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006, 4, e180. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Y.; Li, C.; Yang, T.; Hu, B.; Zhang, M.; Guo, S.; Xiao, H.; Liang, X.-J.; Huang, Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020, 40, 107534. [Google Scholar] [CrossRef] [PubMed]
- Orlandini von Niessen, A.G.O.; Poleganov, M.A.; Rechner, C.; Plaschke, A.; Kranz, L.M.; Fesser, S.; Diken, M.; Löwer, M.; Vallazza, B.; Beissert, T.; et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening. Mol. Ther. 2018, 27, 824–836. [Google Scholar] [CrossRef] [Green Version]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Tanguay, R.M.; Jorquera, R.; Poudrier, J.; St-Louis, M. Tyrosine and its catabolites: From disease to cancer. Acta Biochim. Pol. 1996, 43, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Mangus, D.A.; Evans, M.C.; Jacobson, A. Poly(A)-binding proteins: Multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003, 4, 223. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Jacobson, A. The intimate relationships of mRNA decay and translation. Trends Genet. 2013, 29, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalkanen, A.L.; Coleman, S.J.; Wilusz, J. Determinants and implications of mRNA poly(A) tail size—Does this protein make my tail look big? Semin. Cell Dev. Biol. 2014, 34, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, S.A.; Chipman, L.B.; Nicholson, A.L.; Chen, Y.-H.; Yee, B.A.; Yeo, E.; Coller, J.; Pasquinelli, A.E. Short poly(A) tails are a conserved feature of highly expressed genes. Nat. Struct. Mol. Biol. 2017, 24, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Bidram, M.; Zhao, Y.; Shebardina, N.G.; Baldin, A.V.; Bazhin, A.V.; Ganjalikhany, M.R.; Zamyatnin, A.A.; Ganjalikhani-Hakemi, M. mRNA-based cancer vaccines: A therapeutic strategy for the treatment of melanoma patients. Vaccines 2021, 9, 1060. [Google Scholar] [CrossRef]
- Ziemniak, M.; Strenkowska, M.; Kowalska, J.; Jemielity, J. Potential therapeutic applications of RNA cap analogs. Future Med. Chem. 2013, 5, 1141–1172. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.N.; Diken, M.; Kreiter, S.; Selmi, A.; Kowalska, J.; Jemielity, J.; Darzynkiewicz, E.; Huber, C.; Türeci, Ö.; Sahin, U. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 2010, 17, 961–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Elfakess, R.; Dikstein, R. A translation initiation element specific to mRNAs with very short 5′UTR that also regulates transcription. PLoS ONE 2008, 3, e3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karikó, K.; Muramatsu, H.; Keller, J.M.; Weissman, D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 2012, 20, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Van Gulck, E.R.A.; Ponsaerts, P.; Heyndrickx, L.; Vereecken, K.; Moerman, F.; De Roo, A.; Colebunders, R.; Van den Bosch, G.; Van Bockstaele, D.R.; Van Tendeloo, V.F.I.; et al. Efficient stimulation of HIV-1-specific T cells using dendritic cells electroporated with mRNA encoding autologous HIV-1 Gag and Env proteins. Blood 2006, 107, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Briones, M.C.; Silva-Pilipich, N.; Herrador-Cañete, G.; Vanrell, L.; Smerdou, C. A new generation of vaccines based on alphavirus self-amplifying RNA. Curr. Opin. Virol. 2020, 44, 145–153. [Google Scholar] [CrossRef]
- Lundstrom, K. Self-amplifying RNA viruses as RNA vaccines. Int. J. Mol. Sci. 2020, 21, 5130. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Ljungberg, K.; Liljeström, P. Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines 2015, 14, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Berglund, P.; Rhodes, G.; Parker, S.; Jondal, M.; Liljeström, P. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine 1994, 12, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S. From COVID-19 to cancer mRNA vaccines: Moving from bench to clinic in the vaccine landscape. Front. Immunol. 2021, 12, 679344. [Google Scholar] [CrossRef] [PubMed]
- Deering, R.P.; Kommareddy, S.; Ulmer, J.B.; Brito, L.A.; Geall, A.J. Nucleic acid vaccines: Prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 2014, 11, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Beissert, T.; Perkovic, M.; Vogel, A.; Erbar, S.; Walzer, K.C.; Hempel, T.; Brill, S.; Haefner, E.; Becker, R.; Türeci, Ö.; et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol. Ther. 2020, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Kinnear, E. Using plasmids as DNA vaccines for infectious diseases. Microbiol. Spectr. 2014, 2, 651–668. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. mRNA vaccine era—Mechanisms, drug platform and clinical prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Blakney, A.K.; Zhu, Y.; McKay, P.F.; Bouton, C.; Yeow, J.; Tang, J.; Hu, K.; Samnuan, K.; Grigsby, C.; Shattock, R.J.; et al. Big is beautiful: Enhanced saRNA delivery and immunogenicity by a higher molecular weight, bioreducible, cationic polymer. ACS Nano 2020, 14, 5711–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Wu, H.; Xu, R. Advancing to the era of cancer immunotherapy. Cancer Commun. 2021, 41, 803–829. [Google Scholar] [CrossRef]
- Lorenz, C.; Fotin-Mleczek, M.; Roth, G.; Becker, C.; Dam, T.C.; Verdurmen, W.P.R.; Brock, R.; Probst, J.; Schlake, T. Protein expression from exogenous mRNA: Uptake by receptor-mediated endocytosis and trafficking via the lysosomal pathway. RNA Biol. 2011, 8, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Selmi, A.; Vascotto, F.; Kautz-Neu, K.; Türeci, Ö.; Sahin, U.; von Stebut, E.; Diken, M.; Kreiter, S. Uptake of synthetic naked RNA by skin-resident dendritic cells via macropinocytosis allows antigen expression and induction of T-cell responses in mice. Cancer Immunol. Immunother. 2016, 65, 1075–1083. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Tockary, T.A.; Yoshinaga, N.; Li, J.; Osawa, S.; Gorantla, L.; Fukushima, S.; Osada, K.; Kataoka, K. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J. Drug Target. 2019, 27, 670–680. [Google Scholar] [CrossRef]
- Yen, A.; Cheng, Y.; Sylvestre, M.; Gustafson, H.H.; Puri, S.; Pun, S.H. Serum nuclease susceptibility of mRNA cargo in condensed polyplexes. Mol. Pharm. 2018, 15, 2268–2276. [Google Scholar] [CrossRef]
- Aldosari, B.; Alfagih, I.; Almurshedi, A. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Gan, Z.; Zurla, C.; Levin, J.; Islam, F.Z.; Kalathoor, S.; Sato, M.; Sago, C.D.; Santangelo, P.J.; Dahlman, J.E. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv. Mater. 2019, 32, e1904905. [Google Scholar] [CrossRef]
- Lubich, C.; Allacher, P.; de la Rosa, M.; Bauer, A.; Prenninger, T.; Horling, F.M.; Siekmann, J.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. The mystery of antibodies against polyethylene glycol (PEG)—What do we know? Pharm. Res. 2016, 33, 2239–2249. [Google Scholar] [CrossRef]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Lin, J.; Huang, Y.; Li, L.; Delcassian, D.; Ge, Y.; Shi, Y.; Anderson, D.G. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 2020, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef] [Green Version]
- Ur Rehman, Z.; Hoekstra, D.; Zuhorn, I.S. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: Real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 2013, 7, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.M.P.; Brans, T.; Samal, S.K.; Dubruel, P.; Demeester, J.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Endosomal size and membrane leakiness influence proton sponge-based rupture of endosomal vesicles. ACS Nano 2018, 12, 2332–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriadis, G.J. Entrapment of ribonucleic acids in liposomes. FEBS Lett. 1978, 86, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhang, X.; Dong, Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1530. [Google Scholar] [CrossRef]
- Ma, Z.; Li, J.; He, F.; Wilson, A.; Pitt, B.; Li, S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem. Biophys. Res. Commun. 2005, 330, 755–759. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R.S. Smart biomaterials: Recent advances and future directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Wu, D.; Kumar, J.N.; Cheng, W.; Lay, C.L.; Liu, Y. Redox-responsive hyperbranched poly(amido amine)s with tertiary amino cores for gene delivery. Biomacromolecules 2013, 14, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.D.; Yang, Y.; Leung, E.; Krissansen, G.W. mRNA transfection by a Xentry-protamine cell-penetrating peptide is enhanced by TLR antagonist E6446. PLoS ONE 2018, 13, e0201464. [Google Scholar] [CrossRef] [Green Version]
- Sedic, M.; Senn, J.J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S.J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E.; et al. Safety Evaluation of lipid nanoparticle–formulated modified mRNA in the sprague-dawley rat and cynomolgus monkey. Vet. Pathol. 2018, 55, 341–354. [Google Scholar] [CrossRef]
- Jorritsma, S.; Gowans, E.; Grubor-Bauk, B.; Wijesundara, D. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. EbioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- John, S.; Yuzhakov, O.; Woods, A.; Deterling, J.; Hassett, K.; Shaw, C.A.; Ciaramella, G. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 2018, 36, 1689–1699. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, D.; Pardi, N.; Muramatsu, H.; Karikó, K. HPLC purification of in vitro transcribed long RNA. Methods Mol Biol. 2013, 969, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Shivalingam, A.; Taemaitree, L.; El-Sagheer, A.H.; Brown, T. Squaramides and ureas: A flexible approach to polymerase-compatible nucleic acid assembly. Angew. Chem. Int. Ed. 2020, 59, 11416–11422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Narasimhan, M.; Li, Q.-Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Crommelin, D.J.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Ahmed, T. Immunotherapy for neuroblastoma using mRNA vaccines. Adv. Cancer Biol.—Metastasis 2022, 4, 100033. [Google Scholar] [CrossRef]
- Islam, M.A.; Rice, J.; Reesor, E.; Zope, H.; Tao, W.; Lim, M.; Ding, J.; Chen, Y.; Aduluso, D.; Zetter, B.R.; et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 2021, 266, 120431. [Google Scholar] [CrossRef]
- Jahanafrooz, Z.; Baradaran, B.; Mosafer, J.; Hashemzaei, M.; Rezaei, T.; Mokhtarzadeh, A.; Hamblin, M.R. Comparison of DNA and mRNA vaccines against cancer. Drug Discov. Today 2020, 25, 552–560. [Google Scholar] [CrossRef]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Kramps, T.; Elbers, K. Introduction to RNA vaccines. Methods Mol Biol. 2017, 1499, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lee, J.; Kim, Y.; Song, K.-H.; Cho, E.; Kim, M.; Jung, H.; Kim, T.W. Far beyond cancer immunotherapy: Reversion of multi-malignant phenotypes of immunotherapeutic-resistant cancer by targeting the NANOG signaling axis. Immune Netw. 2020, 20, e7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Papukashvili, D.; Dong, Y.; Wang, X.; Hu, X.; Yang, N.; Cai, J.; Xie, F.; Rcheulishvili, N.; Wang, P.G. Identification of tumor antigens and design of mRNA vaccine for colorectal cancer based on the immune subtype. Front. Cell Dev. Biol. 2022, 9, 783527. [Google Scholar] [CrossRef]
- Chesney, J.A.; Mitchell, R.A.; Yaddanapudi, K. Myeloid-derived suppressor cells—A new therapeutic target to overcome resistance to cancer immunotherapy. J. Leukoc. Biol. 2017, 102, 727–740. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-associated macrophages: Recent insights and therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef]
- Cassetta, L.; Kitamura, T. Macrophage targeting: Opening new possibilities for cancer immunotherapy. Immunology 2018, 155, 285–293. [Google Scholar] [CrossRef]
- Saeed, M.; Gao, J.; Shi, Y.; Lammers, T.; Yu, H. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics 2019, 9, 7981–8000. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Prendergast, G.C. Cancer vaccines: A brief overview. Methods Mol Biol. 2016, 1403, 755–761. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the treatment of prostate cancer: Novel insights and future directions. Futur. Oncol. 2018, 14, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, C.-D.; Wu, X.-H. Therapeutic cancer vaccines: From initial findings to prospects. Immunol. Lett. 2018, 196, 11–21. [Google Scholar] [CrossRef]
- Tsai, H.-J. Clinical cancer chemoprevention: From the hepatitis B virus (HBV) vaccine to the human papillomavirus (HPV) vaccine. Taiwan. J. Obstet. Gynecol. 2015, 54, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Hui, A.-M. The paradigm shift in treatment from COVID-19 to oncology with mRNA vaccines. Cancer Treat. Rev. 2022, 107, 102405. [Google Scholar] [CrossRef]
- Abbott, M.; Ustoyev, Y. Cancer and the immune system: The history and background of immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Velcheti, V.; Schalper, K. Basic Overview of current immunotherapy approaches in cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 298–308. [Google Scholar] [CrossRef]
- Tay, B.; Wright, Q.; Ladwa, R.; Perry, C.; Leggatt, G.; Simpson, F.; Wells, J.; Panizza, B.; Frazer, I.; Cruz, J. Evolution of cancer vaccines—Challenges, achievements, and future directions. Vaccines 2021, 9, 535. [Google Scholar] [CrossRef]
- Maiorano, B.; Schinzari, G.; Ciardiello, D.; Rodriquenz, M.; Cisternino, A.; Tortora, G.; Maiello, E. Cancer vaccines for genitourinary tumors: Recent progresses and future possibilities. Vaccines 2021, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Donninger, H.; Li, C.; Eaton, J.; Yaddanapudi, K. Cancer vaccines: Promising therapeutics or an unattainable dream. Vaccines 2021, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Janczar, S.; Bulas, M.; Walenciak, J.; Baranska, D.; Ussowicz, M.; Młynarski, W.; Zalewska-Szewczyk, B. Pulmonary exacerbation of undiagnosed toxocariasis in intensively-treated high-risk neuroblastoma patients. Children 2020, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-G.; Sang, Y.-B.; Lee, J.-H.; Chon, H.-J. Combining cancer vaccines with immunotherapy: Establishing a new immunological approach. Int. J. Mol. Sci. 2021, 22, 8035. [Google Scholar] [CrossRef]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant viral nanoparticle conjugated with anti-PD-1 peptide for ovarian cancer immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef]
- Liu, C.-C.; Yang, H.; Zhang, R.; Zhao, J.-J.; Hao, D.-J. Tumour-associated antigens and their anti-cancer applications. Eur. J. Cancer Care 2017, 26, e12446. [Google Scholar] [CrossRef]
- Salomon, N.; Vascotto, F.; Selmi, A.; Vormehr, M.; Quinkhardt, J.; Bukur, T.; Schrörs, B.; Löewer, M.; Diken, M.; Türeci, Ö.; et al. A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice. Oncoimmunology 2020, 9, 1771925. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A., III; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Goedegebuure, S.; Gillanders, W. Preclinical and clinical development of neoantigen vaccines. Ann. Oncol. 2017, 28, xii11–xii17. [Google Scholar] [CrossRef]
- Srivastava, P.K. Neoepitopes of cancers: Looking back, looking ahead. Cancer Immunol. Res. 2015, 3, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Szanto, C.L.; Cornel, A.M.; Vijver, S.V.; Nierkens, S. Monitoring immune responses in neuroblastoma patients during therapy. Cancers 2020, 12, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.A.; Cheung, N.K.V. Targets and antibody formats for immunotherapy of neuroblastoma. J. Clin. Oncol. 2020, 38, 1836–1848. [Google Scholar] [CrossRef]
- Modak, S.; Cheung, N.-K.V. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer Investig. 2007, 25, 67–77. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.; Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Hornung, V.; Barchet, W.; Schlee, M.; Hartmann, G. RNA recognition via TLR7 and TLR8. In Toll-Like Receptors (TLRs) and innate immunity; Springer: Berlin/Heidelberg, Germany, 2008; pp. 71–86. [Google Scholar] [CrossRef]
- Wilusz, C.J.; Wormington, M.; Peltz, S.W. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2001, 2, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; Paoletti, E.; Moss, B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J. Biol. Chem. 1975, 250, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3’-O-methyl)GpppG and 7-methyl (3’-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar]
- Weissman, D.; Karikó, K. mRNA: Fulfilling the promise of gene therapy. Mol. Ther. 2015, 23, 1416–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Wolters, P.; Martin, S.; Delbrook, C.; Yates, B.; et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018, 24, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchioni, M.; Nazzani, S.; Preisser, F.; Bandini, M.; Karakiewicz, P.I. Therapeutic strategies for organ-confined and non-organ-confined bladder cancer after radical cystectomy. Expert Rev. Anticancer Ther. 2018, 18, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y. T cell dysfunction and exhaustion in cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Senti, G.; Kündig, T.M. Intralymphatic immunotherapy. World Allergy Organ. J. 2015, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Van der Jeught, K.; Joe, P.T.; Bialkowski, L.; Heirman, C.; Daszkiewicz, L.; Liechtenstein, T.; Escors, D.; Thielemans, K.; Breckpot, K. Intratumoral administration of mRNA encoding a fusokine consisting of IFN-β and the ectodomain of the TGF-β receptor II potentiates antitumor immunity. Oncotarget 2014, 5, 10100–10113. [Google Scholar] [CrossRef] [Green Version]
- Beyaert, S.; Machiels, J.-P.; Schmitz, S. Vaccine-based immunotherapy for head and neck cancers. Cancers 2021, 13, 6041. [Google Scholar] [CrossRef] [PubMed]
- Karan, D.; Holzbeierlein, J.M.; Van Veldhuizen, P.; Thrasher, J.B. Cancer immunotherapy: A paradigm shift for prostate cancer treatment. Nat. Rev. Urol. 2012, 9, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2017, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther.—Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [Green Version]
- Besin, G.; Milton, J.; Sabnis, S.; Howell, R.; Mihai, C.; Burke, K.; Benenato, K.E.; Stanton, M.; Smith, P.; Senn, J.; et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 2019, 3, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Yamamoto, T.N.; Kishton, R.J.; Restifo, N.P. Developing neoantigen-targeted T cell–based treatments for solid tumors. Nat. Med. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Aurisicchio, L.; Pallocca, M.; Ciliberto, G.; Palombo, F. The perfect personalized cancer therapy: Cancer vaccines against neoantigens. J. Exp. Clin. Cancer Res. 2018, 37, 86. [Google Scholar] [CrossRef] [Green Version]
- Wells, D.K.; van Buuren, M.M.; Dang, K.K.; Hubbard-Lucey, V.M.; Sheehan, K.C.; Campbell, K.M.; Lamb, A.; Ward, J.P.; Sidney, J.; Blazquez, A.B.; et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 2020, 183, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Artyomov, M.N.; Mardis, E.R.; Schreiber, R.D. Tumor neoantigens: Building a framework for personalized cancer immunotherapy. J. Clin. Investig. 2015, 125, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Roudko, V.; Greenbaum, B.; Bhardwaj, N. Computational prediction and validation of tumor-associated neoantigens. Front. Immunol. 2020, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Leet, D.E.; Allesøe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221, Erratum in Nature 2018, 555, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinsteyn, A.; Kodysh, J.; Hodes, I.; Mondet, S.; Aksoy, B.A.; Finnigan, J.P.; Bhardwaj, N.; Hammerbacher, J. Computational pipeline for the PGV-001 neoantigen vaccine trial. Front. Immunol. 2018, 8, 1807. [Google Scholar] [CrossRef] [Green Version]
- Esprit, A.; De Mey, W.; Shahi, R.B.; Thielemans, K.; Franceschini, L.; Breckpot, K. Neo-antigen mRNA vaccines. Vaccines 2020, 8, 776. [Google Scholar] [CrossRef]
- Cantwell-Dorris, E.R.; O’Leary, J.J.; Sheils, O.M. BRAFV600E: Implications for carcinogenesis and molecular therapy. Mol. Cancer Ther. 2011, 10, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Boespflug, A.; Caramel, J.; Dalle, S.; Thomas, L. Treatment of NRAS-mutated advanced or metastatic melanoma: Rationale, current trials and evidence to date. Ther. Adv. Med Oncol. 2017, 9, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Nature Biotechnology. The problem with neoantigen prediction. Nat. Biotechnol. 2017, 35, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Gao, Y.; Chen, Y.; Wang, K.; Zhang, S.; Li, G. Identification of novel tumor antigens and the immune landscapes of bladder cancer patients for mRNA vaccine development. Front. Oncol. 2022, 12, 921711. [Google Scholar] [CrossRef] [PubMed]
- Ping, H.; Yu, W.; Gong, X.; Tong, X.; Lin, C.; Chen, Z.; Cai, C.; Guo, K.; Ke, H. Analysis of melanoma tumor antigens and immune subtypes for the development of mRNA vaccine. Investig. New Drugs 2022, 40, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, M.B. Messenger RNA vaccine advances provide treatment possibilities for cancer. Cancer 2022, 128, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, W.; Huang, X.; He, Y.; Gou, X.; Liu, X.; Hu, Z.; Xu, W.; Rahman, K.; Li, S.; et al. Strategies to package recombinant adeno-associated virus expressing the N-terminal gasdermin domain for tumor treatment. Nat. Commun. 2021, 12, 7155. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chiang, C.-Y.; Liu, S.-J.; Chen, H.-W. A novel recombinant Fcγ receptor-targeted survivin combines with chemotherapy for efficient cancer treatment. Biomedicines 2021, 9, 806. [Google Scholar] [CrossRef]
- Wu, C.; Qin, C.; Long, W.; Wang, X.; Xiao, K.; Liu, Q. Tumor antigens and immune subtypes of glioblastoma: The fundamentals of mRNA vaccine and individualized immunotherapy development. J. Big Data 2022, 9, 92. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Yan, Z.; Zhang, M. Identification of tumor antigens and immune subtypes of glioma for mRNA vaccine development. Cancer Med. 2022, 11, 2711–2726. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Li, L.; Ma, P.; Jiang, Y.; Ge, M.; Yu, Y.; Huang, H.; Fang, Y.; Jiang, N.; et al. Identification of tumor antigens and immune subtypes of malignant mesothelioma for mRNA vaccine development. Vaccines 2022, 10, 1168. [Google Scholar] [CrossRef]
- You, W.; Ouyang, J.; Cai, Z.; Chen, Y.; Wu, X. Comprehensive analyses of immune subtypes of stomach adenocarcinoma for mRNA vaccination. Front. Immunol. 2022, 13, 827506. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Wei, Y.; Xiong, Y.; Liu, Q.; Hu, Z.; Zeng, Z.; Tang, F.; Ouyang, Y. Identification of potential antigens for developing mRNA vaccine for immunologically cold mesothelioma. Front. Cell Dev. Biol. 2022, 10, 879278. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Van der Jeught, K.; De Koker, S.; Bialkowski, L.; Heirman, C.; Tjok Joe, P.; Perche, F.; Maenhout, S.; Bevers, S.; Broos, K.; Deswarte, K.; et al. Dendritic cell targeting mRNA Lipopolyplexes combine strong antitumor t-cell immunity with improved inflammatory safety. ACS Nano 2018, 12, 9815–9829. [Google Scholar] [CrossRef]
- Son, S.; Nam, J.; Zenkov, I.; Ochyl, L.J.; Xu, Y.; Scheetz, L.; Shi, J.; Farokhzad, O.C.; Moon, J.J. Sugar-nanocapsules imprinted with microbial molecular patterns for mRNA vaccination. Nano Lett. 2020, 20, 1499–1509. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; Breckpot, K.; Janssens, J.; Van Calenbergh, S.; De Smedt, S.; Dewitte, H. Broadening the message: A nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano 2019, 13, 1655–1669. [Google Scholar] [CrossRef]
- Lou, B.; De Koker, S.; Lau, C.Y.J.; Hennink, W.E.; Mastrobattista, E. mRNA polyplexes with post-conjugated GALA peptides efficiently target, transfect, and activate antigen presenting cells. Bioconjugate Chem. 2019, 30, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Coolen, A.-L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.-Y.; Verrier, B. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Clemençon, R.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Pichon, C. Neutral lipopolyplexes for in vivo delivery of conventional and replicative RNA vaccine. Mol. Ther. -Nucleic Acids 2019, 17, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2016, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef]
- Linch, M.; Papai, Z.; Takacs, I.; Imedio, E.R.; Kühnle, M.-C.; Derhovanessian, E.; Vogler, I.; Renken, S.; Graham, P.; Sahin, U.; et al. 421 A first-in-human (FIH) phase I/IIa clinical trial assessing a ribonucleic acid lipoplex (RNA-LPX) encoding shared tumor antigens for immunotherapy of prostate cancer; preliminary analysis of PRO-MERIT. J. Immunother. Cancer 2021, 9, A451. [Google Scholar] [CrossRef]
- Braiteh, F.; LoRusso, P.; Balmanoukian, A.; Klempner, S.; Camidge, D.R.; Hellmann, M.; Gordon, M.; Bendell, J.; Mueller, L.; Sabado, R.; et al. Abstract CT169: A phase Ia study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in patients with locally advanced or metastatic solid tumors. Cancer Res. 2020, 80, CT169. [Google Scholar] [CrossRef]
- Srikrishna, D.; Sachsenmeier, K. We need to bring R0 < 1 to treat cancer too. Genome Med. 2021, 13, 120. [Google Scholar] [CrossRef]
- Bauman, J.; Burris, H.; Clarke, J.; Patel, M.; Cho, D.; Gutierrez, M.; Julian, R.; Scott, A.; Cohen, P.; Frederick, J.; et al. 798 Safety, tolerability, and immunogenicity of mRNA-4157 in combination with pembrolizumab in subjects with unresectable solid tumors (KEYNOTE-603): An update. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Burris, H.A.; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. JCO 2019, 37 (Suppl. S15), 2523. [Google Scholar] [CrossRef]
- Liang, X.; Li, D.; Leng, S.; Zhu, X. RNA-based pharmacotherapy for tumors: From bench to clinic and back. Biomed. Pharmacother. 2020, 125, 109997. [Google Scholar] [CrossRef]
- Cafri, G.; Gartner, J.J.; Hopson, K.; Meehan, R.S.; Zaks, T.Z.; Robbins, P.; Rosenberg, S.A. Immunogenicity and tolerability of personalized mRNA vaccine mRNA-4650 encoding defined neoantigens expressed by the autologous cancer. J. Clin. Oncol. 2019, 37, 2643. [Google Scholar] [CrossRef]
- De Keersmaecker, B.; Claerhout, S.; Carrasco, J.; Bar, I.; Corthals, J.; Wilgenhof, S.; Neyns, B.; Thielemans, K. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: Link between T-cell activation and clinical responses in advanced melanoma. J. Immunother. Cancer 2019, 8, e000329. [Google Scholar] [CrossRef] [Green Version]
- Wilgenhof, S.; Van Nuffel, A.M.T.; Benteyn, D.; Corthals, J.; Aerts, C.; Heirman, C.; Van Riet, I.; Bonehill, A.; Thielemans, K.; Neyns, B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann. Oncol. 2013, 24, 2686–2693. [Google Scholar] [CrossRef]
- Jansen, Y.; Kruse, V.; Corthals, J.; Schats, K.; Van Dam, P.-J.; Seremet, T.; Heirman, C.; Brochez, L.; Kockx, M.; Thielemans, K.; et al. A randomized controlled phase II clinical trial on mRNA electroporated autologous monocyte-derived dendritic cells (TriMixDC-MEL) as adjuvant treatment for stage III/IV melanoma patients who are disease-free following the resection of macrometastases. Cancer Immunol. Immunother. 2020, 69, 2589–2598. [Google Scholar] [CrossRef]
- Deng, Z.; Tian, Y.; Song, J.; An, G.; Yang, P. mRNA vaccines: The dawn of a new era of cancer immunotherapy. Front. Immunol. 2022, 13, 887125. [Google Scholar] [CrossRef]
- Rausch, S.; Schwentner, C.; Stenzl, A.; Bedke, J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum. Vaccines Immunother. 2014, 10, 3146–3152. [Google Scholar] [CrossRef] [Green Version]
- Kübler, H.; Scheel, B.; Gnad-Vogt, U.; Miller, K.; Schultze-Seemann, W.; Vom Dorp, F.; Parmiani, G.; Hampel, C.; Wedel, S.; Trojan, L.; et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: A first-in-man phase I/IIa study. J. Immunother. Cancer 2015, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Matsui, Y.; Nakajima, T.; Iizasa, T.; Ishikawa, A. Phase III randomized controlled trial of adjuvant chemoimmunotherapy in patients with resected primary lung cancer. Ann. Oncol. 2017, 28, v403. [Google Scholar] [CrossRef]
- Sebastian, M.; Schröder, A.; Scheel, B.; Hong, H.S.; Muth, A.; von Boehmer, L.; Zippelius, A.; Mayer, F.; Reck, M.; Atanackovic, D.; et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 799–812. [Google Scholar] [CrossRef]
- Sabari, J.; Ramirez, K.A.; Schwarzenberger, P.; Ricciardi, T.; Macri, M. Phase 1/2 study of mRNA vaccine therapy + durvalumab (durva) ± tremelimumab (treme) in patients with metastatic non-small cell lung cancer (NSCLC). In Proceedings of the Fourth CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference: Translating Science into Survival, New York, NY, USA, 30 September–3 October 2018. [Google Scholar]
- Papachristofilou, A.; Hipp, M.M.; Klinkhardt, U.; Früh, M.; Sebastian, M.; Weiss, C.; Pless, M.; Cathomas, R.; Hilbe, W.; Pall, G.; et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 38. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol. Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Hamid, O.; Hellman, M.; Carneiro, B. Preliminary safety, antitumor activity and pharmacodynamics results of HIT-IT MEDI1191 (mRNA IL-12) in patients with advanced solid tumors and superficial lesions. In Proceedings of the ESMO Targeted Anticancer Therapies (TAT), Virtual Congress, 1–2 March 2021. [Google Scholar]
- Weide, B.; Carralot, J.-P.; Reese, A.; Scheel, B.; Eigentler, T.K.; Hoerr, I.; Rammensee, H.-G.; Garbe, C.; Pascolo, S. Results of the first phase i/ii clinical vaccination trial with direct injection of mrna. J. Immunother. 2008, 31, 180–188. [Google Scholar] [CrossRef]
- Rittig, S.M.; Haentschel, M.; Weimer, K.J.; Heine, A.; Muller, M.R.; Brugger, W.; Horger, M.S.; Maksimovic, O.; Stenzl, A.; Hoerr, I.; et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ Immune responses and induce clinical benefit in vaccinated patients. Mol. Ther. 2011, 19, 990–999. [Google Scholar] [CrossRef]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef]
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Bialkowski, L.; Van Der Jeught, K.; Bevers, S.; Tjok Joe, P.; Renmans, D.; Heirman, C.; Aerts, J.L.; Thielemans, K. Immune checkpoint blockade combined with IL-6 and TGF-β inhibition improves the therapeutic outcome of mRNA-based immunotherapy. Int. J. Cancer 2018, 143, 686–698. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Xu, Z.; Miao, L.; Huang, L. mRNA vaccine with antigen-specific checkpoint blockade induces an enhanced immune response against established melanoma. Mol. Ther. 2018, 26, 420–434. [Google Scholar] [CrossRef] [Green Version]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A thermostable mRNA vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Parhiz, H.; Brenner, J.S.; Patel, P.N.; Papp, T.E.; Shahnawaz, H.; Li, Q.; Shi, R.; Zamora, M.E.; Yadegari, A.; Marcos-Contreras, O.A.; et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE). J. Control. Release 2021, 344, 50–61. [Google Scholar] [CrossRef]
- Igyártó, B.Z.; Jacobsen, S.; Ndeupen, S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr. Opin. Virol. 2022, 48, 65–72. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 2020, 6, eabb8133. [Google Scholar] [CrossRef]
- Krause, W. Liver-specific X-ray contrast agents. In Contrast Agents II; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2002; Volume 222, pp. 173–200. [Google Scholar]


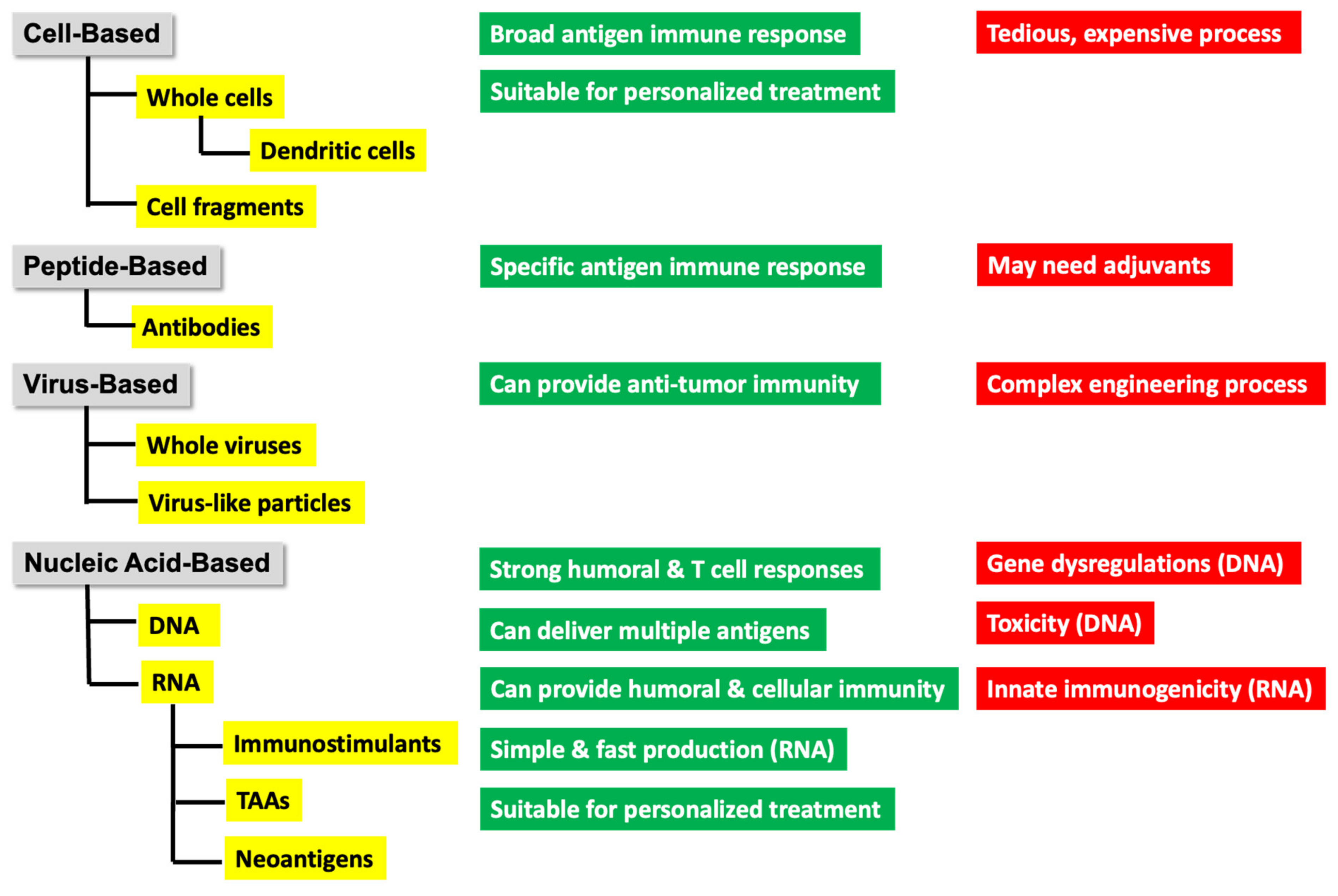
 . Tumor cells expressing neoantigens A
. Tumor cells expressing neoantigens A  , B
, B  , or C
, or C  .
.
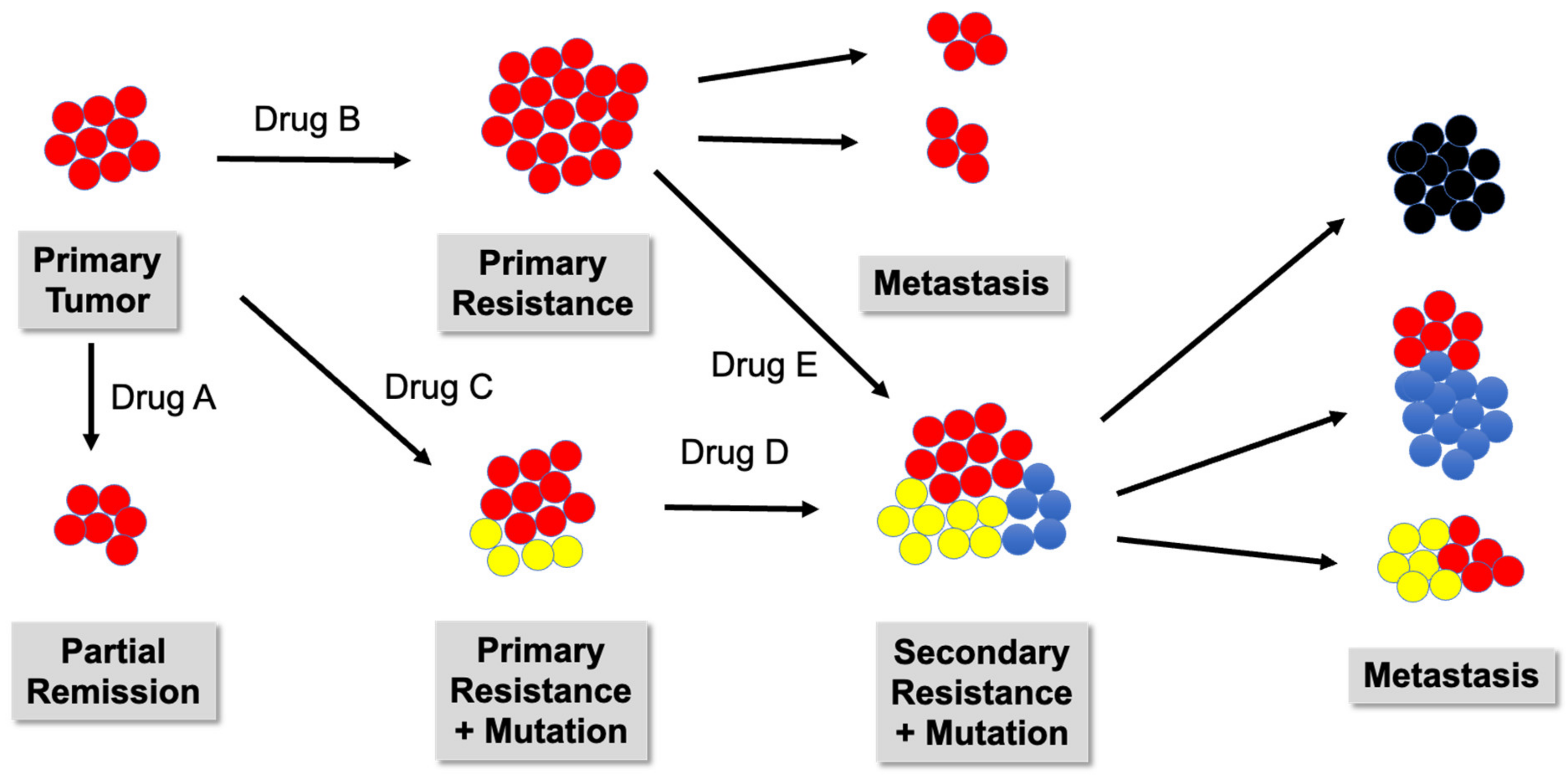
 . Tumor cells expressing neoantigens A
. Tumor cells expressing neoantigens A  , B
, B  , or C
, or C  .
.
| Component | Nucleotides | Function |
|---|---|---|
| 5′ cap | 7-Methyl-G | Essential for ribosome recognition, transcription, protection against ribonucleases |
| 5′ UTR | A, C, G, U; from 3 to several hundred nucleotides | Contributes to stability, localization, and translation efficiency Normally proprietary knowledge |
| Start | AUG within a Kozak sequence | Codes for methione and initiates translation |
| Coding | A, C, G, U | Regulation of splicing Decoding (reading) by ribosomes and translation into the target protein |
| Stop | UAG: amber UAA: ochre UGA: opal | Terminates the translation process |
| 3′ UTR | Contributes to stability, localization, and translation efficiency, potentially involved in disease susecptibility | |
| Poly(A) tail | A | Protects against exonucleases, aids in transport from nucleus to cytosol and in translation |
| Position | Modification | Effect | Reference |
|---|---|---|---|
| 5′ cap |
|
| [17,18,19,20] |
| 5′ UTR |
|
| [21,22,23,24,25,26,27,28,29] |
| Start | |||
| Coding |
|
| [30,31,32,33] |
| Stop | |||
| 3′ UTR |
|
| See 5′ UTR [23,25,34,35,36] |
| Poly(A) tail | Length of the poly(A) tail, ideally > 90 A, shorter sequence is more efficient | Critical role for translation and stability | [20,37,38,39,40] |
| Product | Active Ingredient | Formulation | Storage |
|---|---|---|---|
| Comirnaty, tozinameran, BNT162b2 BioNTech (Mainz, Germany) | Single-stranded, 5′-capped mRNA, encoding the spike antigen [glycoprotein (S)] of SARS-CoV-2 (isolate Wuhan-Hu-1) containing two consecutive proline mutations (P2 S); uridine substituted by N1-methylpseudouridine (1mΨ) | Multidose concentrate to be diluted prior to i.m. injection; dispersion of mRNA in LNPs containing ALC-0315 and ALC-0159 (functional lipids), DSPC and cholesterol (structural lipids) in aqueous cryoprotectant buffer. | −90 °C to −60 °C −25 °C to −15 °C (for 2 weeks) |
| Spikevax, elasomeran/imelasomeran (Omicron BA.1 variant) mRNA-1273 Moderna, (Camebridge, MA, USA) | Single-stranded, 5′-capped mRNA, encoding for the full-length SARS-CoV-2 spike protein modified with 2 proline substitutions within the heptad repeat 1 domain (S-2P); S protein composed of two subunits (S1 and S2) and stabilized in the pre-fusion conformation by two amino acid mutations, K986P and V987P; open reading frame of 3819 nucleotides; contains 1mΨ instead of uridine; undisclosed modification of the 5′ cap | Multidose dispersion for injection with mRNA encapsulated in lipid nanoparticles with the following main components: SM-102, cholesterol, DSPC, and PEG2000-DMG | −50 °C to −15 °C |
| Vaxzevria AZD1222 COVID-19 Vaccine (ChAdOx1-S [recombinant]) AstraZeneca (Cambridge, UK) | Single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) vector expressing the S glycoprotein spike protein of SARS-CoV-2 with a tPA leader sequence; no mutations introduced in the expressed SARS-CoV-2 spike protein; non-encapsulated, icosahedral particles (virions of 80 to 100 nm diameter) containing a single copy of the double-stranded DNA genome | Liquid dosage form for i.m. injection | 2 °C to 8 °C |
| COVID-19 Vaccine Ad26.COV2.S Janssen (Beerse, Belgium) | Recombinant, replication-incompetent adenovirus serotype 26 (Ad26) encoding the SARS-CoV-2 spike (S) protein | Liquid suspension containing 2-hydroxypropyl-β-cyclodextrin for i.m. injection | −25 °C to −15 °C |
| COVID-19 Vaccine (inactivated, adjuvanted) Valneva Valneva Austria GmbH (Wien, Austria) | Purified, inactivated, and adjuvanted whole virus SARS-CoV-2 (Italian strain (LAZ-INMI1-isl/2020, GISAID Accession number: EPI_ISL_410545)) vaccine grown on Vero cell culture | Liquid suspension for i.m. injection adjuvanted with hydrated aluminium hydroxide and CpG 1018 and recombinant human albumin produced in yeast | 2 °C to 8 °C |
| Nuvaxovid NVX-CoV2373 COVID-19 vaccine (recombinant, adjuvanted) Novavax CZ (Jevany, Czechia) | Protein product of a recombinant SARS- CoV-2 S-gene (Wuhan-Hu-1) encoding the 1260 amino acid spike protein (the full-length 1273 amino acid protein minus the signal peptide); S gene codon optimized for expression in Spodoptera frugiperda (Sf9) insect cells; five amino acid changes introduced, including three in the S1/S2 furin cleavage site (RRAR to QQAQ) and two in the HR1 domain | Aqueous buffered dispersion for i.m. injection, co-formulated with Matrix-M1 adjuvant | 2 °C to 8 °C |
| Tumor Type | Neoantigen | Reference |
|---|---|---|
| Bladder cancer | AP2S1, P3H4, and RAC3 | [174] |
| Melanoma | PTPRC, SIGLEC10, CARD11, LILRB1, and ADAMDEC1 | [175] |
| Colorectal, NSCLC, and pancreatic cancers | KRAS | [176] |
| Esophageal squamous cell carcinoma (ESCC) | NLRC5, FCRL4, TMEM229B, and LCP2 | [177] |
| Soft tissue sarcoma | HLTF, ITGA10, PLCG1, and TTC3 | [178] |
| Glioblastoma | ADAMTSL4, COL6A1, CTSL, CYTH4, EGFLAM, LILRB2, MPZL2, SAA2, and LSP1 | [179] |
| Glioma | NAT1, FRRS1, GTF2H2C, BRCA2, GRAP, NR5A2, ABCB4, ZNF90, ERCC6L, and ZNF813 | [180] |
| Malignant mesothelioma | FAM134B, ALDH3A2, SAV1, RORC, and FN1 | [181] |
| Stomach adenocarcinoma | ADAMTS18, COL10A1, PPEF1, and STRA6 | [182] |
| Mesothelioma | AUNIP, FANCI, LASP1, PSMD8, and XPO5 | [183] |
| Name | mRNA | Indications | Admin. | Reference |
|---|---|---|---|---|
| BNT111 FixVac | 4 TAAs: tyrosinase, NY-ESO-1, MAGE A3, TPTE | Advanced melanoma Phase I: Lipo-MERIT trial ± checkpoint inhibitor PD1 Phase II: + cemiplimab | Intravenous (i.v.) liposomal RNA (RNA-LPX) | [197] NCT02410733 NCT04526899 |
| BNT112 FixVac | 5 prostate cancer-specific antigens: kallikrein-2, kallikrein-3, acid phosphatase prostate, HOXB13, NK3 homeobox 1 | Prostate cancer Phase I/II + cemiplimab PRO-MERIT trial | i.v. RNA-LPX | [198] NCT04382898 |
| BNT113 FixVac | HPV16-E6 and -E7 | HPV16+ head and neck cancer; AHEAD-MERIT Phase II + pembrolizumab HARE-40 Phase I/II | i.v. RNA-LPX | NCT04534205 NCT03418480 |
| BNT115 W_ova1 Vaccine FixVac | 3 ovarian cancer TAAs | Ovarian cancer Phase I + carboplatin/paclitaxel | i.v. | NCT04163094 |
| BNT116 FixVac | 6 mRNAs each of which encodes for a different TAA | NSCLC Phase I/II + cemiplimab Phase I + cemiplimab or docetaxel, LuCa-MERIT-1 | i.v. Liposomes | NCT05557591 NCT05142189 |
| BNT121 IVAC MUTANOME | Personalized vaccine | Metastatic melanoma Phase I ± RBL001/RBL002 | Intranodal | [3,172] NCT02035956 |
| BNT122 (RO7198457 autogene cevumeran) iNeST | 20 patient-specific antigens | Multiple solid tumors Phase I | i.v. | [199] NCT03289962 |
| Melanoma Phase II + pembrolizumab | i.v. | NCT03815058 | ||
| NSCLC (adjuvant) Phase II + atezolizumab | i.v. | NCT04267237 | ||
| CRC Phase II | i.v. | NCT04486378 | ||
| Pancreatic cancer Phase I + atezolizumab + mFOLFIRINOX | i.v. | NCT04161755 | ||
| BNT114 + BNT122 Personalized | IVAC_W_bre1_uID and IVAC_W_bre1_uID/IVAC_M_uID | Triple Negative Breast Cancer TNBC-MERIT | i.v. | NCT02316457 |
| SAR441000 (BNT131) | IL-12sc, IL-15sushi, GM-CSF, IFNα | Solid tumors Phase I ± cemiplimab | Intratumoral | NCT03871348 |
| BNT141 | Encoded antibodies | Multiple solid tumors Phase I/II ± nab-paclitaxel and gemcitabine | i.v. | NCT04683939 |
| BNT142 | Encoded antibodies | Multiple solid CLDN6+ tumors Phase I/II | i.v. | NCT05262530 |
| BNT151 | Encoded cytokines: optimized IL-2 | Multiple solid tumors (optimized IL-2) Phase I/II | i.v. | NCT04455620 |
| BNT152 | Encoded cytokines: IL-2, IL-7 | Multiple solid tumors Phase I/II | i.v. | NCT04455620 NCT04710043 |
| BNT153 | Encoded cytokines: IL-2, IL-7 | Multiple solid tumors Phase I | i.v. | NCT04710043 |
| mRNA-2416 | OX40L | Advanced malignancies Phase I/II ± durvalumab | Intratumoral LNP | [3] NCT03323398 |
| mRNA-2752 | OX40L, IL-23, IL-36γ | Advanced malignancies Phase I/II ± durvalumab | Intratumoral LNP | [200] NCT03739931 |
| mRNA-4157 (V941) | Up to 34 neoantigens, personalized | High-risk melanoma, solid tumors KEYNOTE-603 Phase I + pembrolizumab KEYNOTE-942 Phase II + pembrolizumab | i.m. LNP | [201,202,203,204] NCT03313778 NCT03897881 |
| mRNA-4650 NCI-4650 | Up to 20 antigens + up to 15 HLA class I candidate neoantigens | Gastric or rectal cancer Phase I Melanoma Phase I/II | i.m. | [204,205] NCT03480152 |
| mRNA-5671 Merck V941 | 4 KRAS mutations (G12D, G13D, G12C, and G12V), personalized | CRC, NSCLC, pancreatic adenocarcinoma Phase I ± pembrolizumab | i.m. | [204] NCT03948763 |
| ECI-006 | 5 TAAs + 3 DC-activating antigens | Melanoma Phase I ± standard anti-PD-1 | Intranodal TriMix | [204] NCT03394937 |
| TriMix | 3 mRNA encoding CD70, CD40L, and a constitutively active form of TLR4 | Breast cancer Phase I | Intratumoral | NCT03788083 |
| TriMixDC-MEL IPI | MAGE-A3, MAGE-C2, tyrosinase, and gp100 | Melanoma Phase II + ipilimumab | Intratumoral | [206] NCT01302496 |
| TriMix-DC | Melanoma Phase I Phase I/II | i.v. and intradermal | [207] NCT01066390 | |
| TriMix-DC + TLR-DC | Melanoma Phase I Phase I/II | i.v. (mRNA) intranodal (DCs) | [207] NCT01530698 | |
| TriMix: DC + mRNA (CD70, CD40) + TLR4 | Tyrosinase, gp100, MAGE-A3, or MAGE-C2 | Breast cancer Phase II + ipilimumab | Intratumoral | [206,207] NCT01302496 |
| TriMixDC-MEL: Autologous monocyte-derived mRNA co-electroporated DCs + mRNA | CD40L, CD70, caTLR4 | Melanoma Phase I | i.v. | [208] |
| CV8102 | TLR7/8, RIG-1 | Skin cancer Phase I | Intradermal Protamine | [209] NCT03291002 |
| TL | Hepatocellular carcinoma Phase I + IMA970A + cyclophosphamide | Intradermal Protamine | NCT03203005 | |
| CV9103 RNActive® | 4 antigens for prostate cancer: PSA, PSMA, PSCA, STEAP | Prostate cancer Phase I/II Phase I/II | Intradermal | [210,211] NCT00906243 NCT00831467 |
| CV9104 Mixture of 6 mRNAs, each encoding 1 antigen | PSA, PSCA, PSMA, STEAP1, PAP, MUC1 | Prostate cancer Phase I/II Phase II | Intradermal or needle-free injection device (Tropis®, London, UK) Protamine | [210,212] NCT01817738 NCT02140138 |
| CV9201 | 5 mRNAs: NY-ESO-1, MAGE C1, MAGE C2, survivin, TBG | NSCLC Phase I/II | Intradermal | [213] NCT00923312 NCT03164772 |
| CV9202 BI 1361849 | 6 mRNAs encoding 6 different antigens: NY-ESO-1, MAGE C1, MAGE C2, TPBG, survivin, MUC1 | NSCLC Phase I + local radiation Phase I/II ± durvalumab and tremelimumab | Intradermal | [214,215,216] NCT01915524 NCT03164772 |
| MEDI1191 | IL-12 | Advanced solid tumors, prostate, breast cancer, NSCLC Phase I + durvalumab | Intratumoral LNP | [204,217] NCT03946800 |
| Naked mRNA | Melan-A, MAGE-A1, MAGE-A3, survivin, GP100, and tyrosinase | Melanoma Phase I/II + GM-CSF Phase I/II + GM-CSF | Intradermal GM-CSF as adjuvant | [218] NCT00204516 NCT00204607 |
| Naked mRNA | MUC1, CEA, Her-2/neu, telomerase, survivin, MAGE-A1 | Renal cell cancer Phase I + durvalumab | Intradermal GM-CSF as adjuvant | [219] |
| SW1115C3 | Cancer TSAs, personalized | Solid tumors Phase I | Subcutaneous | [209] NCT05198752 |
| Tumor mRNA + pp65 flLAMP | Glioma, glioblastoma Phase I | i.v. RNA-LP (DOTAP liposome) | NCT04573140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause, W. mRNA—From COVID-19 Treatment to Cancer Immunotherapy. Biomedicines 2023, 11, 308. https://doi.org/10.3390/biomedicines11020308
Krause W. mRNA—From COVID-19 Treatment to Cancer Immunotherapy. Biomedicines. 2023; 11(2):308. https://doi.org/10.3390/biomedicines11020308
Chicago/Turabian StyleKrause, Werner. 2023. "mRNA—From COVID-19 Treatment to Cancer Immunotherapy" Biomedicines 11, no. 2: 308. https://doi.org/10.3390/biomedicines11020308
APA StyleKrause, W. (2023). mRNA—From COVID-19 Treatment to Cancer Immunotherapy. Biomedicines, 11(2), 308. https://doi.org/10.3390/biomedicines11020308




