Hand Grip Strength Relative to Waist Circumference as a Means to Identify Men and Women Possessing Intact Mobility in a Cohort of Older Adults with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Procedure
2.2. Eligibility Criteria
2.3. Exposure Assessment
2.3.1. Hand Grip Strength (HGS) Assessment
2.3.2. Anthropometry
2.4. Outcome Assessment
2.4.1. Usual Gait Speed (UGS)
2.4.2. Timed Up and Go Test (TUG)
2.4.3. Two-Minute Walk Test (2MWT)
2.4.4. Mobility Status
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.1.1. Clinical Characteristics
3.1.2. Mobility Characteristics
3.2. Determination of Optimal HGS Indices for Analysis Using Univariate Analysis
3.3. Identification of Confounders
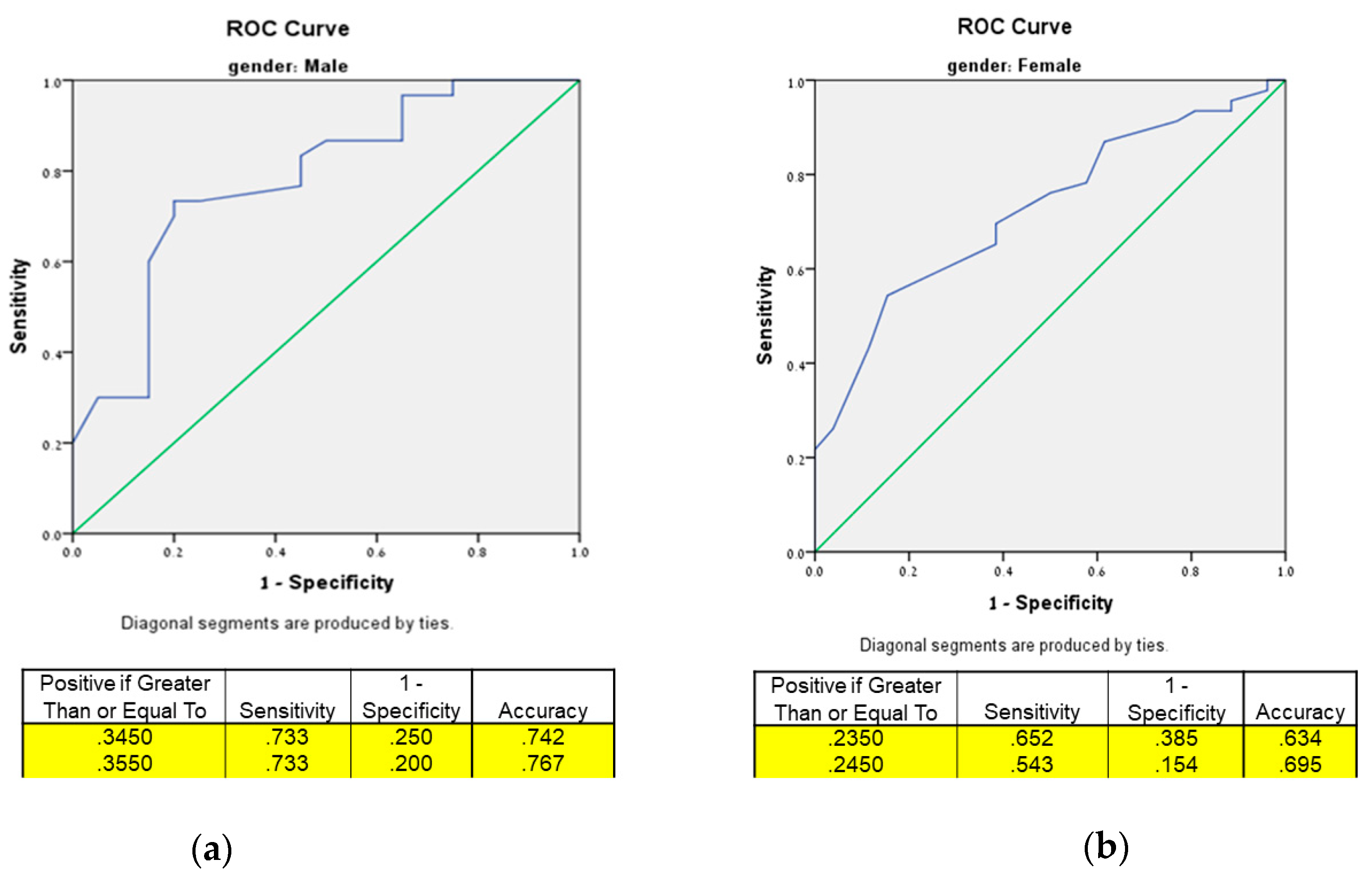
3.4. Optimum Thresholds for HGS Indices Using Receiver Operating Characteristic (ROC) Curve Analysis and Testing Their Prediction for Intact Mobility Using Odds Ratios (ORs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soubra, R.; Chkeir, A.; Novella, J.-L. A Systematic Review of Thirty-One Assessment Tests to Evaluate Mobility in Older Adults. Biomed Res. Int. 2019, 2019, 1354362. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, E.; Sieber, C.C.; Kob, R. Mobility in Older Community-Dwelling Persons: A Narrative Review. Front. Physiol. 2020, 11, 881. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cooper, R.; Shardell, M.; Simonsick, E.M.; Schrack, J.A.; Kuh, D. Age-Related Change in Mobility: Perspectives From Life Course Epidemiology and Geroscience. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Schrack, J.A.; Santanasto, A.J.; Studenski, S.A.; Ferrucci, L.; Glynn, N.W. Pittsburgh Fatigability Scale: One-Page Predictor of Mobility Decline in Mobility-Intact Older Adults. J. Am. Geriatr. Soc. 2018, 66, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Musich, S.; Wang, S.S.; Ruiz, J.; Hawkins, K.; Wicker, E. The Impact of Mobility Limitations on Health Outcomes among Older Adults. Geriatr. Nurs. 2018, 39, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Macri, E.M.; Lewis, J.A.; Khan, K.M.; Ashe, M.C.; de Morton, N.A. The de Morton Mobility Index: Normative Data for a Clinically Useful Mobility Instrument. J. Aging Res. 2012, 2012, 353252. [Google Scholar] [CrossRef] [PubMed]
- De Fátima Ribeiro Silva, C.; Ohara, D.G.; Matos, A.P.; Pinto, A.C.P.N.; Pegorari, M.S. Short Physical Performance Battery as a Measure of Physical Performance and Mortality Predictor in Older Adults: A Comprehensive Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 10612. [Google Scholar] [CrossRef] [PubMed]
- Avin, K.G.; Hanke, T.A.; Kirk-Sanchez, N.; McDonough, C.M.; Shubert, T.E.; Hardage, J.; Hartley, G. Management of Falls in Community-Dwelling Older Adults: Clinical Guidance Statement From the Academy of Geriatric Physical Therapy of the American Physical Therapy Association. Phys. Ther. 2015, 95, 815–834. [Google Scholar] [CrossRef]
- Caron, N.; Peyrot, N.; Caderby, T.; Verkindt, C.; Dalleau, G. Effect of Type 2 Diabetes on Energy Cost and Preferred Speed of Walking. Eur. J. Appl. Physiol. 2018, 118, 2331–2338. [Google Scholar] [CrossRef]
- IJzerman, T.H.; Schaper, N.C.; Melai, T.; Meijer, K.; Willems, P.J.B.; Savelberg, H.H.C.M. Lower Extremity Muscle Strength Is Reduced in People with Type 2 Diabetes, with and without Polyneuropathy, and Is Associated with Impaired Mobility and Reduced Quality of Life. Diabetes Res. Clin. Pract. 2012, 95, 345–351. [Google Scholar] [CrossRef]
- Gregg, E.W.; Beckles, G.L.; Williamson, D.F.; Leveille, S.G.; Langlois, J.A.; Engelgau, M.M.; Narayan, K.M. Diabetes and Physical Disability among Older U.S. Adults. Diabetes Care 2000, 23, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Shiraiwa, T.; Takahara, M.; Iwamoto, M.; Kuribayashi, N.; Nomura, T.; Yamada, M.; Sone, H.; Araki, S.-I. Applications of Physical Performance Measures to Routine Diabetes Care for Frailty Prevention Concept: Fundamental Data with Grip Strength, Gait Speed, Timed Chair Stand Speed, Standing Balance, and Knee Extension Strength. BMJ Open Diabetes Res. Care 2020, 8, e001562. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Tra, Y.; Yeh, H.-C.; Egan, J.M.; Ferrucci, L.; Brancati, F.L. Quadriceps Strength, Quadriceps Power, and Gait Speed in Older U.S. Adults with Diabetes: Results from the National Health and Nutrition Examination Survey (NHANES), 1999–2002. J. Am. Geriatr. Soc. 2013, 61, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Bianchi, L.; Lauretani, F.; Lauretani, F.; Bandinelli, S.; Guralnik, J.M.; Zuliani, G.; Ferrucci, L. Role of Muscle Mass and Muscle Quality in the Association Between Diabetes and Gait Speed. Diabetes Care 2012, 35, 1672–1679. [Google Scholar] [CrossRef]
- Wages, N.P.; Simon, J.E.; Clark, L.A.; Amano, S.; Russ, D.W.; Manini, T.M.; Clark, B.C. Relative Contribution of Muscle Strength, Lean Mass, and Lower Extremity Motor Function in Explaining between-Person Variance in Mobility in Older Adults. BMC Geriatr. 2020, 20, 255. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘Timed Up and Go’ Test: More than Meets the Eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef]
- Kang, L.; Jia, L.; Han, P.; Zhang, W.; Ma, Y.; Fu, L.; Yu, H.; Chen, X.; Wang, L.; Hou, L.; et al. Combined Effect of Obesity and Mobility Limitation with Incidence of Type 2 Diabetes and Mortality in Chinese Elderly. Rejuvenation Res. 2017, 20, 375–382. [Google Scholar] [CrossRef]
- Eckstrom, E.; Parker, E.M.; Shakya, I. Coordinated Care Plan to Prevent Older Adult Falls. Available online: https://stacks.cdc.gov/view/cdc/78040 (accessed on 13 November 2022).
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, But Not Muscle Mass, Is Associated with Mortality in the Health, Aging and Body Composition Study Cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Simonsick, E.M.; Newman, A.B.; Visser, M.; Goodpaster, B.; Kritchevsky, S.B.; Rubin, S.; Nevitt, M.C.; Harris, T.B. Mobility Limitation in Self-Described Well-Functioning Older Adults: Importance of Endurance Walk Testing. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 841–847. [Google Scholar] [CrossRef]
- Riwniak, C.; Simon, J.E.; Wages, N.P.; Clark, L.A.; Manini, T.M.; Russ, D.W.; Clark, B.C. Comparison of a Multi-Component Physical Function Battery to Usual Walking Speed for Assessing Lower Extremity Function and Mobility Limitation in Older Adults. J. Nutr. Health Aging 2020, 24, 906–913. [Google Scholar] [CrossRef]
- Bodilsen, A.C.; Klausen, H.H.; Petersen, J.; Beyer, N.; Andersen, O.; Jørgensen, L.M.; Juul-Larsen, H.G.; Bandholm, T. Prediction of Mobility Limitations after Hospitalization in Older Medical Patients by Simple Measures of Physical Performance Obtained at Admission to the Emergency Department. PLoS ONE 2016, 11, e0154350. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, E.J.; Richardson, J.; McCallum, C.A.; Wilhelm, M. The Predictive Validity of Physical Performance Measures in Determining Markers of Preclinical Disability in Community-Dwelling Middle-Aged and Older Adults: A Systematic Review. Phys. Ther. 2018, 98, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Wallace, D.; Chandler, J.M.; Duncan, P.W.; Rooney, E.; Fox, M.; Guralnik, J.M. Physical Performance Measures in the Clinical Setting. J. Am. Geriatr. Soc. 2003, 51, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Studenski, S.; Ferrucci, L. A Diagnosis of Dismobility—Giving Mobility Clinical Visibility. JAMA 2014, 311, 2061–2062. [Google Scholar] [CrossRef]
- Milanović, Z.; Pantelić, S.; Trajković, N.; Sporiš, G.; Kostić, R.; James, N. Age-Related Decrease in Physical Activity and Functional Fitness among Elderly Men and Women. Clin. Interv. Aging 2013, 8, 549–556. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Brown, T.; Kiel, D.P.; et al. Establishing the Link Between Lean Mass and Grip Strength Cut Points with Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1317–1323. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Wang, Y.-C. Four-Meter Gait Speed: Normative Values and Reliability Determined for Adults Participating in the NIH Toolbox Study. Arch. Phys. Med. Rehabil. 2019, 100, 509–513. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Lee, S.K.; Shin, C. Normalized Hand Grip and Back Muscle Strength as Risk Factors for Incident Type 2 Diabetes Mellitus: 16 Years of Follow-Up in a Population-Based Cohort Study. Diabetes Metab. Syndr. Obes. 2021, 14, 741–750. [Google Scholar] [CrossRef]
- Ho, F.K.W.; Celis-Morales, C.A.; Petermann-Rocha, F.; Sillars, A.; Welsh, P.; Welsh, C.; Anderson, J.; Lyall, D.M.; Mackay, D.F.; Sattar, N.; et al. The Association of Grip Strength with Health Outcomes Does Not Differ If Grip Strength Is Used in Absolute or Relative Terms: A Prospective Cohort Study. Age Ageing 2019, 48, 684–691. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus Is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-Analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; McClure, R.; Villani, A. Adiposity Is Inversely Associated with Strength in Older Adults with Type 2 Diabetes Mellitus. Eur. Geriatr. Med. 2020, 11, 451–458. [Google Scholar] [CrossRef]
- Brandão, M.P.; Cardoso, M.F. Obesity in Older Type 2 Diabetic Patients: Does Working Environment Add Vulnerability? Int. J. Environ. Res. Public Health 2018, 15, 2677. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Saudek, C.D.; Brancati, F.L.; Selvin, E. Association of Diabetes, Comorbidities, and A1C With Functional Disability in Older Adults. Diabetes Care 2010, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Matsuhisa, M. Clinical Impact of Sarcopenia and Dynapenia on Diabetes. Diabetol. Int. 2019, 10, 183–187. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Cheng, K.K.; Lam, T.H.; Xu, L. Association of a Composite Score of Relative Grip Strength and Timed up and Go Test with Incident Type 2 Diabetes Mellitus: Guangzhou Biobank Cohort Study. Aging 2021, 13, 18376–18391. [Google Scholar] [CrossRef]
- Lombardo, M.; Padua, E.; Campoli, F.; Panzarino, M.; Mîndrescu, V.; Annino, G.; Iellamo, F.; Bellia, A. Relative Handgrip Strength Is Inversely Associated with the Presence of Type 2 Diabetes in Overweight Elderly Women with Varying Nutritional Status. Acta Diabetol. 2021, 58, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Saraiva, B.; Nascimento, D.D.C.; Oliveira, S.C.; Neto, I.S.; Valduga, R.; Santos, N.G.; Tibana, R.A.; Prestes, J.; Willardson, J.M.; et al. Relative Handgrip Strength as a Simple Tool to Evaluate Impaired Heart Rate Recovery and a Low Chronotropic Index in Obese Older Women. Int. J. Exerc. Sci. 2018, 11, 844–855. [Google Scholar]
- Boonpor, J.; Parra-Soto, S.; Petermann-Rocha, F.; Ferrari, G.; Welsh, P.; Pell, J.P.; Sattar, N.; Gill, J.M.R.; Ho, F.K.; Gray, S.R.; et al. Associations between Grip Strength and Incident Type 2 Diabetes: Findings from the UK Biobank Prospective Cohort Study. BMJ Open Diabetes Res. Care 2021, 9, e001865. [Google Scholar] [CrossRef]
- Momma, H.; Sawada, S.S.; Kato, K.; Gando, Y.; Kawakami, R.; Miyachi, M.; Huang, C.; Nagatomi, R.; Tashiro, M.; Ishizawa, M.; et al. Physical Fitness Tests and Type 2 Diabetes Among Japanese: A Longitudinal Study from the Niigata Wellness Study. J. Epidemiol. 2019, 29, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ntuk, U.E.; Celis-Morales, C.A.; Mackay, D.F.; Sattar, N.; Pell, J.P.; Gill, J.M.R. Association between Grip Strength and Diabetes Prevalence in Black, South-Asian, and White European Ethnic Groups: A Cross-Sectional Analysis of 418 656 Participants in the UK Biobank Study. Diabet. Med. 2017, 34, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip Strength Cutpoints for the Identification of Clinically Relevant Weakness. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef] [PubMed]
- De Souza Vasconcelos, K.S.; Domingues Dias, J.M.; de Carvalho Bastone, A.; Alvarenga Vieira, R.; de Souza Andrade, A.C.; Rodrigues Perracini, M.; Oliveira Guerra, R.; Corrêa Dias, R. Handgrip Strength Cutoff Points to Identify Mobility Limitation in Community-Dwelling Older People and Associated Factors. J. Nutr. Health Aging 2016, 20, 306–315. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Travison, T.G.; Zhu, H.; Magaziner, J.; Binder, E.F.; Pahor, M.; Correa-de-Araujo, R.; Cawthon, P.M.; Bhasin, S.; Orwig, D.; et al. Application of Cut-Points for Low Muscle Strength and Lean Mass in Mobility-Limited Older Adults. J. Am. Geriatr. Soc. 2020, 68, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, J.; Stenholm, S.; Rantanen, T.; Heliövaara, M.; Sainio, P.; Koskinen, S. Hand-Grip Strength Cut-Points to Screen Older Persons at Risk for Mobility Limitation. J. Am. Geriatr. Soc. 2010, 58, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Souza Saraiva, W.; Prestes, J.; Schwerz Funghetto, S.; Navalta, J.W.; Tibana, R.A.; da Cunha Nascimento, D. Relation Between Relative Handgrip Strength, Chronological Age and Physiological Age with Lower Functional Capacity in Older Women. Open Access J. Sports Med. 2019, 10, 185–190. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in Daily Practice: Assessment and Management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef]
- Dugdale, D.C.; Epstein, R.; Pantilat, S.Z. Time and the Patient–Physician Relationship. J. Gen. Intern. Med. 1999, 14, S34–S40. [Google Scholar] [CrossRef]
- Kabeya, Y.; Uchida, J.; Toyoda, M.; Katsuki, T.; Oikawa, Y.; Kato, K.; Kawai, T.; Shimada, A.; Atsumi, Y.; Higaki, M. Factors Affecting Consultation Length in a Japanese Diabetes Practice. Diabetes Res. Clin. Pract. 2017, 126, 54–59. [Google Scholar] [CrossRef]
- Kitagawa, N.; Okamura, T.; Kitagawa, N.; Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Handgrip Measurement as a Useful Benchmark for Locomotive Syndrome in Patients with Type 2 Diabetes Mellitus: A KAMOGAWA-DM Cohort Study. J. Diabetes Investig. 2020, 11, 1602–1611. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S113–S124. [Google Scholar] [CrossRef]
- Buch, A.; Eldor, R.; Kis, O.; Keinan-Boker, L.; Dunsky, A.; Rubin, A.; Lopez, A.; Sofer, Y.; Osher, E.; Marcus, Y.; et al. The Effect of Circuit Resistance Training, Empagliflozin or “Vegeterranean Diet” on Physical and Metabolic Function in Older Subjects with Type 2 Diabetes: A Study Protocol for a Randomized Control Trial (CEV-65 Trial). BMC Geriatr. 2019, 19, 228. [Google Scholar] [CrossRef]
- American Diabetes Association Executive Summary: Standards of Medical Care in Diabetes—2014. Diabetes Care 2014, 37 (Suppl. S1), S5–S13. [CrossRef] [PubMed]
- Fried, L.P.; Borhani, N.O.; Enright, P.; Furberg, C.D.; Gardin, J.M.; Kronmal, R.A.; Kuller, L.H.; Manolio, T.A.; Mittelmark, M.B.; Newman, A.; et al. The Cardiovascular Health Study: Design and Rationale. Ann. Epidemiol. 1991, 1, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.H.Y.; de Craen, A.J.M.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.M.; Westendorp, R.G.J.; Maier, A.B. Accuracy of Direct Segmental Multi-Frequency Bioimpedance Analysis in the Assessment of Total Body and Segmental Body Composition in Middle-Aged Adult Population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Ben-Yehuda, A.; Rouach, V.; Maier, A.B.; Greenman, Y.; Izkhakov, E.; Stern, N.; Eldor, R. Validation of a Multi-Frequency Bioelectrical Impedance Analysis Device for the Assessment of Body Composition in Older Adults with Type 2 Diabetes. Nutr. Diabetes 2022, 12, 45. [Google Scholar] [CrossRef]
- WHO. Consultation on Obesity. In Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Heo, M.; Faith, M.S.; Pietrobelli, A.; Heymsfield, S.B. Percentage of Body Fat Cutoffs by Sex, Age, and Race-Ethnicity in the US Adult Population from NHANES 1999–2004. Am. J. Clin. Nutr. 2012, 95, 594–602. [Google Scholar] [CrossRef]
- Choquette, S.; Bouchard, D.R.; Doyon, C.Y.; Sénéchal, M.; Brochu, M.; Dionne, I.J. Relative Strength as a Determinant of Mobility in Elders 67–84 Years of Age. A Nuage Study: Nutrition as a Determinant of Successful Aging. J. Nutr. Health Aging 2010, 14, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kallen, M.; Slotkin, J.; Griffith, J.; Magasi, S.; Salsman, J.; Nowinski, C.; Gershon, R. NIH Toolbox Technical Manual Domain. Available online: https://staging.healthmeasures.net/images/nihtoolbox/Technical_Manuals/Sensation/Toolbox_Taste_Intensity_Test_Technical_Manual-_edits_1-22-14.pdf (accessed on 13 November 2022).
- Butland, R.J.; Pang, J.; Gross, E.R.; Woodcock, A.A.; Geddes, D.M. Two-, Six-, and 12-Minute Walking Tests in Respiratory Disease. Br. Med. J. Clin. Res. Ed. 1982, 284, 1607–1608. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Wang, Y.-C.; Gershon, R.C. Two-Minute Walk Test Performance by Adults 18 to 85 Years: Normative Values, Reliability, and Responsiveness. Arch. Phys. Med. Rehabil. 2015, 96, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Roedersheimer, K.M.; Pereira, G.F.; Jones, C.W.; Braz, V.A.; Mangipudi, S.A.; Platts-Mills, T.F. Self-Reported vs. Performance-Based Assessments of a Simple Mobility Task among Older Adults in the Emergency Department. Ann. Emerg. Med. 2016, 67, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, E.; Calvani, R.; Marzetti, E.; Cesari, M. Physical Functional Assessment in Older Adults. J. Frailty Aging 2021, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Swank, C.; Almutairi, S.; Medley, A. Proposing Development and Utility of a Mobility Composite Measure in Patients with a Neurologic Disorder. Rehabil. Res. Pract. 2017, 2017, 8619147. [Google Scholar] [CrossRef] [PubMed]
- Varela, S.; Ayán, C.; Cancela, J.M. Batteries Assessing Health Related Fitness in the Elderly: A Brief Review. Eur. Rev. Aging Phys. Act. 2008, 5, 97–105. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.-W.; Peng, T.-C.; Chen, W.-L.; Han, D.-S.; Chen, C.-L.; Yang, W.-S. Impact of Adiposity on Muscle Function and Clinical Events among Elders with Dynapenia, Presarcopenia and Sarcopenia: A Community-Based Cross-Sectional Study. Aging 2021, 13, 7247–7258. [Google Scholar] [CrossRef]
- Sandhu, R.; Koley, S. A Study of Handgrip Strength in Patients with Type-2 Diabetes Mellitus and Its Association with Some Anthropometric Variables. Int. J. Health Sci. Res. 2018, 8, 82–89. [Google Scholar]
- Angleman, S.B.; Harris, T.B.; Melzer, D. The Role of Waist Circumference in Predicting Disability in Periretirement Age Adults. Int. J. Obes. 2006, 30, 364–373. [Google Scholar] [CrossRef]
- Stenholm, S.; Koster, A.; Alley, D.E.; Houston, D.K.; Kanaya, A.; Lee, J.S.; Newman, A.B.; Satterfield, S.; Simonsick, E.M.; Visser, M.; et al. Joint Association of Obesity and Metabolic Syndrome with Incident Mobility Limitation in Older Men and Women—Results from the Health, Aging, and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65A, 84–92. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Wang, W.; Sun, M.; Tian, H.; Wei, L.; Wu, Y. Dynapenic Abdominal Obesity and the Effect on Long-Term Gait Speed and Falls in Older Adults. Clin. Nutr. 2022, 41, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Day, K.; Kwok, A.; Evans, A.; Mata, F.; Verdejo-Garcia, A.; Hart, K.; Ward, L.C.; Truby, H. Comparison of a Bioelectrical Impedance Device against the Reference Method Dual Energy X-Ray Absorptiometry and Anthropometry for the Evaluation of Body Composition in Adults. Nutrients 2018, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, X.; Guo, Q.; Wang, J.; Zhang, W.; Shen, S.; Han, P.; Ma, Y.; Kang, L.; Wang, M.; et al. Clinical Relevance of Different Handgrip Strength Indexes and Mobility Limitation in the Elderly Adults. J. Gerontol. Ser. A 2016, 71, 96–102. [Google Scholar] [CrossRef]
- Manini, T.M.; Patel, S.M.; Newman, A.B.; Travison, T.G.; Kiel, D.P.; Shardell, M.D.; Pencina, K.M.; Wilson, K.E.; Kelly, T.L.; Massaro, J.M.; et al. Identification of Sarcopenia Components That Discriminate Slow Walking Speed: A Pooled Data Analysis. J. Am. Geriatr. Soc. 2020, 68, 1419–1428. [Google Scholar] [CrossRef]
- Welmer, A.-K.; Angleman, S.; Rydwik, E.; Fratiglioni, L.; Qiu, C. Association of Cardiovascular Burden with Mobility Limitation among Elderly People: A Population-Based Study. PLoS ONE 2013, 8, e65815. [Google Scholar] [CrossRef] [PubMed]
- Lieberz, D.; Borgeson, H.; Dobson, S.; Ewings, L.; Johnson, K.; Klaysmat, K.; Schultz, A.; Tasson, R.; Borstad, A.L. A Physical Therapy Mobility Checkup for Older Adults: Feasibility and Participant Preferences from a Discrete Choice Experiment. J. Patient Cent. Res. Rev. 2022, 9, 24–34. [Google Scholar] [CrossRef]
- Fritschi, C.; Bronas, U.G.; Park, C.G.; Collins, E.G.; Quinn, L. Early Declines in Physical Function among Aging Adults with Type 2 Diabetes. J. Diabetes Complicat. 2017, 31, 347–352. [Google Scholar] [CrossRef]
- Haskell, W.L. Health Consequences of Physical Activity: Understanding and Challenges Regarding Dose-Response. Med. Sci. Sports Exerc. 1994, 26, 649–660. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total N = 122 | Women N = 72 | Men N = 50 | p |
|---|---|---|---|---|
| Age (years) | 70.3 (4.4) | 70.3 (4.4) | 70.1 (3.9) | 0.82 |
| Height (cm) | 163 (8.8) | 158 (5) | 171 (6) | <0.001 * |
| Weight (kg) | 85.4 (16.5) | 79.9 (15) | 93.2 (15.6) | <0.001 * |
| BMI (kg/m2) | 32.2 (6.1) | 32.3 (6.6) | 31.9 (5.3) | 0.68 |
| BMI ≥ 30 (%) 1 | 63.9 | 64.0 | 63.8 | 0.68 |
| WC (cm) | 111.4 (14.7) | 109.7 (15.4) | 113.9 (13.4) | 0.12 |
| WC ≥ 102 cm men (%) 2 WC ≥ 88 cm women (%) 2 | 94.2 | 97.2 | 90.0 | 0.12 |
| SMM (kg) | 31.4 (12) | 27.5 (10.3) | 38.3 (11.5) | <0.001 * |
| BFM (kg) | 34.9 (11) | 35.8 (10.4) | 35.1 (6.7) | <0.001 * |
| PBF Men ≥ 32.3; women ≥ 44.1 (%) 3 | 40.3 (7.5) | 44.0 (5.6) | 35.1 (6.8) | <0.001 * |
| Diabetes duration (years) | 13.7 (9.3) | 14.2 (8.7) | 12.8 (10.2) | 0.44 |
| HbA1c (%) | 7.4 (1.1) | 7.4 (1) | 7.56 (1.2) | 0.43 |
| Vitamin D (<25 ng/mL) (%) | 31.1 (16.8) | 37.5 (15.9) (n = 67) | 40.5 (18.2) (n = 42) | 0.98 |
| Hypertension (%) | 59.9 | 62.0 | 58.4 | 0.68 |
| Diabetes complications 4 (%) | 39.4 | 31.9 | 42.0 | 0.25 |
| Polypharmacy (≥8 drugs) (%) | 32.1 | 33.3 | 30.6 (n = 49) | 0.75 |
| Physical activitys 5 (%) | 50.8 | 51.4 | 50.0 | 0.88 |
| HGS Indices | ||||
| HGS (absolute) (kg) | 31.3 (9.4) | 24.9 (4.6) | 40.44 (6.6) | <0.001 * |
| HGS/BW (kg/kg) | 0.37 (0.1) | 0.32 (0.07) | 0.44 (0.1) | <0.001 * |
| HGS/height (kg/m) | 19.03 (5) | 15.82 (2.8) | 23.64 (3.6) | <0.001 * |
| HGS/BMI (kg/kg/m2) | 1.01 (0.3) | 0.80 (0.2) | 1.30 (0.31) | <0.001 * |
| HGS/WC (kg/cm) | 0.28 (0.1) | 0.23 (0.05) | 0.36 (0.08) | <0.001 * |
| HGS/SMM (kg/kg) | 1.04 (0.29) | 0.99 (0.28) | 1.12 (0.28) | <0.001 * |
| HGS/BFM (kg/kg) | 1.0 (0.5) | 0.76 (26) | 1.36 (0.55) | <0.001 * |
| HGS/PBF (kg/%) | 0.84 (0.4) | 0.58 (0.13) | 1.21 (0.38) | <0.001 * |
| Test | Total N = 122 (%) | Women N = 72 | Men N = 50 | p |
|---|---|---|---|---|
| UGS (m/sec) | 1.11 (0.2) | 1.08 (0.2) | 1.14 (0.2) | 0.06 |
| TUG (sec) | 10.68 (0.2) | 10.97 (2.5) | 10.25 (1.8) | 0.03 * |
| 2MWT (m) | 162.9 (31.3) | 154.2 (28.2) | 175.6 (31.5) | <0.001 * |
| Pass UGS (%) 1 | 83.6 | 87.5 | 78.0 | 0.16 |
| Pass TUG (%) 2 | 79.5 | 77.8 | 82.0 | 0.57 |
| Pass 2MWT (%) 3 | 73.7 | 75.0 | 71.4 | 0.66 |
| Pass all tests (%) | 63.3 | 63.9 | 60.0 | 0.66 |
| Fail 1 test only (%) | 20.4 | 20.9 | 20.0 | 0.878 |
| Fail > 1 test | 16.4 | 13.9 | 20.0 | 0.977 |
| Test | AUC (95% CI) | p-Value | Cut-Off | Sensitivity % | Specificity % | Accuracy |
|---|---|---|---|---|---|---|
| Men | ||||||
| UGS | 0.758 (0.579, 0.936) | 0.01 * | 0.305 | 0.821 | 0.636 | 0.728 |
| TUG | 0.827 (0.704, 0.950) | 0.002 * | 0.355 | 0.634 | 1.0 | 0.817 |
| 2MWT | 0.779 (0.644, 0.913) | 0.003 * | 0.335 | 0.686 | 0.857 | 0.771 |
| Pass all | 0.781 (0.649, 0.913) | 0.01 * | 0.355 | 0.733 | 0.800 | 0.767 |
| Women | ||||||
| UGS | 0.631 (0.402, 0.861) | 0.2 | 0.180 | 0.825 | 0.556 | 0.690 |
| TUG | 0.725 (0.602, 0.848) | 0.006 * | 0.235 | 0.500 | 0.933 | 0.719 |
| 2MWT | 0.751 (0.623, 0.880) | 0.002 * | 0.215 | 0.685 | 0.722 | 0.704 |
| Pass all | 0.725 (0.609, 0.842) | 0.002 * | 0.245 | 0.543 | 0.846 | 0.695 |
| Test | Gender | N | OR | 95% CI |
|---|---|---|---|---|
| UGS | Men | 50 | 8.0 | (1.3–34.9) |
| Women | 72 | 0.4 | (0.37–4.3) | |
| TUG | Men | 49 | * | * |
| Women | 72 | 15 | (1.8–121.4) | |
| 2MWT | Men | 49 | 13.1 | (2.5–68.7) |
| Women | 72 | 5.6 | (1.7–18.4) | |
| Pass all | Men | 50 | 8.2 | (2.2–30.1) |
| Women | 72 | 3.0 | (1.1–8.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, O.S.; Buch, A.; Eldor, R.; Moran, D.S. Hand Grip Strength Relative to Waist Circumference as a Means to Identify Men and Women Possessing Intact Mobility in a Cohort of Older Adults with Type 2 Diabetes. Biomedicines 2023, 11, 352. https://doi.org/10.3390/biomedicines11020352
Kis OS, Buch A, Eldor R, Moran DS. Hand Grip Strength Relative to Waist Circumference as a Means to Identify Men and Women Possessing Intact Mobility in a Cohort of Older Adults with Type 2 Diabetes. Biomedicines. 2023; 11(2):352. https://doi.org/10.3390/biomedicines11020352
Chicago/Turabian StyleKis, Ofer S., Assaf Buch, Roy Eldor, and Daniel S. Moran. 2023. "Hand Grip Strength Relative to Waist Circumference as a Means to Identify Men and Women Possessing Intact Mobility in a Cohort of Older Adults with Type 2 Diabetes" Biomedicines 11, no. 2: 352. https://doi.org/10.3390/biomedicines11020352
APA StyleKis, O. S., Buch, A., Eldor, R., & Moran, D. S. (2023). Hand Grip Strength Relative to Waist Circumference as a Means to Identify Men and Women Possessing Intact Mobility in a Cohort of Older Adults with Type 2 Diabetes. Biomedicines, 11(2), 352. https://doi.org/10.3390/biomedicines11020352







