Clinical and Therapeutic Evaluation of the Ten Most Prevalent CRB1 Mutations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Clinical Evaluation
2.3. Base Editing Analysis
2.4. Prime Editing Analysis
3. Results and Discussion
3.1. Clinical Results
| Variant # | cDNA Change | Protein Change | Exon | Reported Alleles (n) | Proportion of Alleles (%) |
|---|---|---|---|---|---|
| 1 | c.2843G>A | p.(Cys948Tyr) | 9 | 260 | 12.48 |
| 2 | c.2401A>T | p.(Lys801*) | 7 | 73 | 3.50 |
| 3 | c.2234C>T | p.(Thr745Met) | 7 | 72 | 3.45 |
| 4 | c.2290C>T | p.(Arg764Cys) | 7 | 64 | 3.07 |
| 5 | c.2688T>A | p.(Cys896*) | 8 | 43 | 2.06 |
| 6 | c.498_506del | p.(Ile167_Gly169del) | 2 | 42 | 2.02 |
| 7 | c.613_619del | p.(Ile205Aspfs*13) | 2 | 34 | 1.63 |
| 8 | c.1576C>T | p.(Arg526*) | 6 | 30 | 1.44 |
| 9 | c.3307G>A | p.(Gly1103Arg) | 9 | 21 | 1.01 |
| 10 | c.614T>C | p.(Ile205Thr) | 2 | 20 | 0.96 |
| Total | 659 | 31.62 | |||
3.2. Evaluation of Clinical Results
3.3. Therapeutic Development for CRB1-Linked Inherited Retinal Dystrophies
3.3.1. Evaluation of Base Editing
| Mutation | Strand | Position | Spacer (5′ to 3′) | PAM | Bystanders (BS) | BS Effects | |
|---|---|---|---|---|---|---|---|
| ABE | c.1576C>T | − | 4 | TTCaGAAAAGTAGAAGAGCC | ATT | BS1 and BS2 | BS1 + BS2 = Phe525Pro BS1 alone = Phe525Ser (isoform B = 152) (isoform A&C = 525) |
| 5 | CTTCaGAAAAGTAGAAGAGC | CAT | BS1 | ||||
| 6 | GCTTCaGAAAAGTAGAAGAG | CCA | N/A | ||||
| 7 | TGCTTCaGAAAAGTAGAAGA | GCC | N/A | ||||
| c.2234C>T | − | 4 | AGCaTTCGGACAAACATGGA | GAG | N/A | Leu746Pro (isoform A) Leu373Pro (isoform B) | |
| 5 | AAGCaTTCGGACAAACATGG | AGA | N/A | ||||
| 6 | GAAGCaTTCGGACAAACATG | GAG | N/A | ||||
| 7 | TGAAGCaTTCGGACAAACAT | GGA | BS1 | ||||
| c.2290C>T | − | 4 | CACaGATATATTGATAAGTG | CTG | BS2 | BS1 is a silent; BS2 Ile763Thr (isoform A) Ile390Thr (isoform B) | |
| 5 | ACACaGATATATTGATAAGT | GCT | BS2 | ||||
| 6 | GACACaGATATATTGATAAG | TGC | BS1 | ||||
| 7 | AGACACaGATATATTGATAA | GTG | BS1 | ||||
| c.2843G>A | + | 4 | TAGaTATTGCAAATGCTGTT | TTT | BS2 | BS1 is intronic (−2); BS2 Ile949Val (isoform A) Ile5676Val (isoform B) | |
| 5 | TTAGaTATTGCAAATGCTGT | TTT | BS2 | ||||
| 6 | ATTAGaTATTGCAAATGCTG | TTT | BS1 | ||||
| 7 | CATTAGaTATTGCAAATGCT | GTT | BS1 | ||||
| c.3307G>A | + | 4 | ATCaGAGGCATTTATCTCTC | TTA | BS2 | BS2 is silent; BS1 Ile1102Val (isoform A) Ile729Val (isoform B) | |
| 5 | AATCaGAGGCATTTATCTCT | CTT | BS2 | ||||
| 6 | AAATCaGAGGCATTTATCTC | TCT | N/A | ||||
| 7 | GAAATCaGAGGCATTTATCT | CTC | BS1 | ||||
| CBE | c.614T>C | + | 4 | AAAcAGGAAGATATACTTGT | ATC | N/A | N/A |
| 5 | GAAAcAGGAAGATATACTTG | TAT | |||||
| 6 | TGAAAcAGGAAGATATACTT | GTA | |||||
| 7 | ATGAAAcAGGAAGATATACT | TGT | |||||
| 8 | AATGAAAcAGGAAGATATAC | TTG |
3.3.2. Evaluation of Prime Editing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stone, E.M.; Andorf, J.L.; Whitmore, S.S.; DeLuca, A.P.; Giacalone, J.C.; Streb, L.M.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E.; Sheffield, V.C.; et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 2017, 124, 1314–1331. [Google Scholar] [CrossRef]
- Talib, M.; van Schooneveld, M.J.; van Genderen, M.M.; Wijnholds, J.; Florijn, R.J.; ten Brink, J.B.; Schalij-Delfos, N.E.; Dagnelie, G.; Cremers, F.P.M.; Wolterbeek, R.; et al. Genotypic and Phenotypic Characteristics of CRB1-Associated Retinal Dystrophies: A Long-Term Follow-up Study. Ophthalmology 2017, 124, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Pellissier, L.P.; Wijnholds, J. The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog. Retin. Eye Res. 2014, 40, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, A.I.; ten Brink, J.B.; de Kok, Y.J.; van Soest, S.; van den Born, L.I.; van Driel, M.A.; van de Pol, D.J.; Payne, A.M.; Bhattacharya, S.S.; Kellner, U.; et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 1999, 23, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Burke, T.; Oll, M.; Yzer, S.; Lee, W.; Xie, Y.A.; Allikmets, R. Whole exome sequencing identifies CRB1 defect in an unusual maculopathy phenotype. Ophthalmology 2014, 121, 1773–1782. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Aleman, T.S.; Pianta, M.J.; Sumaroka, A.; Schwartz, S.B.; Smilko, E.E.; Milam, A.H.; Sheffield, V.C.; Stone, E.M. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum. Mol. Genet. 2003, 12, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, A.I.; Heckenlively, J.R.; van den Born, L.I.; de Kok, Y.J.M.; van der Velde-Visser, S.D.; Kellner, U.; Jurklies, B.; van Schooneveld, M.J.; Blankenagel, A.; Rohrschneider, K.; et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am. J. Hum. Genet. 2001, 69, 198–203. [Google Scholar] [CrossRef]
- Bujakowska, K.; Audo, I.; Mohand-Säid, S.; Lancelot, M.E.; Antonio, A.; Germain, A.; Eveillard, T.Ĺ.; Letexier, Ḿ.; Saraiva, J.P.; Lonjou, C.; et al. CRB1 mutations in inherited retinal dystrophies. Hum. Mutat. 2012, 33, 306–315. [Google Scholar] [CrossRef]
- Henderson, R.H.; Mackay, D.S.; Li, Z.; Moradi, P.; Sergouniotis, P.; Russell-Eggitt, I.; Thompson, D.A.; Robson, A.G.; Holder, G.E.; Webster, A.R.; et al. Phenotypic variability in patients with retinal dystrophies due to mutations in CRB1. Br. J. Ophthalmol. 2011, 95, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.L.; Salles, M.V.; Costa, K.A.; Filippelli-Silva, R.; Martin, R.P.; Sallum, J.M.F. The correlation between CRB1 variants and the clinical severity of Brazilian patients with different inherited retinal dystrophy phenotypes. Sci. Rep. 2017, 7, 8654. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, C.; Michel, D.; Lane-Guermonprez, L.; Delgrossi, M.-H.; Médina, E.; Arsanto, J.-P.; Le Bivic, A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell 2004, 15, 1324–1333. [Google Scholar] [CrossRef]
- Roh, M.H.; Makarova, O.; Liu, C.-J.; Shin, K.; Lee, S.; Laurinec, S.; Goyal, M.; Wiggins, R.; Margolis, B. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 2002, 157, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.M.; Pellissier, L.P.; Wijnholds, J. The CRB1 complex: Following the trail of Crumbs to a feasible gene therapy strategy. Front. Neurosci. 2017, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Willoughby, J.J.; Christensen, A.K.; Jensen, A.M. Mosaic Eyes is a novel component of the Crumbs complex and negatively regulates photoreceptor apical size. Development 2006, 133, 4849–4859. [Google Scholar] [CrossRef]
- Laprise, P.; Beronja, S.; Silva-Gagliardi, N.F.; Pellikka, M.; Jensen, A.M.; McGlade, C.J.; Tepass, U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell 2006, 11, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Gosens, I.; Sessa, A.; den Hollander, A.I.; Letteboer, S.J.F.; Belloni, V.; Arends, M.L.; Le Bivic, A.; Cremers, F.P.M.; Broccoli, V.; Roepman, R. FERM protein EPB41L5 is a novel member of the mammalian CRB-MPP5 polarity complex. Exp. Cell Res. 2007, 313, 3959–3970. [Google Scholar] [CrossRef]
- Gamblin, C.L.; Hardy, É.J.-L.; Chartier, F.J.-M.; Bisson, N.; Laprise, P. A bidirectional antagonism between aPKC and Yurt regulates epithelial cell polarity. J. Cell Biol. 2014, 204, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, A.I.; Davis, J.; van der Velde-Visser, S.D.; Zonneveld, M.N.; Pierrottet, C.O.; Koenekoop, R.K.; Kellner, U.; van den Born, L.I.; Heckenlively, J.R.; Hoyng, C.B.; et al. CRB1 mutation spectrum in inherited retinal dystrophies. Hum. Mutat. 2004, 24, 355–369. [Google Scholar] [CrossRef]
- Pellikka, M.; Tanentzapf, G.; Pinto, M.; Smith, C.; McGlade, C.J.; Ready, D.F.; Tepass, U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 2002, 416, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Van de Pavert, S.A.; Kantardzhieva, A.; Malysheva, A.; Meuleman, J.; Versteeg, I.; Levelt, C.; Klooster, J.; Geiger, S.; Seeliger, M.W.; Rashbass, P.; et al. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J. Cell Sci. 2004, 117, 4169–4177. [Google Scholar] [CrossRef]
- Kantardzhieva, A.; Gosens, I.; Alexeeva, S.; Punte, I.M.; Versteeg, I.; Krieger, E.; Neefjes-Mol, C.A.; den Hollander, A.I.; Letteboer, S.J.F.; Klooster, J.; et al. MPP5 recruits MPP4 to the CRB1 complex in photoreceptors. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2192–2201. [Google Scholar] [CrossRef]
- Pellissier, L.P.; Lundvig, D.M.S.; Tanimoto, N.; Klooster, J.; Vos, R.M.; Richard, F.; Sothilingam, V.; Garcia Garrido, M.; Le Bivic, A.; Seeliger, M.W.; et al. CRB2 acts as a modifying factor of CRB1-related retinal dystrophies in mice. Hum. Mol. Genet. 2014, 23, 3759–3771. [Google Scholar] [CrossRef]
- Pellissier, L.P.; Quinn, P.M.; Alves, C.H.; Vos, R.M.; Klooster, J.; Flannery, J.G.; Heimel, J.A.; Wijnholds, J. Gene therapy into photoreceptors and Müller glial cells restores retinal structure and function in CRB1 retinitis pigmentosa mouse models. Hum. Mol. Genet. 2015, 24, 3104–3118. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.M.; Buck, T.M.; Mulder, A.A.; Ohonin, C.; Alves, C.H.; Vos, R.M.; Bialecka, M.; van Herwaarden, T.; van Dijk, E.H.C.; Talib, M.; et al. Human iPSC-Derived Retinas Recapitulate the Fetal CRB1 CRB2 Complex Formation and Demonstrate that Photoreceptors and Müller Glia Are Targets of AAV5. Stem Cell Rep. 2019, 12, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.A.; Cochran, K.; Kozlowski, C.; Wang, J.; Alexander, G.; Cady, M.A.; Spencer, W.J.; Ruzycki, P.A.; Clark, B.S.; Laeremans, A.; et al. Comprehensive identification of mRNA isoforms reveals the diversity of neural cell-surface molecules with roles in retinal development and disease. Nat. Commun. 2020, 11, 3328. [Google Scholar] [CrossRef]
- Alves, C.H.; Sanz, A.S.; Park, B.; Pellissier, L.P.; Tanimoto, N.; Beck, S.C.; Huber, G.; Murtaza, M.; Richard, F.; Sridevi Gurubaran, I.; et al. Loss of CRB2 in the mouse retina mimics human retinitis pigmentosa due to mutations in the CRB1 gene. Hum. Mol. Genet. 2013, 22, 35–50. [Google Scholar] [CrossRef]
- Pellissier, L.P.; Alves, C.H.; Quinn, P.M.; Vos, R.M.; Tanimoto, N.; Lundvig, D.M.S.; Dudok, J.J.; Hooibrink, B.; Richard, F.; Beck, S.C.; et al. Targeted ablation of CRB1 and CRB2 in retinal progenitor cells mimics Leber congenital amaurosis. PLoS Genet. 2013, 9, e1003976. [Google Scholar] [CrossRef]
- Quinn, P.M.; Alves, C.H.; Klooster, J.; Wijnholds, J. CRB2 in immature photoreceptors determines the superior-inferior symmetry of the developing retina to maintain retinal structure and function. Hum. Mol. Genet. 2018, 27, 3137–3153. [Google Scholar] [CrossRef]
- Quinn, P.M.; Mulder, A.A.; Henrique Alves, C.; Desrosiers, M.; de Vries, S.I.; Klooster, J.; Dalkara, D.; Koster, A.J.; Jost, C.R.; Wijnholds, J. Loss of CRB2 in Müller glial cells modifies a CRB1-associated retinitis pigmentosa phenotype into a Leber congenital amaurosis phenotype. Hum. Mol. Genet. 2019, 28, 105–123. [Google Scholar] [CrossRef]
- Alves, C.H.; Boon, N.; Mulder, A.A.; Koster, A.J.; Jost, C.R.; Wijnholds, J. CRB2 loss in rod photoreceptors is associated with progressive loss of retinal contrast sensitivity. Int J Mol Sci. 2019, 20, 4069. [Google Scholar] [CrossRef]
- Mehalow, A.K.; Kameya, S.; Smith, R.S.; Hawes, N.L.; Denegre, J.M.; Young, J.A.; Bechtold, L.; Haider, N.B.; Tepass, U.; Heckenlively, J.R.; et al. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum. Mol. Genet. 2003, 12, 2179–2189. [Google Scholar] [CrossRef]
- Alves, C.H.; Pellissier, L.P.; Vos, R.M.; Garcia Garrido, M.; Sothilingam, V.; Seide, C.; Beck, S.C.; Klooster, J.; Furukawa, T.; Flannery, J.G.; et al. Targeted ablation of Crb2 in photoreceptor cells induces retinitis pigmentosa. Hum. Mol. Genet. 2014, 23, 3384–3401. [Google Scholar] [CrossRef]
- Cho, S.H.; Nahar, A.; Kim, J.; Lee, M.; Kozmik, Z.; Kim, S. Targeted deletion of Crb1/Crb2 in the optic vesicle models key features of leber congenital amaurosis 8. Dev. Biol. 2019, 453, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Van De Pavert, S.A.; Meuleman, J.; Malysheva, A.; Aartsen, W.M.; Versteeg, I.; Tonagel, F.; Kamphuis, W.; McCabe, C.J.; Seeliger, M.W.; Wijnholds, J. A single amino acid substitution (Cys249Trp) in Crb1 causes retinal degeneration and deregulates expression of pituitary tumor transforming gene Pttg1. J. Neurosci. 2007, 27, 564–573. [Google Scholar] [CrossRef]
- Buck, T.M.; Vos, R.M.; Alves, C.H.; Wijnholds, J. AAV-CRB2 protects against vision loss in an inducible CRB1 retinitis pigmentosa mouse model. Mol. Ther. Methods Clin. Dev. 2020, 20, 423–441. [Google Scholar] [CrossRef]
- Boon, N.; Alves, C.H.; Mulder, A.A.; Andriessen, C.A.; Buck, T.M.; Quinn, P.M.J.; Vos, R.M.; Koster, A.J.; Jost, C.R.; Wijnholds, J. Defining Phenotype, Tropism, and Retinal Gene Therapy Using Adeno-Associated Viral Vectors (AAVs) in New-Born Brown Norway Rats with a Spontaneous Mutation in Crb1. Int. J. Mol. Sci. 2021, 22, 3563. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Heyer, W.D.; Ehmsen, K.T.; Liu, J. Regulation of homologous recombination in eukaryotes. Annu. Rev. Genet. 2010, 44, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Park, J.E.; Paa, P.; Rajakumar, P.D.; Prekop, H.T.; Chew, Y.T.; Manivannan, S.N.; Chew, W.L. Programmable C:G to G:C genome editing with CRISPR-Cas9-directed base excision repair proteins. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.L.D.; Levi, S.R.; Eulau, E.; Tsai, Y.T.; Quinn, P.M.J. Prime Editing for Inherited Retinal Diseases. Front. Genome Ed. 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bellingrath, J.S.; McClements, M.E.; Kaukonen, M.; Fischer, M.D.; Maclaren, R.E. In silico analysis of pathogenic CRB1 single nucleotide variants and their amenability to base editing as a potential lead for therapeutic intervention. Genes 2021, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.L.D.; Jenny, L.A.; Maumenee, I.H.; Tsang, S.H.; Quinn, P.M.J. Analysis of CRB1 Pathogenic Variants Correctable with CRISPR Base and Prime Editing. Adv. Exp. Med. Biol. 2023, 139, 319–328. [Google Scholar]
- Liu, B.; Dong, X.; Cheng, H.; Zheng, C.; Chen, Z.; Rodríguez, T.C.; Liang, S.Q.; Xue, W.; Sontheimer, E.J. A split prime editor with untethered reverse transcriptase and circular RNA template. Nat. Biotechnol. 2022, 40, 1388–1393. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6140, Phenylalanine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenylalanine (accessed on 10 May 2022).
- Chan, A.; Duker, J.S.; Ko, T.H.; Fujimoto, J.G.; Schuman, J.S. Normal macular thickness measurements in healthy eyes using stratus optical coherence tomography. Arch. Ophthalmol. 2006, 124, 193–198. [Google Scholar] [CrossRef]
- Khan, K.N.; Robson, A.; Mahroo, O.A.R.; Arno, G.; Inglehearn, C.F.; Armengol, M.; Waseem, N.; Holder, G.E.; Carss, K.J.; Raymond, L.F.; et al. A clinical and molecular characterisation of CRB1-associated maculopathy. Eur. J. Hum. Genet. 2018, 26, 687–694. [Google Scholar] [CrossRef]
- Mucciolo, D.P.; Murro, V.; Giorgio, D.; Passerini, I.; Sodi, A.; Virgili, G.; Rizzo, S. Long-term follow-up of a CRB1-associated maculopathy. Ophthalmic Genet. 2018, 39, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L.; Pierce, E.A.; Laster, A.M.; Daiger, S.P.; Birch, D.G.; Ash, J.D.; Iannaccone, A.; Flannery, J.G.; Sahel, J.A.; Zack, D.J.; et al. Inherited retinal degenerations: Current landscape and knowledge gaps. Transl. Vis. Sci. Technol. 2018, 7, 6. [Google Scholar] [CrossRef]
- Mairot, K.; Smirnov, V.; Bocquet, B.; Labesse, G.; Arndt, C.; Defoort-dhellemmes, S.; Zanlonghi, X.; Hamroun, D.; Picot, M. CRB1 -Related Retinal Dystrophies in a Cohort of 50 Patients: A Reappraisal in the Light of Specific Müller Cell and Photoreceptor CRB1 Isoforms. Int. J. Mol. Sci. 2021, 22, 12642. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; Van Cauwenbergh, C.; De Zaeytijd, J.; Van Wynsberghe, D.; De Baere, E.; Boon, C.J.F.; Leroy, B.P. CRB1-Associated retinal dystrophies in a Belgian cohort: Genetic characteristics and long-Term clinical follow-up. Br. J. Ophthalmol. 2022, 106, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; Boon, C.J.F. Retinal dystrophies and the road to treatment: Clinical requirements and considerations. Asia-Pacific J. Ophthalmol. 2020, 9, 159–179. [Google Scholar] [CrossRef]
- Varela, M.D.; Georgiou, M.; Alswaiti, Y.; Kabbani, J.; Fujinami, K.; Fujinami-Yokokawa, Y.; Khoda, S.; Mahroo, O.A.; Robson, A.G.; Webster, A.R.; et al. CRB1-associated Retinal Dystrophies: Genetics, Clinical Characteristics and Natural History. Am. J. Ophthalmol. 2022, 246, 107–121. [Google Scholar] [CrossRef]
- Nguyen, X.T.A.; Talib, M.; van Schooneveld, M.J.; Wijnholds, J.; van Genderen, M.M.; Schalij-Delfos, N.E.; Klaver, C.C.W.; Talsma, H.E.; Fiocco, M.; Florijn, R.J.; et al. CRB1-Associated Retinal Dystrophies: A Prospective Natural History Study in Anticipation of Future Clinical Trials. Am. J. Ophthalmol. 2022, 234, 37–48. [Google Scholar] [CrossRef]
- Talib, M.; van Schooneveld, M.J.; Wijnholds, J.; van Genderen, M.M.; Schalij-Delfos, N.E.; Talsma, H.E.; Florijn, R.J.; ten Brink, J.B.; Cremers, F.P.M.; Thiadens, A.A.H.J.; et al. Defining inclusion criteria and endpoints for clinical trials: A prospective cross-sectional study in CRB1-associated retinal dystrophies. Acta Ophthalmol. 2021, 99, e402–e414. [Google Scholar] [CrossRef]
- Mathijssen, I.B.; Florijn, R.J.; Born, L.I.V.A.N.D.E.N.; Zekveld-vroon, R.C.; Brink, J.B.T.E.N.; Plomp, A.S.; Baas, F.; Meijers-heijboer, H.; Bergen, A.A.B. LONG-TERM FOLLOW-UP OF PATIENTS WITH RETINITIS PIGMENTOSA TYPE 12 CAUSED BY CRB1 MUTATIONS A Severe Phenotype With Considerable. Retina 2016, 37, 161–172. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Tsang, S.H.; Yi-Ting, T. Dual Expression Vector for Gene Augmentation for Crumbs Complex Homologue 1 (Crb1) Mutations. U.S. Patent 20220186253, 16 June 2022. [Google Scholar]
- Kay, J.; Ray, T. Compositions and Methods for the Diagnosis and Treatment of Retinopathies. U.S. Patent US 20090155176A1, 18 June 2009. [Google Scholar]
- Kantor, A.; Mcclements, M.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Levy, J.M.; Yeh, W.H.; Pendse, N.; Davis, J.R.; Hennessey, E.; Butcher, R.; Koblan, L.W.; Comander, J.; Liu, Q.; Liu, D.R. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 2020, 4, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Suh, S.; Foik, A.T.; Leinonen, H.; Newby, G.A.; Gao, X.D.; Banskota, S.; Hoang, T.; Du, S.W.; Dong, Z.; et al. In vivo base editing rescues cone photoreceptors in a mouse model of early-onset inherited retinal degeneration. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Chen, Y.; Wu, G.; Wen, J.; Wu, J.; Liu, Q.; Li, Y.; Kang, R.; Hu, S.; Wang, J.; et al. Dual-AAV delivering split prime editor system for in vivo genome editing. Mol. Ther. 2021, 30, 283–294. [Google Scholar] [CrossRef]
- Jang, H.; Jo, D.H.; Cho, C.S.; Shin, J.H.; Seo, J.H.; Yu, G.; Gopalappa, R.; Kim, D.; Cho, S.-R.; Kim, J.H.; et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. Nat. Biomed. Eng. 2022, 6, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liang, S.Q.; Liu, B.; Liu, P.; Kwan, S.Y.; Wolfe, S.A.; Xue, W. A flexible split prime editor using truncated reverse transcriptase improves dual-AAV delivery in mouse liver. Mol. Ther. 2022, 30, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Choi, E.H.; Leinonen, H.; Foik, A.T.; Newby, G.A.; Yeh, W.H.; Dong, Z.; Kiser, P.D.; Lyon, D.C.; Liu, D.R.; et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat. Biomed. Eng. 2021, 5, 169–178. [Google Scholar] [CrossRef]
- Tornabene, P.; Trapani, I.; Centrulo, M.; Marrocco, E.; Minopoli, R.; Lupo, M.; Iodice, C.; Gesualdo, C.; Simonelli, F.; Surace, E.M.; et al. Inclusion of a degron reduces levelsof undesired inteins after AAV-mediated proteintrans-splicing in the retina. Mol. Ther. Methods Clin. Dev. 2021, 23, 448–459. [Google Scholar] [CrossRef]
- McClements, M.E.; Maclaren, R.E. Adeno-associated virus (AAV) dual vector strategies for gene therapy encoding large transgenes. Yale J. Biol. Med. 2017, 90, 611–623. [Google Scholar]
- Davis, J.R.; Wang, X.; Witte, I.P.; Huang, T.P.; Levy, J.M.; Raguram, A.; Banskota, S.; Seidah, N.G.; Musunuru, K.; Liu, D.R. Efficient in vivo base editing via single adeno-associated viruses with size-optimized genomes encoding compact adenine base editors. Nat. Biomed. Eng. 2022, 6, 1272–1283. [Google Scholar] [CrossRef]
- Salman, A.; Kantor, A.; McClements, M.E.; Marfany, G.; Trigueros, S.; MacLaren, R.E. Non-Viral Delivery of CRISPR/Cas Cargo to the Retina Using Nanoparticles: Current Possibilities, Challenges, and Limitations. Pharmaceutics 2022, 14, 1842. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Barrera, M.; Ryals, R.C.; Gautam, M.; Jozic, A.; Landry, M.; Korzun, T.; Gupta, M.; Acosta, C.; Stoddard, J.; Reynaga, R.; et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci. Adv. 2023, 9, eadd4623. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Marx, A.; Bronstein, A.M. Codon-specific Ramachandran plots show amino acid backbone conformation depends on identity of the translated codon. Nat. Commun. 2022, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 145742, Proline. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Proline (accessed on 10 May 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5951, Serine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Serine (accessed on 10 May 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6287, Valine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Valine (accessed on 10 May 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6306, l-Isoleucine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/l-Isoleucine (accessed on 10 May 2022).
- Anna, A.; Monika, G. Splicing mutations in human genetic disorders: Examples, detection, and confirmation. J. Appl. Genet. 2018, 59, 253–268. [Google Scholar] [CrossRef]
- Rogan, P.K.; Caminsky, N.; Mucaki, E.J. Interpretation of mRNA splicing mutations in genetic disease: Review of the literature and guidelines for information-theoretical analysis. F1000Research 2014, 3, 282. [Google Scholar]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, J.; Zhang-Ding, Z.; Xin, C.; Liu, M.; Wang, Y.; Ai, C.; Hu, J. In-depth assessment of the PAM compatibility and editing activities of Cas9 variants. Nucleic Acids Res. 2021, 49, 8785–8795. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Herrera-Carrillo, E.; Berkhout, B. Delineation of the Exact Transcription Termination Signal for Type 3 Polymerase III. Mol. Ther. Nucleic Acids 2018, 10, 36–44. [Google Scholar] [CrossRef]
- Mairhofer, J.; Wittwer, A.; Cserjan-Puschmann, M.; Striedner, G. Preventing T7 RNA polymerase read-through transcription-A synthetic termination signal capable of improving bioprocess stability. ACS Synth. Biol. 2015, 4, 265–273. [Google Scholar] [CrossRef]
- Song, H.; Kang, C. Sequence-specific termination by T7 RNA polymerase requires formation of paused conformation prior to the point of RNA release. Genes to Cells 2001, 6, 291–301. [Google Scholar] [CrossRef]
- Jeng, S.T.; Gardner, J.F.; Gumport, R.I. Transcription termination by bacteriophage T7 RNA polymerase at Rho-independent terminators. J. Biol. Chem. 1990, 265, 3823–3830. [Google Scholar] [CrossRef] [PubMed]

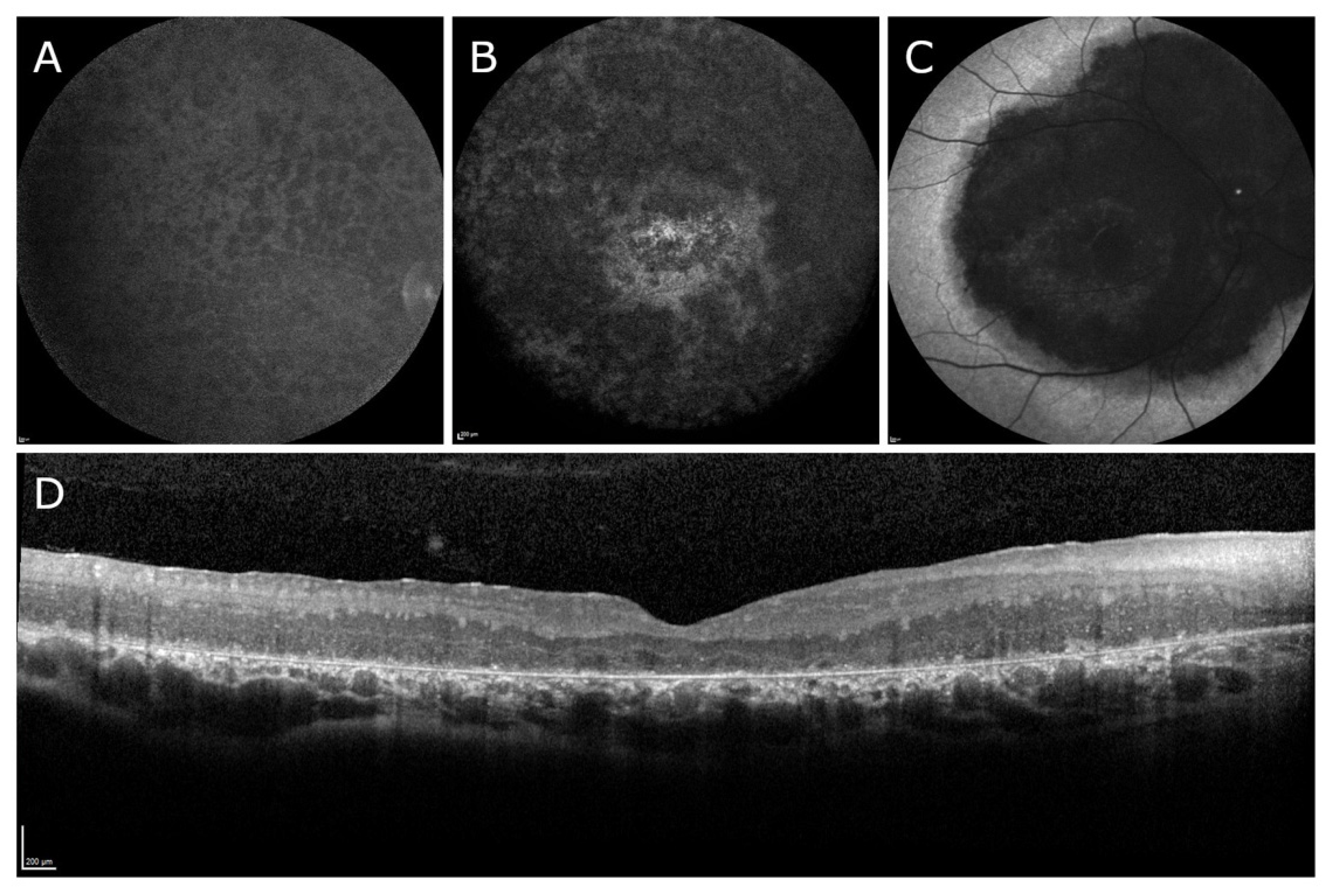
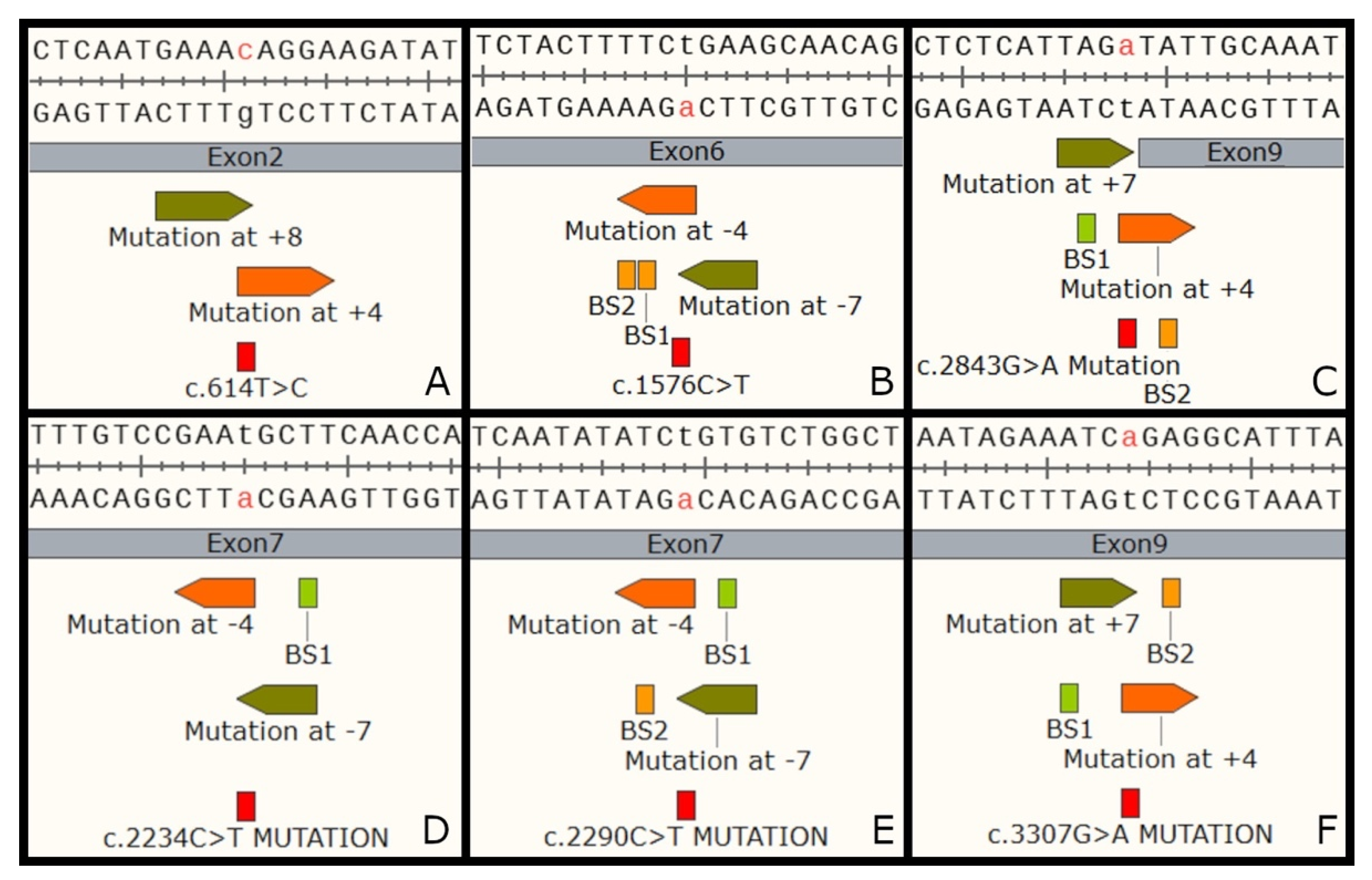
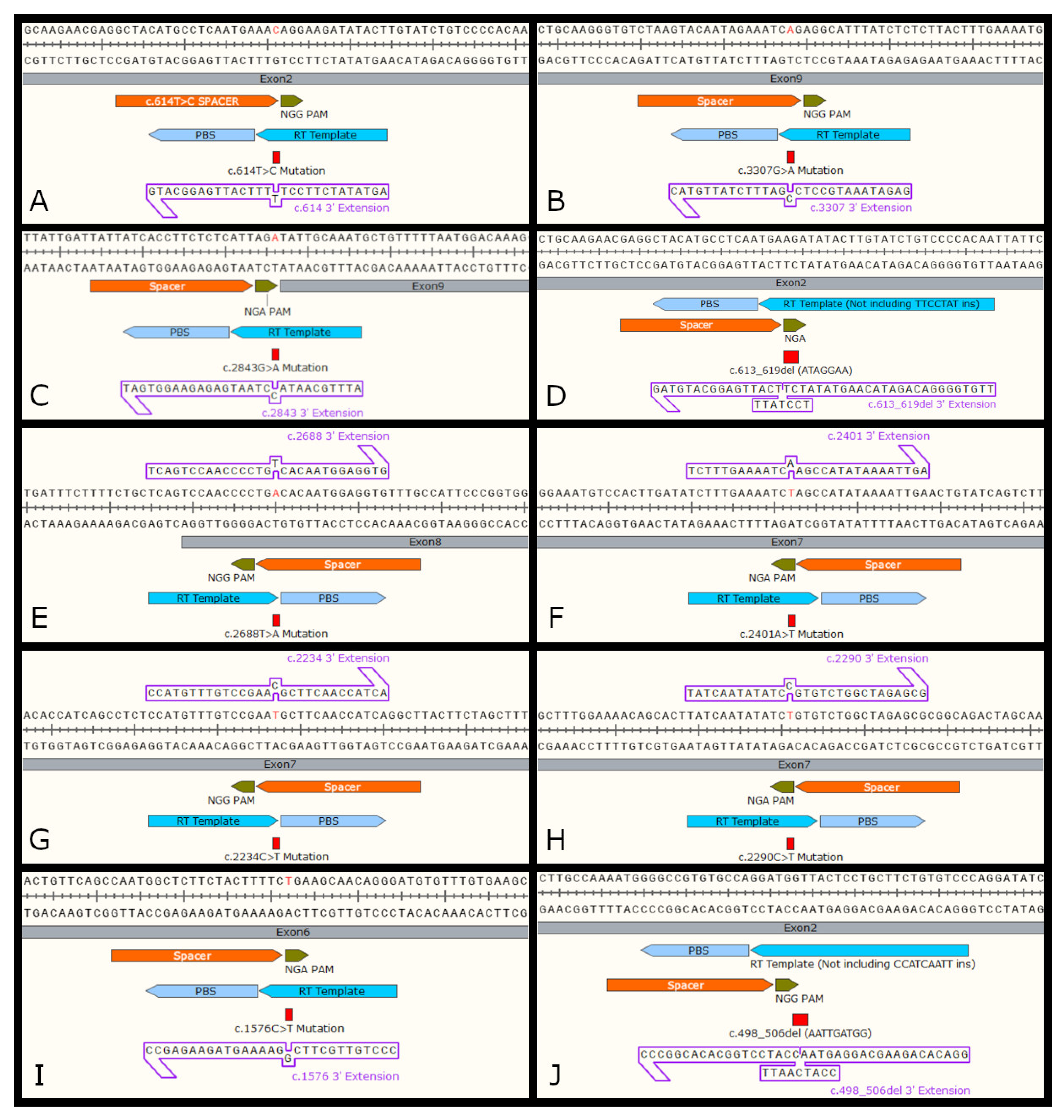
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age and sex | 44F | 8M | 26M | 24M | 49F | 40M | 20F | 9M | 5M |
| Age at onset * | childhood | 3 | birth | 4 | childhood | 12 | 4 | 8 | 2 |
| BCVA (LogMar) OD, OS | 2.8, 2.4 | 0.9, 0.9 | 1.9, 1.9 | 2.4, 2.4 | 2.4, 2.4 | 0.9, 1.3 | 1.6, 1.5 | 0.5, 0.5 | 1.1, 0.9 |
| Follow-up (years) | 1 | 5 | 9 | 2 | n/a | 8 | 1 | 1 | 3 |
| Number of visits | 2 | 4 | 6 | 2 | 1 | 17 | 3 | 7 | 8 |
| Retinal thickness (microns) | 423 | 489 | 437 | 327 | n/a | 382 | 292 | 296 | 515 |
| Genetic variant 1 ‡ | c.2401A>T p.Lys801Ter | c.2843G>A p.Cys948Tyr | c.2843G>A p.Cys948Tyr | c.3307G>A p.Gly1103Arg | c.2234C>T p.Thr745Met | c.2843G>A p.Cys948Tyr | c.3307G>A p.Gly1103Arg | c.3307G>A p.Gly1103Arg | c.3307G>A p.Gly1103Arg |
| Genetic variant 2 | c.2706C>G p.Cys902Trp | c.2480G>T p.Gly827Val | c.2245_2247del p.749delSer | homozygous | c.257_258dup p.Asn87* | c.498_506delAATTGATGG; p.Ile167_Gly169del | homozygous | homozygous | homozygous |
| Mutation | PAM | Edit Position | Spacer | 3′ Extension |
|---|---|---|---|---|
| c.2843G>A | AGA | +6 | TATTATCACCTTCTCTCATT | ATTTGCAATACCTAATGAGAGAAGGTGAT |
| c.2234C>T | CGG | −1 | AGCCTGATGGTTGAAGCATT | CCATGTTTGTCCGAACGCTTCAACCATCA |
| c.2401A>T | AGA | −4 | CAGTTCAATTTTATATGGCT ** | TCTTTGAAAATCAAGCCATATAAAATTGA |
| c.2290C>T | AGA | −4 | GCCGCGCTCTAGCCAGACAC | TATCAATATATCCGTGTCTGGCTAGAGCG |
| c.2688T>A | GGG | −1 | CAAACACCTCCATTGTGACA | TCAGTCCAACCCCTGTCACAATGGAGGTG |
| c.613_619del | AGA | +4 | GAGGCTACATGCCTCAATGA | TTGTGGGGACAGATACAAGTATATCTTCCTATTTCATTGAGGCATGTAG |
| c.498_506del | TGG | +6 | AATGGGGCCGTGTGCCAGGA | GGACACAGAAGCAGGAGTAACCATCAATTCCATCCTGGCACACGGCCC |
| c.1576C>T | TGA | +4 | CAATGGCTCTTCTACTTTTC ** | CCCTGTTGCTTCGGAAAAGTAGAAGAGCC |
| c.3307G>A | AGG | +2 | CTAAGTACAATAGAAATCAG | GAGATAAATGCCTCCGATTTCTATTGTAC |
| c.614T>C | AGG | +3 | GCTACATGCCTCAATGAAAC | AGTATATCTTCCTTTTTCATTGAGGCATG** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes da Costa, B.; Kolesnikova, M.; Levi, S.R.; Cabral, T.; Tsang, S.H.; Maumenee, I.H.; Quinn, P.M.J. Clinical and Therapeutic Evaluation of the Ten Most Prevalent CRB1 Mutations. Biomedicines 2023, 11, 385. https://doi.org/10.3390/biomedicines11020385
Lopes da Costa B, Kolesnikova M, Levi SR, Cabral T, Tsang SH, Maumenee IH, Quinn PMJ. Clinical and Therapeutic Evaluation of the Ten Most Prevalent CRB1 Mutations. Biomedicines. 2023; 11(2):385. https://doi.org/10.3390/biomedicines11020385
Chicago/Turabian StyleLopes da Costa, Bruna, Masha Kolesnikova, Sarah R. Levi, Thiago Cabral, Stephen H. Tsang, Irene H. Maumenee, and Peter M. J. Quinn. 2023. "Clinical and Therapeutic Evaluation of the Ten Most Prevalent CRB1 Mutations" Biomedicines 11, no. 2: 385. https://doi.org/10.3390/biomedicines11020385
APA StyleLopes da Costa, B., Kolesnikova, M., Levi, S. R., Cabral, T., Tsang, S. H., Maumenee, I. H., & Quinn, P. M. J. (2023). Clinical and Therapeutic Evaluation of the Ten Most Prevalent CRB1 Mutations. Biomedicines, 11(2), 385. https://doi.org/10.3390/biomedicines11020385







