Transcription of Autophagy Associated Gene Expression as Possible Predictors of a Colorectal Cancer Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Materials
2.3. Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nature 2007, 9, 1102–1109. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Klionsky, D.J. Development by Self-Digestion: Molecular Mechanisms and Biological Functions of Autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2017, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Klionsky, D.J. Autophagy: From phenomenology toą molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 2007, 8, 931–937. [Google Scholar] [CrossRef]
- Lu, G.; Wang, Y.; Shi, Y.; Zhang, Z.; Huang, C.; He, W.; Wang, C.; Shen, H. Autophagy in health and disease: From molecular mechanisms to therapeutic target. Medcomm 2022, 3, e150. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Sargazi, S.; Sheervalilou, R.; Rokni, M.; Shirvaliloo, M.; Shahraki, O.; Rezaei, N. The role of autophagy in controlling SARS-CoV-2 infection: An overview on virophagy-mediated molecular drug targets. Cell Biol. Int. 2021, 45, 1599–1612. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Mishra, P.K. Editorial: Autophagy-Mediated Cell Survival and Death in Disease Progression and Treatment. Front. Cell Dev. Biol. 2022, 10, 916347. [Google Scholar] [CrossRef]
- Yu, L.; Wan, F.; Dutta, S.; Welsh, S.; Liu, Z.; Freundt, E.; Baehrecke, E.H.; Lenardo, M. Autophagic programmed cell death by selective catalase degradation. Proc. Natl. Acad. Sci. 2006, 103, 4952–4957. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Kundu, M.K.; Dey, A.; Rahman, H.; et al. Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells. Life 2022, 12, 811. [Google Scholar] [CrossRef]
- Badadani, M. Autophagy Mechanism, Regulation, Functions, and Disorders. Cell Biol. 2012, 2012, 927064. [Google Scholar] [CrossRef] [Green Version]
- Barnard, R.A.; Regan, D.P.; Hansen, R.J.; Maycotte, P.; Thorburn, A.; Gustafson, D.L. Autophagy Inhibition Delays Early but Not Late-Stage Metastatic Disease. Experiment 2016, 358, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.Y.; Xia, B.; White, E. Autophagy-Mediated Tumor Promotion. Cell 2013, 155, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Fung, C.; Lock, R.; Gao, S.; Salas, E.; Debnath, J. Induction of Autophagy during Extracellular Matrix Detachment Promotes Cell Survival. Mol. Biol. Cell 2008, 19, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Luo, R.Z.; Lu, Y.; Zhang, X.; Yu, Q.; Khare, S.; Kondo, S.; Kondo, Y.; Yu, Y.; Mills, G.B.; et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Investig. 2008, 118, 3917–3929. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.H.; Jeong, E.G.; Lee, J.W.; Kim, M.S.; Kim, S.H.; Kim, S.S.; Yoo, N.J.; Lee, S.H. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. Apmis 2007, 115, 1344–1349. [Google Scholar] [CrossRef]
- Tang, H.; Da, L.; Mao, Y.; Li, Y.; Li, D.; Xu, Z.; Li, F.; Wang, Y.; Tiollais, P.; Li, T.; et al. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 ex-pression. Hepatology 2009, 49, 60–71. [Google Scholar] [CrossRef]
- Karantza-Wadsworth, V.; White, E. Role of autophagy in breast cancer. Autophagy 2007, 3, 610–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, J.H.; Jin, L.; Lin, S.M.; Yang, Y.; Sui, Y.X.; Shi, H. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010, 294, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Influence of autophagy on the efficacy of radiotherapy. Radiat. Oncol. 2017, 12, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classen, F.; Kranz, P.; Riffkin, H.; Pompsch, M.; Wolf, A.; Göpelt, K.; Baumann, M.; Baumann, J.; Brockmeier, U.; Metzen, E. Autophagy induced by ionizing radiation promotes cell death over survival in human colorectal cancer cells. Exp. Cell Res. 2019, 374, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Benoit, A.; Hasmim, M.; Duhem, C.; Vogin, G.; Berchem, G.; Noman, M.Z.; Janji, B. Targeting Cytoprotective Autophagy to Enhance Anticancer Therapies. Front. Oncol. 2021, 11, 626309. [Google Scholar] [CrossRef]
- Garbar, C.; Mascaux, C.; Giustiniani, J.; Merrouche, Y.; Bensussan, A. Chemotherapy treatment induces an increase of autophagy in the luminal breast cancer cell MCF7, but not in the triple-negative MDA-MB231. Sci. Rep. 2017, 7, 7201. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.A.Z.; Romagnoli, G.G.; Kaneno, R. Inhibiting autophagy to prevent drug resistance and improve anti-tumor therapy. Life Sci. 2020, 265, 118745. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Dereń-Wagemann, I.; Kiełbiński, M.; Kuliczkowski, K. Autofagia-proces o dwóch obliczach. Acta Haematol. Pol. 2013, 44, 383–391. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Muc-Wierzgoń, M.; Waniczek, D.; Fatyga, E.; Klakla, K.; Mazurek, U.; Wierzgoń, J. Autophagy-related gene expression in colorectal cancer patients. J. Biol. Regul. Homeost. Agents 2017, 31, 923–927. [Google Scholar]
- Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/pcr-qpcr-dpcr/real-time-pcr-enzymes-and-kits/probe-based-one-step-qrt-pcr/quantitect-rt-pcr-kits (accessed on 28 December 2022).
- Ponchel, F.; Toomes, C.; Bransfield, K.; Leong, F.T.; Douglas, S.H.; Field, S.L.; Bell, S.M.; Combaret, V.; Puisieux, A.; Mighell, A.J.; et al. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Al-Joufi, F.A.; Setia, A.; Salem-Bekhit, M.M.; Sahu, R.K.; Alqahtani, F.Y.; Widyowati, R.; Aleanizy, F.S. Molecular Pathogenesis of Colorectal Cancer with an Emphasis on Recent Advances in Biomarkers, as Well as Nanotechnology-Based Diagnostic and Therapeutic Approaches. Nanomaterials 2022, 12, 169. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Zmarzły, N.; Grabarek, B.; Mazurek, U.; Muc-Wierzgoń, M. Gene involved in the regulation of different types of autophagy and their participation in cancer pathogenesis. Oncotarget 2018, 9, 34413–34428. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef]
- Fulda, S. Autophagy in Cancer Therapy. Front. Oncol. 2017, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-L.; Chen, F.-F.; Chang, S.-F.; Chen, C.-N.; Lung, J.; Lo, C.-H.; Lee, F.-H.; Lu, Y.-C.; Hung, C.-H. Expression of Beclin Family Proteins Is Associated with Tumor Progression in Oral Cancer. PLoS ONE 2015, 10, e0141308. [Google Scholar] [CrossRef]
- Hill, S.M.; Wrobel, L.; Rubinsztein, D.C. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2018, 26, 617–629. [Google Scholar] [CrossRef]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation–at the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Sun, C.; Tian, D.; Li, Y.; Gao, X.; He, S.; Li, T. Expression and clinical significances of Beclin1, LC3 and mTOR in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 3882–3891. [Google Scholar]
- Zhang, M.Y.; Gou, W.F.; Zhao, S.; Mao, X.Y.; Zheng, Z.H.; Takano, Y.; Zheng, H.C. Beclin 1 expression is closely linked to colorectal carcinogenesis and distant metastasis of colorectal carci-noma. Int. J. Mol. Sci. 2014, 15, 14372–14385. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Li, G.; Huang, C.; Hou, Z.; Yang, X.; Luo, X.; Feng, Y.; Wang, G.; Hu, J.; Cao, Z. The autophagy-independent role of BECN1 in colorectal cancer metastasis through regulating STAT3 signaling pathway activation. Cell Death Dis. 2020, 11, 304. [Google Scholar] [CrossRef]
- Prerna, K.; Dubey, V.K. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int. J. Biol. Macromol. 2022, 204, 258–273. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zhao, S.; Guo, X.; Xu, Y.; Zheng, Z.; Lu, H.; Zheng, H. Effects of Beclin 1 overexpression on aggressive phenotypes of colon cancer cells. Oncol. Lett. 2018, 17, 2441–2450. [Google Scholar] [CrossRef]
- Shen, H.; Yin, L.; Deng, G.; Guo, C.; Han, Y.; Li, Y.; Cai, C.; Fu, Y.; Liu, S.; Zeng, S. Knockdown of Beclin-1 impairs epithelial-mesenchymal transition of colon cancer cells. J. Cell. Biochem. 2018, 119, 7022–7031. [Google Scholar] [CrossRef]
- Park, J.K.; Huang, S.; Wu, T.T. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carci-nomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther. 2013, 14, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zhao, Z.; Li, J.; Li, J.; Luo, Y.; Li, W.; You, W.; Zhang, Y.; Li, Z.; Yang, J.; et al. Nuclear Beclin 1 Destabilizes Retinoblastoma Protein to Promote Cell Cycle Progression and Colorectal Cancer Growth. Cancers 2022, 14, 4735. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lu, C.; Zhang, L. Beclin-1 Expression is a Predictor of Clinical Outcome in Patients with Esophageal Squamous Cell Carcinoma and Correlated to Hypoxia-Inducible Factor (HIF)-1α Expression. Pathol. Oncol. Res. 2009, 15, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.-B.; Shi, Y.-H.; Zhou, J.; Qiu, S.-J.; Xu, Y.; Dai, Z.; Shi, G.-M.; Wang, X.-Y.; Ke, A.-W.; Wu, B.; et al. Association of Autophagy Defect with a Malignant Phenotype and Poor Prognosis of Hepatocellular Carcinoma. Cancer Res 2008, 68, 9167–9175. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-J.; Li, H.-R.; Huang, Y.; Jiang, W.-Q.; Xu, R.-H.; Huang, H.-Q.; Lv, Y.; Xia, Z.-J.; Zhu, X.-F.; Lin, T.-Y.; et al. Beclin 1 expression: A predictor of prognosis in patients with extranodal natural killer T-cell lymphoma, nasal type. Autophagy 2010, 6, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Li, B.X.; Li, C.Y.; Peng, R.Q.; Wu, X.J.; Wang, H.Y.; Wan, D.S.; Zhu, X.F.; Zhang, X.S. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy 2009, 5, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Pitiakoudis, M.; Gatter, K.C.; Harris, A.L. Beclin 1 over- and underex-pression in colorectal cancer: Distinct patterns relate to prognosis and tumour hypoxia. Br J Cancer. 2010, 103, 1209–1214. [Google Scholar] [CrossRef]
- Hu, D.; Huo, Y.; Xue, Y.; Feng, H.; Sun, W.; Wang, H.; Wu, J.; Wang, X. Clinical application of autophagy proteins as prognostic biomarkers in colorectal cancer: A meta-analysis. Futur. Oncol. 2022, 18, 3537–3549. [Google Scholar] [CrossRef]
- Li, J.; Hou, N.; Faried, A.; Tsutsumi, S.; Kuwano, H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur. J. Cancer 2010, 46, 1900–1909. [Google Scholar] [CrossRef]
- Apel, A.; Herr, I.; Schwarz, H.; Rodemann, H.P.; Mayer, A. Blocked Autophagy Sensitizes Resistant Carcinoma Cells to Radiation Therapy. Cancer Res 2008, 68, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.-L.; Li, D.; Hu, F.; Song, J.-R.; Zhang, S.-S.; Deng, W.-J.; Sun, K.; Zhao, Q.-D.; Xie, X.-Q.; Song, Y.-J.; et al. Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett. 2012, 320, 171–179. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 Antiapoptotic Proteins Inhibit Beclin 1-Dependent Autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Maiuri, M.C.; Le Toumelin, G.; Criollo, A.; Rain, J.C.; Gautier, F.; Juin, P.; Tasdemir, E.; Pierron, G.; Troulinaki, K.; Tavernarakis, N.; et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007, 26, 2527–2539. [Google Scholar] [CrossRef]
- Grasso, S.; Pereira, G.J.; Palmeira-Dos-Santos, C.; Calgarotto, A.K.; Martínez-Lacaci, I.; Ferragut, J.A.; Smaili, S.S.; Bincoletto, C. Autophagy regulates Selumetinib (AZD6244) induced-apoptosis in colorectal cancer cells. Eur. J. Med. Chem. 2016, 122, 611–618. [Google Scholar] [CrossRef]
- Palmeira-Dos-Santos, C.; Pereira, G.J.S.; Barbosa, C.M.V.; Jurkiewicz, A.; Smaili, S.S.; Bincoletto, C. Comparative study of autophagy inhibition by 3MA and CQ on Cytarabine-induced death of leukaemia cells. J. Cancer Res. Clin. Oncol. 2014, 140, 909–920. [Google Scholar] [CrossRef]
- Djavaheri-Mergny, M.; Maiuri, M.C.; Kroemer, G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene 2010, 29, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Rubinsztein, D.C. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: An effect rescued by Bcl-xL. Cell Death Differ. 2010, 17, 268–277. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The Role of Autophagy in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirtoli, L.; Cevenini, G.; Tini, P.; Vannini, M.; Oliveri, G.; Marsili, S.; Mourmouras, V.; Rubino, G.; Miracco, C. The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy 2009, 5, 930–936. [Google Scholar] [CrossRef] [Green Version]
- Cuervo, A.M.; Dice, J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef]
- Kiffin, R.; Christian, C.; Knecht, E.; Cuervo, A.M. Activation of Chaperone-mediated Autophagy during Oxidative Stress. Mol. Biol. Cell 2004, 15, 4829–4840. [Google Scholar] [CrossRef] [Green Version]
- Wanh, Y.; Lu, J. Chaperone-Mediated Autophagy in Neurodegenerative Diseases: Molecular Mechanisms and Pharmacological Opportunities. Cells 2022, 1, 2250–2255. [Google Scholar]
- Sooparb, S.; Price, S.R.; Shaoguang, J.; Franch, H.A. Suppression of chaperone-mediated autophagy in the renal cortex during acute diabetes mellitus. Kidney Int. 2004, 65, 2135–2144. [Google Scholar] [CrossRef] [Green Version]
- Vogiatzi, T.; Xilouri, M.; Vekrellis, K.; Stefanis, L. Wild Type α-Synuclein Is Degraded by Chaperone-mediated Autophagy and Macroautophagy in Neuronal Cells. J. Biol. Chem. 2008, 283, 23542–23556. [Google Scholar] [CrossRef] [Green Version]
- Auzmendi-Iriarte, J.; Matheu, A. Impact of Chaperone-Mediated Autophagy in Brain Aging: Neurodegenerative Diseases and Glioblastoma. Front. Aging Neurosci. 2021, 12, 630743. [Google Scholar] [CrossRef]
- Wang, Y.; Martinez-Vicente, M.; Krüger, U.; Kaushik, S.; Wong, E.; Mandelkow, E.-M.; Cuervo, A.M.; Mandelkow, E. Tau fragmentation, aggregation and clearance: The dual role of lysosomal processing. Hum. Mol. Genet. 2009, 18, 4153–4170. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, P.; Song, W.; Sun, X. Degradation of regulator of calcineurin 1 (RCAN1) is mediated by both chaper-one-mediated autophagy and ubiquitin proteasome pathways. FASEB J. 2009, 23, 3383–3392. [Google Scholar] [CrossRef]
- Kon, M.; Kiffin, R.; Koga, H.; Chapochnick, J.; Macian, F.; Varticovski, L.; Cuervo, A.M. Chaperone-Mediated Autophagy Is Required for Tumor Growth. Sci. Transl. Med. 2011, 3, 109ra117. [Google Scholar] [CrossRef] [Green Version]
- Saha, T. LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy 2012, 8, 1643–1656. [Google Scholar] [CrossRef] [Green Version]
- Eskelinen, E.-L.; Saftig, P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Liu, Y.; Liu, L.; Chen, M.; Wang, X.; Yang, J.; Gong, Y.; Ding, B.-S.; Wei, Y.; Wei, X. Tumor cells induce LAMP2 a expression in tumor-associated macrophage for cancer progression. Ebiomedicine 2019, 40, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Furuta, K.; Ikeda, M.; Nakayama, Y.; Nakamura, K.; Tanaka, M.; Hamasaki, N.; Himeno, M.; Hamilton, S.R.; August, J.T. Expression of Lysosome-Associated Membrane Proteins in Human Colorectal Neoplasms and Inflammatory Diseases. Am. J. Pathol. 2001, 159, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Dennis, J.W.; Laferté, S.; Waghorne, C.; Breitman, M.L.; Kerbel, R.S. β1-6 Branching of Asn-Linked Oligosaccharides Is Directly Associated with Metastasis. Science 1987, 236, 582–585. [Google Scholar] [CrossRef]
- Laferté, S.; Dennis, J.W. Glycosylation-dependent collagen-binding activities of two membrane glycoproteins in MDAY-D2 tumor cells. Cancer Res 1988, 48, 4743–4748. [Google Scholar]
- Bierhuizen, M.; Maemura, K.; Fukuda, M. Expression of a differentiation antigen and poly-N-acetyllactosaminyl O-glycans directed by a cloned core 2 beta-1,6-N-acetylglucosaminyltransferase. J. Biol. Chem. 1994, 269, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Yang, Z.-Y.; Wang, D.; Yang, X.-Y.; Wang, J.; Li, L.; Wen, Q.; Gao, L.; Bian, X.-W.; Yu, S.-C. The role of lysosomes in cancer development and progression. Cell Biosci. 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Lichter-Konecki, U.; Moter, S.E.; Krawisz, B.R.; Schlotter, M.; Hipke, C.; Konecki, D.S. Expression patterns of murine lyso-some-associated membrane protein 2 (LAMP-2) transcripts during morphogenesis. Differentiation 1999, 65, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Silvian, L.F. PINK1/Parkin Pathway Activation for Mitochondrial Quality Control—Which Is the Best Molecular Target for Therapy? Front. Aging Neurosci. 2022, 14, 890823. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, A.; Boland, M.; Macleod, K. Mitophagy and cancer. Cancer Metab. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Parganlija, D.; Klinkenberg, M.; Dominguez-Bautista, J.A.; Hetzel, M.; Gispert, S.; Chimi, M.A.; Dröse, S.; Mai, S.; Brandt, U.; Auburger, G.; et al. Loss of PINK1 Impairs Stress-Induced Autophagy and Cell Survival. PLoS ONE 2014, 9, e95288. [Google Scholar] [CrossRef] [Green Version]
- Shirihai, O.S.; Song, M.; Dorn, G.W. II How mitochondrial dynamism orchestrates mitophagy. Circ. Res. 2015, 116, 1835–1849. [Google Scholar] [CrossRef] [Green Version]
- Durcan, T.M.; Fon, E.A. The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015, 29, 989–999. [Google Scholar]
- Li, S.; Zhang, J.; Liu, C.; Wang, Q.; Yan, J.; Hui, L.; Jia, Q.; Shan, H.; Tao, L.; Zhang, M. The Role of Mitophagy in Regulating Cell Death. Oxidative Med. Cell. Longev. 2021, 2021, 6617256. [Google Scholar] [CrossRef]
- Vara-Perez, M.; Felipe-Abrio, B.; Agostinis, P. Mitophagy in Cancer: A Tale of Adaptation. Cells 2019, 8, 493. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; He, R.; Shen, M. PINK1/Parkin-Mediated Mitophagy Regulation by Reactive Oxygen Species Alleviates Rocaglamide A-Induced Apoptosis in Pancreatic Cancer Cells. Front Pharmacol. 2019, 10, 968. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Zou, T.; Qu, J.; Chen, X.; Zhang, S.; Lin, Z. Cyclovirobuxine D induced-mitophagy through the p65/BNIP3/LC3 axis potentiates its apoptosis-inducing effects in lung cancer cells. Int. J. Mol. Sci. 2021, 22, 5820. [Google Scholar] [CrossRef]
- Delgado, M.E.; Dyck, L.; Laussmann, M.A.; Rehm, M. Modulation of apoptosis sensitivity through the interplay with au-tophagic and proteasomal degradation pathways. Cell Death Dis. 2014, 5, e1011. [Google Scholar] [CrossRef] [Green Version]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Mao, L.; Liu, H.; Zhang, R. PINK1/Parkin-mediated mitophagy inhibits warangalone-induced mitochondrial apoptosis in breast cancer cells. Aging 2021, 13, 12955–12972. [Google Scholar] [CrossRef]
- Sekine, S. PINK1 import regulation at a crossroad of mitochondrial fate: The molecular mechanisms of PINK1 import. J. Biochem. 2020, 167, 217–224. [Google Scholar]
- Yin, K.; Lee, J.; Liu, Z. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 acti-vation and reducing acetyl-CoA production. Cell Death Differ. 2021, 28, 2421–2435. [Google Scholar] [CrossRef]
- Wang, M.; Luan, S.; Fan, X.; Wang, J.; Huang, J.; Gao, X.; Han, D. The emerging multifaceted role of PINK1 in cancer biology. Cancer Sci. 2022, 113, 4037–4047. [Google Scholar] [CrossRef]
- Salih, D.A.; Brunet, A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008, 20, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z. FoxO transcription factors in mitochondrial homeostasis. Biochem. J. 2022, 479, 525–536. [Google Scholar] [CrossRef]
- Essers, M.; Weijzen, S.; De Vries-Smits, A.M.M.; Saarloos, I.; De Ruiter, N.D.; Bos, J.L.; Burgering, B.M.T. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, E.L.; Brunet, A. FOXO transcription factors in ageing and cancer. Acta Physiol. 2007, 192, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.T.; Lee, D.F.; Xia, W.; Golfman, L.S.; Ou-Yang, F.; Yang, J.Y.; Zou, Y.; Bao, S.; Hanada, N.; Saso, H.; et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004, 117, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seoane, J.; Le, H.-V.; Shen, L.; Anderson, S.A.; Massagué, J. Integration of Smad and Forkhead Pathways in the Control of Neuroepithelial and Glioblastoma Cell Proliferation. Cell 2004, 117, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Modur, V.; Nagarajan, R.; Evers, B.M.; Milbrandt, J. FOXO Proteins Regulate Tumor Necrosis Factor-related Apoptosis Inducing Ligand Expression. J. Biol. Chem. 2002, 277, 47928–47937. [Google Scholar] [CrossRef]
- Chae, Y.-C.; Kim, J.-Y.; Park, J.W.; Kim, K.-B.; Oh, H.; Lee, K.-H.; Seo, S.-B. FOXO1 degradation via G9a-mediated methylation promotes cell proliferation in colon cancer. Nucleic Acids Res. 2018, 47, 1692–1705. [Google Scholar] [CrossRef] [Green Version]
- Giovannucci, E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am. J. Clin. Nutr. 2007, 86, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Venkateswaran, V.; Haddad, A.Q.; Fleshner, N.E.; Fan, R.; Sugar, L.M.; Nam, R.; Klotz, L.H.; Pollak, M. Association of Diet-Induced Hyperinsulinemia With Accelerated Growth of Prostate Cancer (LNCaP) Xenografts. Gynecol. Oncol. 2007, 99, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Wysocki, P.J.; Wierusz-Wysocka, B. Obesity, hyperinsulinemia and breast cancer: Novel targets and a novel role for metformin. Expert Rev. Mol. Diagn. 2010, 10, 509–519. [Google Scholar] [CrossRef]
- Ding, J.; Li, T.; Wang, X.; Zhao, E.; Choi, J.-H.; Yang, L.; Zha, Y.; Dong, Z.; Huang, S.; Asara, J.M.; et al. The Histone H3 Methyltransferase G9A Epigenetically Activates the Serine-Glycine Synthesis Pathway to Sustain Cancer Cell Survival and Proliferation. Cell Metab. 2013, 18, 896–907. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Massey, S.; Ahmad, I.; Sadaf; Akhter, N.; Habib, M.; Mustafa, S.; Deo, S.V.S.; Husain, S.A. FOXO1 Gene Downregulation and Promoter Methylation Exhibits Significant Correlation With Clinical Parameters in Indian Breast Cancer Patients. Front. Genet. 2022, 13, 842943. [Google Scholar] [CrossRef]
- Zhang, J.; He, P.; Xi, Y.; Geng, M.; Chen, Y.; Ding, J. Down-regulation of G9a triggers DNA damage response and inhibits colorectal cancer cells proliferation. Oncotarget 2015, 6, 2917–2927. [Google Scholar] [CrossRef] [Green Version]
- Daitoku, H.; Sakamaki, J.; Fukamizu, A. Regulation of FoxO transcription factors by acetylation and protein–protein interac-tions. Biochim. Biophys. Acta 2011, 1813, 1954–1960. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lyu, Z.; Qin, Y.; Wang, X. FOXO1 promotes tumor progression by increased M2 macrophage infiltration in esoph-ageal squamous cell carcinoma. Theranostics 2020, 10, 11535–11548. [Google Scholar] [CrossRef]
- Han, C.-Y.; Cho, K.-B.; Choi, H.-S.; Han, H.-K.; Kang, K.-W. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinog. 2008, 29, 1837–1844. [Google Scholar] [CrossRef]
- Liang, B.; Kong, D.; Liu, Y.; Liang, N.; He, M.; Ma, S.; Liu, X. Autophagy inhibition plays the synergetic killing roles with radiation in the mul-ti-drug resistant SKVCR ovarian cancer cells. Radiat. Oncol. 2012, 7, 213. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, X.; Guo, L.; Wu, X.; He, C.; Zhang, S.; Xiao, Y.; Yang, Y.; Hao, D. Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol. Med. Rep. 2015, 12, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Gewirtz, D.A. The Challenge of Developing Autophagy Inhibition as a Therapeutic Strategy. Cancer Res. 2016, 76, 5610–5614. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Demaria, S.; Formenti, S.C.; Kroemer, G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat. Rev. Clin. Oncol. 2017, 14, 247–258. [Google Scholar] [CrossRef]
- Chen, N.; Karantza, V. Autophagy as a therapeutic target in cancer. Cancer Biol. Ther. 2011, 11, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakifahmetoglu-Norberg, H.; Xia, H.-G.; Yuan, J. Pharmacologic agents targeting autophagy. J. Clin. Investig. 2015, 125, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.; Kim, J. Novel pharmacological modulators of autophagy: An updated patent review (2012–2015). Expert Opin. Ther. Pat. 2016, 26, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.; Pacheco, R.R.; Kmeid, M.; Chen, A.; Lee, H. Tumor Stroma Ratio and Its Significance in Locally Advanced Colorectal Cancer. Curr. Oncol. 2022, 29, 3232–3241. [Google Scholar] [CrossRef]
- Xu, L.; Luo, H.; Wang, R.; Wu, W.W.; Phue, J.-N.; Shen, R.-F.; Juhl, H.; Wu, L.; Alterovitz, W.-L.; Simonyan, V.; et al. Novel reference genes in colorectal cancer identify a distinct subset of high stage tumors and their associated histologically normal colonic tissues. BMC Med. Genet. 2019, 20, 138. [Google Scholar] [CrossRef]

| Feature | Value |
|---|---|
| Tumor location | Colon: 26 (66.67%); Rectum: 13 (33.33%) |
| T | I: 1 (2.56%); II: 16 (41.03%); III: 20 (51.28%); IV: 2 (5.13%) |
| N | N0: 24 (61.54%); N1: 4 (10.26%); N2: 11 (28.21%) |
| M | M0: 36 (92.31%); M1: 3 (7.69%) |
| TNM staging | I: 14 (35.9%); II: 9 (23.08%); III: 13 (33.33%); IV: 3 (7.69%) |
| Gene | Primer Sequences 5′ → 3′ | |
|---|---|---|
| Starter Forward | Starter Reverse | |
| BECN 1 | CAGTATCAGAGAGAATACAGTG | TGGAAGGTTGCATTAAAGAC |
| LAMP-2 | AACAAAGAGCAGACTGTTTC | CAGCTGTAGAATACTTTCCTTG |
| PINK 1 | GGACGCTGTTCCTCGTTA | ATCTGCGATCACCAGCCA |
| FOXO 1 | GTCAAGACAACGACACATAG | AAACTAAAAGGGAGTTGGTG |
| Colon Samples | Av | Me | Q1 | Q3 | SD | ANOVA Test | Post Hoc (LSD) Test |
|---|---|---|---|---|---|---|---|
| BECN1 | |||||||
| CSI | 13,901 | 10,245.00 | 13,901 | 10,245.00 | 19,344 | p = 0.759580 | NS |
| CSII | 9292 | 5981.00 | 9292 | 5981.00 | 13,083 | ||
| CSIII | 12,258 | 6141.50 | 12,258 | 6141.50 | 17,626 | ||
| CSIV | 4934 | 2629.00 | 4934 | 2629.00 | 5475 | ||
| C | 8,530,219 | 10,560.00 | 8,530,219 | 10,560.00 | 57,606,226 | ||
| LAMP-2 | |||||||
| CSI | 23,389 | 14,735.00 | 8417.00 | 23,340.00 | 25,031 | p = 0.617352 | NS |
| CSII | 5,856,396 | 22,940.00 | 12,870.00 | 35,040.00 | 26,703,506 | ||
| CSIII | 27,636 | 19,830.00 | 9676.00 | 47,850.00 | 22,862 | ||
| CSIV | 17,467 | 17,775.00 | 14,455.00 | 20,895.00 | 4931 | ||
| C | 3,786,237 | 33,850.00 | 14,310.00 | 51,660.00 | 20,487,421 | ||
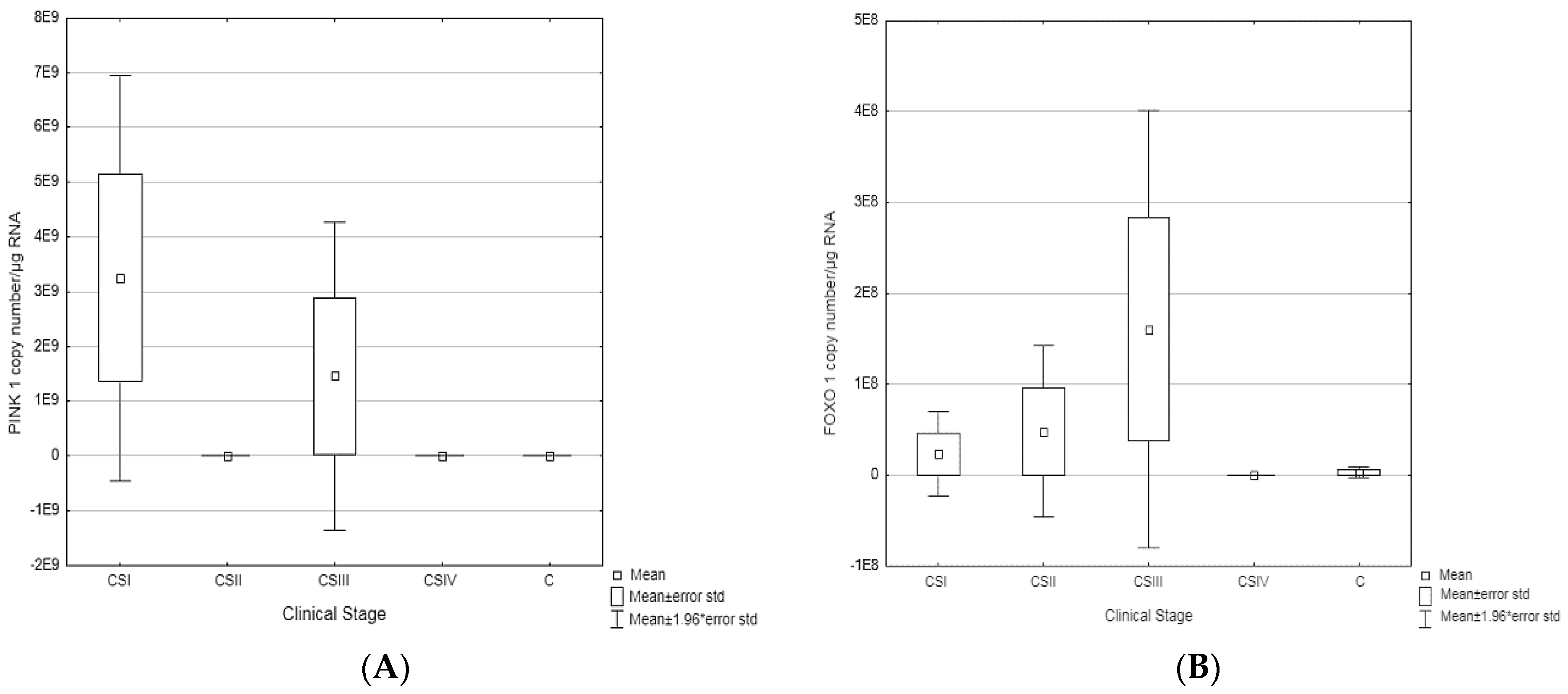
| PINK1 | |||||||
| CSI | 2248 × 109 | 1405 | 2248 × 109 | 1.405 | 8879 × 109 | p = 0.044194 | CSII vs. CSI p = 0.024910 CSI vs. C p = 0.004188 |
| CSII | 1257 × 104 | 1745.00 | 1257 × 104 | 1745.00 | 3342 × 104 | ||
| CSIII | 1452 × 109 | 153.00 | 1452 × 109 | 153.00 | 8245 × 109 | ||
| CSIV | 3586 × 104 | 7202.00 | 3586 × 104 | 7202.00 | 7905 × 104 | ||
| C | 2285 × 104 | 11.780 | 2285 × 104 | 11.780 | 1809 × 105 | ||
| FOXO1 | |||||||
| CSI | 23,485,542 | 337.200 | 134.8500 | 3658.50 | 81,347,958 | p = 0.219372 | CSIII vs. C p = 0.020474 |
| CSII | 47,997,855 | 563.250 | 50.4000 | 5226.00 | 179,571,390 | ||
| CSIII | 1,606,633,388 | 2143.500 | 48.1000 | 34,430.00 | 575,189,334 | ||
| CSIV | 6128 | 275.500 | 74.3345 | 12,685.00 | 10,116 | ||
| C | 3,116,668 | 262.800 | 1.3780 | 14,070.00 | 23,029,138 | ||
| Gene | Clinical Stage | |||
|---|---|---|---|---|
| CSI vs. C | CSII vs. C | CSIII vs. C | CSIV vs. C | |
| BECN1 | ↓ | ↓ | ↓ | ↓ |
| LAMP-2 | ↓ | ↑ | ↓ | ↓ |
| PINK1 | ↑ | ↓ | ↑ | ↑ |
| FOXO1 | ↑ | ↑ | ↑ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, M.; Muc-Wierzgoń, M.; Dzięgielewska-Gęsiak, S.; Fatyga, E.; Waniczek, D. Transcription of Autophagy Associated Gene Expression as Possible Predictors of a Colorectal Cancer Prognosis. Biomedicines 2023, 11, 418. https://doi.org/10.3390/biomedicines11020418
Bednarczyk M, Muc-Wierzgoń M, Dzięgielewska-Gęsiak S, Fatyga E, Waniczek D. Transcription of Autophagy Associated Gene Expression as Possible Predictors of a Colorectal Cancer Prognosis. Biomedicines. 2023; 11(2):418. https://doi.org/10.3390/biomedicines11020418
Chicago/Turabian StyleBednarczyk, Martyna, Małgorzata Muc-Wierzgoń, Sylwia Dzięgielewska-Gęsiak, Edyta Fatyga, and Dariusz Waniczek. 2023. "Transcription of Autophagy Associated Gene Expression as Possible Predictors of a Colorectal Cancer Prognosis" Biomedicines 11, no. 2: 418. https://doi.org/10.3390/biomedicines11020418






