Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines
Abstract
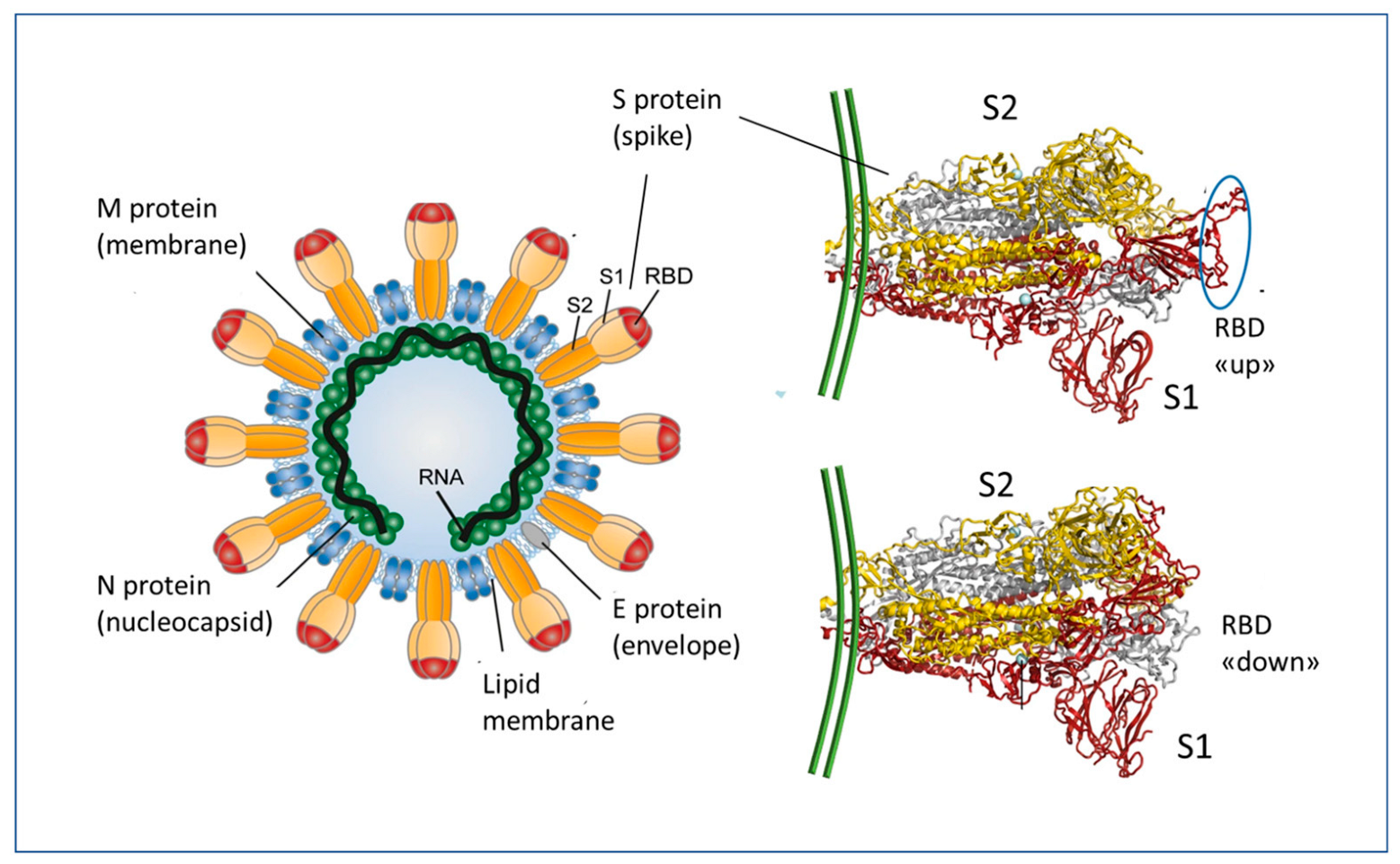
:1. Introduction
2. Essentials of mRNA Vaccines Design and Functioning
3. The Immune Response to the SARS-CoV-2 and to the mRNA Vaccines
3.1. The Importance of the Route of Entry
3.2. Immunization Pathways of the SARS-CoV-2 and mRNA Vaccines
3.3. Differences between Contact with the Whole Virus and Vaccine-Derived Spike Protein
4. LNP Biodistribution and Spike Detection
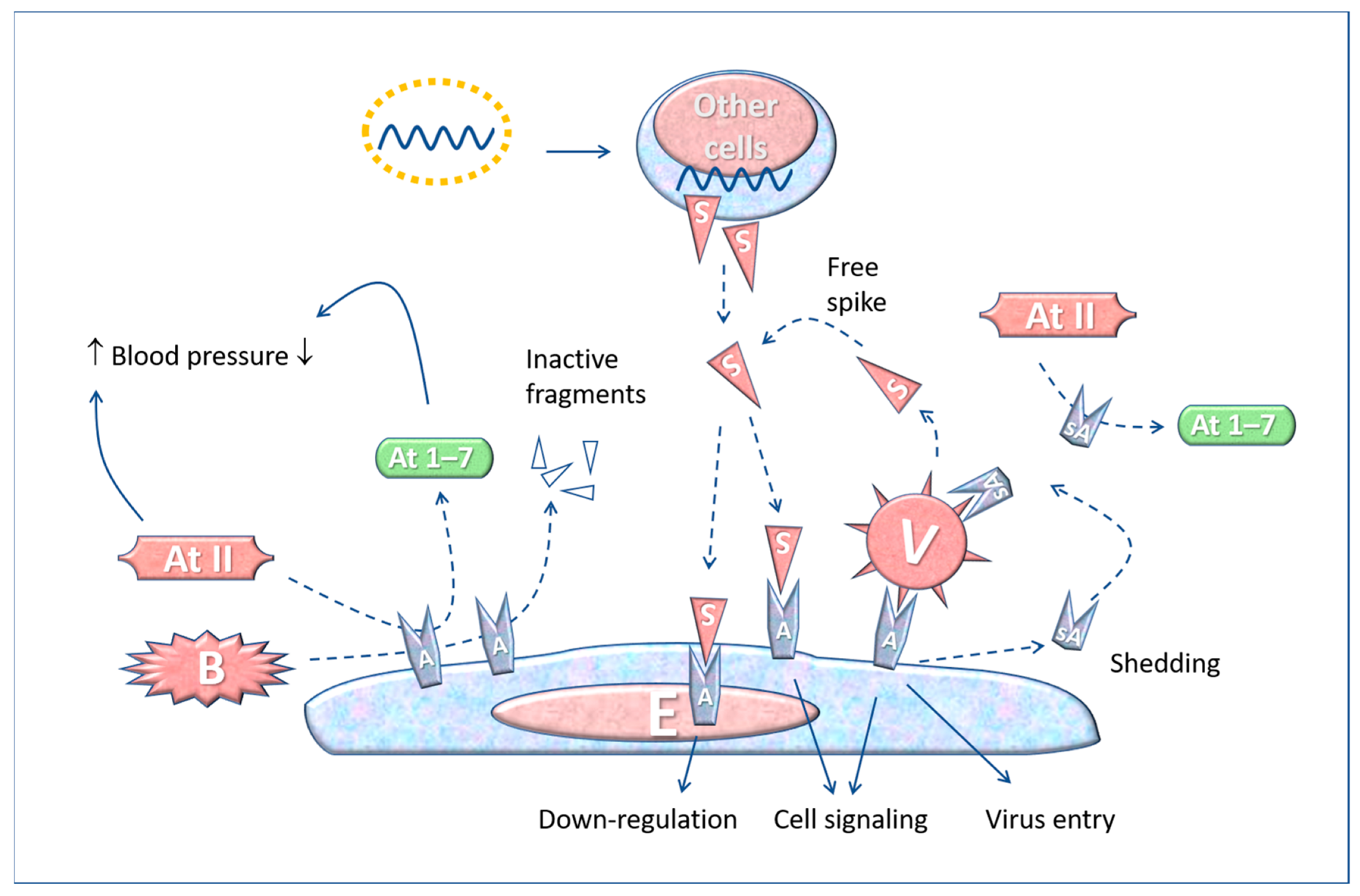
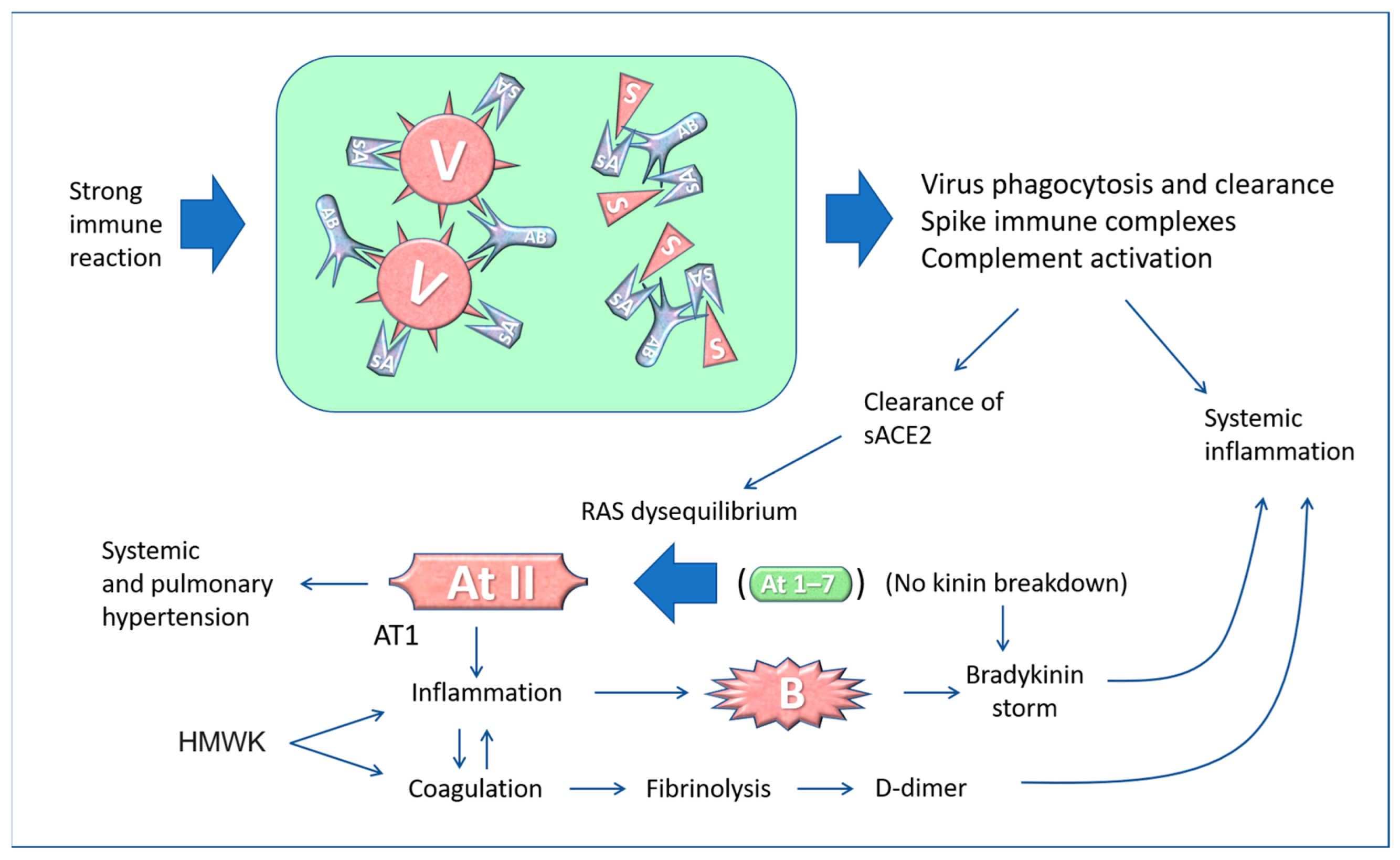
5. The “Active” Spike and the Renin-Angiotensin System
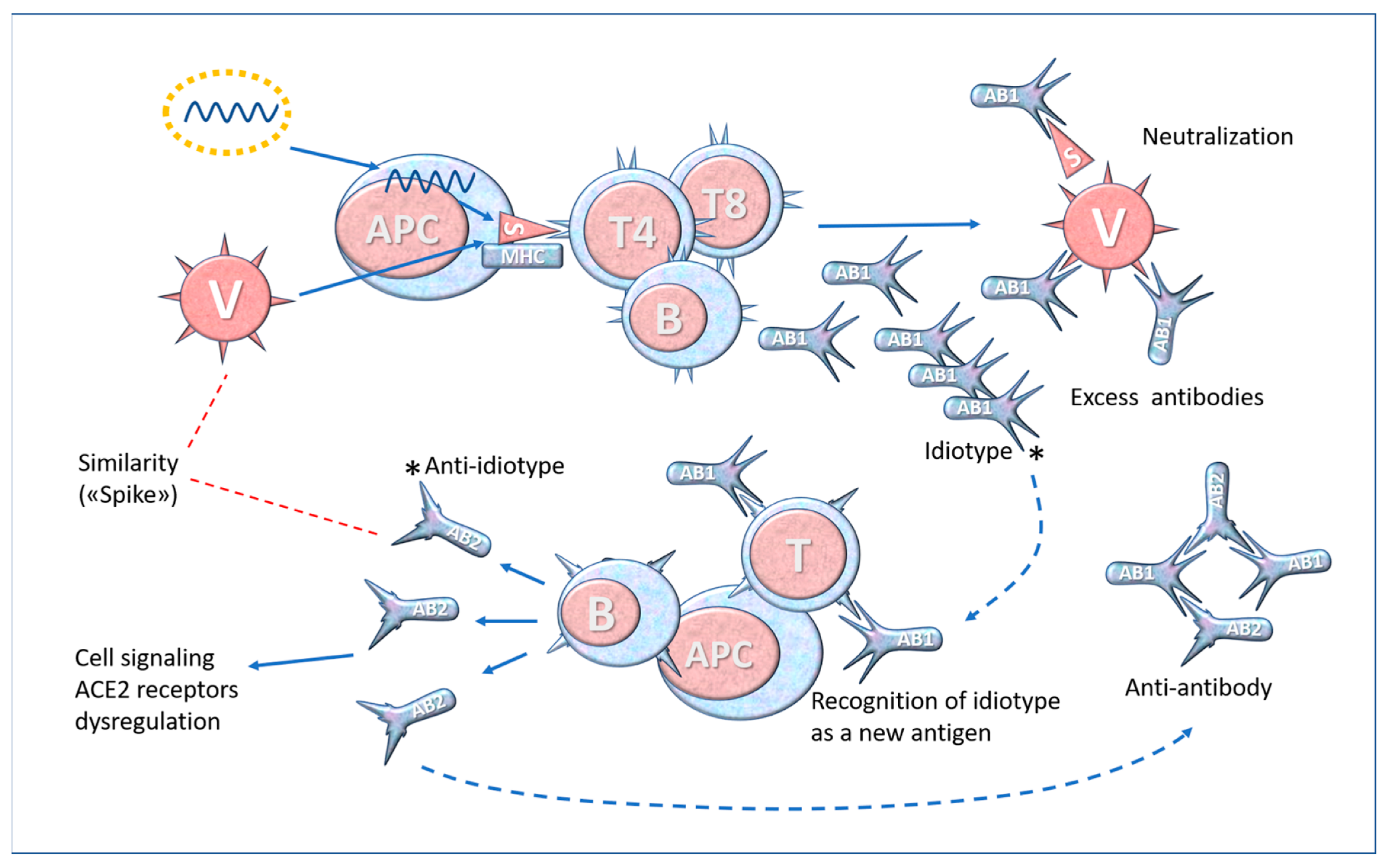
6. Molecular Mimicry and Anti-Idiotype Antibodies
7. The “Boost” and Trained Immunity
8. Overview and Prospects
8.1. Causality Assessment
8.2. Diagnostic and Therapeutic Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; Van Der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. Ebiomedicine 2020, 60, 102976. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. COVID-19: ACE2centric Infective Disease? Hypertension 2020, 76, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Profile of SARS-CoV-2. Wien. Klin. Wochenschr. 2020, 132, 635–644. [Google Scholar]
- Juibari, A.D.; Rezadoost, M.H.; Soleimani, M. The key role of Calpain in COVID-19 as a therapeutic strategy. Inflammopharmacology 2022, 30, 1479–1491. [Google Scholar] [CrossRef]
- Zhao, H.; Meng, X.; Peng, Z.; Lam, H.; Zhang, C.; Zhou, X.; Chan, J.F.; Kao, R.Y.T.; To, K.K.; Yuen, K.Y. Fusion-inhibition peptide broadly inhibits influenza virus and SARS-CoV-2, including Delta and Omicron variants. Emerg. Microbes Infect. 2022, 11, 926–937. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Wilder-Smith, A. What is the vaccine effect on reducing transmission in the context of the SARS-CoV-2 delta variant? Lancet Infect. Dis. 2022, 22, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Solante, R.; Alvarez-Moreno, C.; Burhan, E.; Chariyalertsak, S.; Chiu, N.-C.; Chuenkitmongkol, S.; Dung, D.V.; Hwang, K.-P.; Ibarra, J.O.; Kiertiburanakul, S.; et al. Expert review of global real-world data on COVID-19 vaccine booster effectiveness and safety during the omicron-dominant phase of the pandemic. Expert Rev. Vaccines 2022, 22, 1–16. [Google Scholar] [CrossRef]
- Addo, I.Y.; Dadzie, F.A.; Okeke, S.R.; Boadi, C.; Boadu, E.F. Duration of immunity following full vaccination against SARS-CoV-2: A systematic review. Arch. Public Health 2022, 80, 200. [Google Scholar]
- Kerr, S.; Bedston, S.; Bradley, D.T.; Joy, M.; Lowthian, E.; Mulholland, R.M.; Akbari, A.; Hobbs, F.D.R.; Katikireddi, S.V.; de Lusignan, S.; et al. Waning of first- and second-dose ChAdOx1 and BNT162b2 COVID-19 vaccinations: A pooled target trial study of 12.9 million individuals in England, Northern Ireland, Scotland and Wales. Int. J. Epidemiol. 2022. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R.; et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021, 7, 99. [Google Scholar] [CrossRef]
- Yamamoto, K. Adverse effects of COVID-19 vaccines and measures to prevent them. Virol. J. 2022, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Kouhpayeh, H.; Ansari, H. Adverse events following COVID-19 vaccination: A systematic review and meta-analysis. Int. Immunopharmacol. 2022, 109, 108906. [Google Scholar] [CrossRef]
- Cosentino, M.; Marino, F. Understanding the Pharmacology of COVID-19 mRNA Vaccines: Playing Dice with the Spike? Int. J. Mol. Sci. 2022, 23, 10881. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, B.; Bai, Y.; Shao, Y.; Xiao, Y.; Tang, S. The resurgence risk of COVID-19 in China in the presence of immunity waning and ADE: A mathematical modelling study. Vaccine 2022, 40, 7141–7150. [Google Scholar] [CrossRef] [PubMed]
- Ozbay Kurt, F.G.; Lepper, A.; Gerhards, C.; Roemer, M.; Lasser, S.; Arkhypov, I.; Bitsch, R.; Bugert, P.; Altevogt, P.; Gouttefangeas, C.; et al. Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front. Immunol. 2022, 13, 1012526. [Google Scholar] [CrossRef] [PubMed]
- Karlstad, Ø.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600. [Google Scholar] [CrossRef]
- Sun, C.L.F.; Jaffe, E.; Levi, R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci. Rep. 2022, 12, 6978. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M. A Possible Case of Hypertensive Crisis With Intracranial Haemorrhage After an mRNA Anti-COVID-19 Vaccine. Angiology 2022, 73, 87. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, S.Y.; Ahn, J.G.; Park, S.J.; Shoenfeld, Y.; Kronbichler, A.; Koyanagi, A.; Dragioti, E.; Tizaoui, K.; Hong, S.H.; et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: A pharmacovigilance analysis using WHO international database. J. Med. Virol. 2021, 94, 1085–1095. [Google Scholar] [CrossRef]
- Almas, T.; Rehman, S.; Mansour, E.; Khedro, T.; Alansari, A.; Malik, J.; Alshareef, N.; Nagarajan, V.R.; Al-Awaid, A.H.; Alsufyani, R.; et al. Epidemiology, clinical ramifications, and cellular pathogenesis of COVID-19 mRNA-vaccination-induced adverse cardiovascular outcomes: A state-of-the-heart review. Biomed. Pharmacother. 2022, 149, 112843. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, A.; Salameh, M.A.; Laswi, I.; Mohammed, I.; Mhaimeed, O.; Mhaimeed, N.; Mhaimeed, N.; Paul, P.; Mushannen, M.; Elshafeey, A.; et al. Neurological Immune-Related Adverse Events After COVID-19 Vaccination: A Systematic Review. J. Clin. Pharmacol. 2021, 62, 291–303. [Google Scholar] [CrossRef]
- Afshar, Z.M.; Sharma, A.; Babazadeh, A.; Alizadeh-Khatir, A.; Sio, T.T.; Moghadam, M.A.T.; Pirzaman, A.T.; Mojadad, A.; Hosseinzadeh, R.; Barary, M.; et al. A review of the potential neurological adverse events of COVID-19 vaccines. Acta Neurol. Belg. 2022, 1–36. [Google Scholar] [CrossRef]
- Pour Mohammad, A.; Mashayekhi, F.; Seirafianpour, F.; Gholizadeh Mesgarha, M.; Goodarzi, A. COVID-19 and COVID-19 vaccine-related dermatological reactions: An interesting case series with a narrative review of the potential critical and non-critical mucocutaneous adverse effects related to virus, therapy, and the vaccination. Clin. Case Rep. 2022, 10, e05775. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Lavine, N.; Ohayon, A.; Seida, R.; Alwani, A.; Alrais, M.; Zoubi, M.; Bragazzi, N.L. COVID-19 Vaccination and the Rate of Immune and Autoimmune Adverse Events Following Immunization: Insights From a Narrative Literature Review. Front. Immunol. 2022, 13, 872683. [Google Scholar] [CrossRef]
- Mingot-Castellano, M.E.; Butta, N.; Canaro, M.; Gomez Del Castillo Solano, M.D.C.; Sánchez-González, B.; Jiménez-Bárcenas, R.; Pascual-Izquierdo, C.; Caballero-Navarro, G.; Ureña, L.E.; González-López, T.J.; et al. COVID-19 Vaccines and Autoimmune Hematologic Disorders. Vaccines 2022, 10, 961. [Google Scholar] [CrossRef]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Balbin, C.A.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K.; et al. Potential Autoimmunity Resulting from Molecular Mimicry between SARS-CoV-2 Spike and Human Proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef]
- Crawford, N.W.; Clothier, H.; Hodgson, K.; Selvaraj, G.; Easton, M.L.; Buttery, J.P. Active surveillance for adverse events following immunization. Expert Rev. Vaccines 2014, 13, 265–276. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Causality assessment of adverse events following immunization: The problem of multifactorial pathology. F1000Research 2020, 9, 170. [Google Scholar] [CrossRef]
- Bellavite, P.; Donzelli, A. Adverse events following measles-mumps-rubella-varicella vaccine: An independent perspective on Italian pharmacovigilance data. F1000Research 2020, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; de Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef]
- Sulemankhil, I.; Abdelrahman, M.; Negi, S.I. Temporal Association Between the COVID-19 Ad26.COV2.S Vaccine and Acute Myocarditis: A Case Report and Literature Review. Cardiovasc. Revasc. Med. 2022, 38, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Evans, J.P.; Liu, S.L. Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 2021, 297, 100847. [Google Scholar] [CrossRef]
- Campbell, R.A.; Boilard, E.; Rondina, M.T. Is there a role for the ACE2 receptor in SARS-CoV-2 interactions with platelets? J. Thromb. Haemost. 2021, 19, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, A.; Filippini, F.; Bardelli, M.; Zani, A.; Chiodelli, P.; Messali, S.; Caruso, A.; Caccuri, F. SARS-CoV-2 Infects Human ACE2-Negative Endothelial Cells through an alphavbeta3 Integrin-Mediated Endocytosis Even in the Presence of Vaccine-Elicited Neutralizing Antibodies. Viruses 2022, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- De Beuckelaer, A.; Pollard, C.; Van Lint, S.; Roose, K.; Van Hoecke, L.; Naessens, T.; Udhayakumar, V.K.; Smet, M.; Sanders, N.; Lienenklaus, S.; et al. Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses. Mol. Ther. 2016, 24, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lagniton, P.N.; Liu, Y.; Xu, R.-H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, A.M.; McCullough, P.A. Synthetic mRNAs; Their Analogue Caps and Contribution to Disease. Diseases 2021, 9, 57. [Google Scholar] [CrossRef]
- Orlandini von Niessen, A.G.; Poleganov, M.A.; Rechner, C.; Plaschke, A.; Kranz, L.M.; Fesser, S.; Diken, M.; Löwer, M.; Vallazza, B.; Beissert, T.; et al. Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3’ UTRs Identified by Cellular Library Screening. Mol. Ther. 2019, 27, 824–836. [Google Scholar] [CrossRef]
- McKernan, K.; Kyriakopoulos, A.M.; McCullough, P.A. Differences in Vaccine and SARS-CoV-2 Replication Derived mRNA: Implications for Cell Biology and Future Disease. OSF Preprints 2021. [Google Scholar] [CrossRef]
- Mauro, V.P.; Chappell, S.A. A critical analysis of codon optimization in human therapeutics. Trends Mol. Med. 2014, 20, 604–613. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Roltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Fertig, T.E.; Chitoiu, L.; Marta, D.S.; Ionescu, V.-S.; Cismasiu, V.B.; Radu, E.; Angheluta, G.; Dobre, M.; Serbanescu, A.; Hinescu, M.E.; et al. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines 2022, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Castruita, J.A.S.; Vest Schneider, U.; Mollerup, S.; Leineweber, T.D.; Weis, N.; Bukh, J.; Pedersen, M.S.; Westh, H. SARS-CoV -2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID -19 vaccination. APMIS 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Simon, J.F.; Hagen, A.; Gordon, S.M. Effectiveness of the Coronavirus Disease 2019 (COVID-19) Bivalent Vaccine. medRxiv 2022. [Google Scholar] [CrossRef]
- Wagenhäuser, I.; Reusch, J.; Gabel, A.; Krone, L.B.; Kurzai, O.; Petri, N.; Krone, M. Bivalent BNT162b2mRNA original/Omicron BA.4-5 booster vaccination: Adverse reactions and inability to work compared to the monovalent COVID-19 booster. medRxiv 2022. [Google Scholar] [CrossRef]
- Hwang, I. Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Mol. Cells 2013, 36, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Pasare, C. Location, location, location: Tissue-specific regulation of immune responses. J. Leukoc. Biol. 2013, 94, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.A.; Zheng, S.G.; Gray, J.D. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008, 29, 429–435. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Drummer, H.E.; Van, H.; Klock, E.; Zheng, S.; Wei, Z.; Boo, I.; Center, R.J.; Li, F.; Bhat, P.; Ffrench, R.; et al. Dimeric IgA is a specific biomarker of recent SARS-CoV-2 infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Viant, C.; Gaebler, C.; Cipolla, M.; Hoffmann, H.H.; Oliveira, T.Y.; Oren, D.A.; et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021, 13, eabf1555. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal. Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine 2021, 75, 103788. [Google Scholar] [CrossRef]
- Azzi, L.; Gasperina, D.D.; Veronesi, G.; Shallak, M.; Maurino, V.; Baj, A.; Gianfagna, F.; Cavallo, P.; Dentali, F.; Tettamanti, L.; et al. Mucosal immune response after the booster dose of the BNT162b2 COVID-19 vaccine. Ebiomedicine 2023, 88, 104435. [Google Scholar] [CrossRef]
- Ivanova, E.N.; Devlin, J.C.; Buus, T.B.; Koide, A.; Shwetar, J.; Cornelius, A.; Samanovic, M.I.; Herrera, A.; Mimitou, E.P.; Zhang, C.; et al. SARS-CoV-2 mRNA vaccine elicits a potent adaptive immune response in the absence of IFN-mediated inflammation observed in COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Committee, F.A. Vaccines and Related Biological Products Advisory Committee December 17, 2020 in, Food and Drug Administration. 2020. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-17-2020-meeting-announcement (accessed on 24 July 2022).
- Moore, M.J. mRNA Platform and Mechanism of Action of mRNA-1273 in, FDA document: Emergency Use Authorization (EUA) Application for mRNA-1273. 2020. Available online: https://www.fda.gov/media/144583/download (accessed on 24 July 2022).
- Plotkin, S.A. Vaccines: The fourth century. Clin. Vaccine Immunol. 2009, 16, 1709–1719. [Google Scholar] [CrossRef]
- Reif, D.M.; McKinney, B.A.; Motsinger, A.A.; Chanock, S.J.; Edwards, K.M.; Rock, M.T.; Moore, J.H.; Crowe, J.J.E. Genetic Basis for Adverse Events after Smallpox Vaccination. J. Infect. Dis. 2008, 198, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Kennedy, R.B.; McKinney, B.A.; Ovsyannikova, I.G.; Lambert, N.D.; Jacobson, R.M.; Oberg, A.L. Vaccinomics, adversomics, and the immune response network theory: Individualized vaccinology in the 21st century. Semin. Immunol. 2013, 25, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, Y. The ontology of genetic susceptibility factors (OGSF) and its application in modeling genetic susceptibility to vaccine adverse events. J. Biomed. Semant. 2014, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, E.; McDonald, J.; Fireman, B.; Naleway, A.; Glanz, J.; Jackson, L.A.; Donahue, J.G.; Jacobsen, S.J.; Weintraub, E.; et al. Risk factors and familial clustering for fever 7–10 days after the first dose of measles vaccines. Vaccine 2017, 35, 1615–1621. [Google Scholar] [CrossRef]
- Asandei, A.; Mereuta, L.; Schiopu, I.; Park, J.; Seo, C.H.; Park, Y.; Luchian, T. Non-Receptor-Mediated Lipid Membrane Permeabilization by the SARS-CoV-2 Spike Protein S1 Subunit. ACS Appl. Mater. Interfaces 2020, 12, 55649–55658. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after Covid-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.; Stevenson, J.; Høeg, T.B. BNT162b2 Vaccine-Associated Myo/Pericarditis in Adolescents: A Stratified Risk-Benefit Analysis. Eur. J. Clin. Investig. 2022, 52, e13759. [Google Scholar] [CrossRef]
- Atalis, A.; Keenum, M.C.; Pandey, B.; Beach, A.; Pradhan, P.; Vantucci, C. Nanoparticle-delivered TLR4 and RIG-I agonists enhance immune response to SARS-CoV-2 subunit vaccine. J. Control Release 2022, 347, 476–488. [Google Scholar] [CrossRef]
- Vervaeke, P.; Borgos, S.; Sanders, N.; Combes, F. Regulatory guidelines and preclinical tools to study the biodistribution of RNA therapeutics. Adv. Drug Deliv. Rev. 2022, 184, 114236. [Google Scholar] [CrossRef]
- Di, J.; Du, Z.; Wu, K.; Jin, S.; Wang, X.; Li, T.; Xu, Y. Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size. Pharm. Res. 2022, 39, 105–114. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Miller, B.L. Monitoring Serum Spike Protein with Disposable Photonic Biosensors Following SARS-CoV-2 Vaccination. Sensors 2021, 21, 5857. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vascul. Pharmacol. 2021, 137, 106823. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Gychka, S.G. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines 2021, 9, 36. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Mörz, M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines 2022, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Galbusera, M.; Pezzotta, A.; Gastoldi, S.; Imberti, B.; Perna, A.; Ruggenenti, P.; Donadelli, R.; Benigni, A.; et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front. Immunol. 2022, 13, 827146. [Google Scholar] [CrossRef]
- De Michele, M.; d’Amati, G.; Leopizzi, M.; Iacobucci, M.; Berto, I.; Lorenzano, S.; Mazzuti, L.; Turriziani, O.; Schiavo, O.G.; Toni, D. Evidence of SARS-CoV-2 spike protein on retrieved thrombi from COVID-19 patients. J. Hematol. Oncol. 2022, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaidan, L.; Mestiri, S.; Raza, A.; Merhi, M.; Inchakalody, V.P.; Fernandes, Q.; Taib, N.; Uddin, S.; Dermime, S. The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target. Tumour. Biol. 2021, 43, 177–196. [Google Scholar] [CrossRef]
- Wu, M.L.; Liu, F.L.; Sun, J.; Li, X.; He, X.Y.; Zheng, H.Y.; Zhou, Y.H.; Yan, Q.; Chen, L.; Yu, G.Y.; et al. SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury. Signal Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef]
- Nagashima, S.; Dutra, A.A.; Arantes, M.P.; Zeni, R.C.; Klein, C.K.; de Oliveira, F.C.; Piper, G.W.; Brenny, I.D.; Pereira, M.R.C.; Stocco, R.B.; et al. COVID-19 and Lung Mast Cells: The Kallikrein–Kinin Activation Pathway. Int. J. Mol. Sci. 2022, 23, 1714. [Google Scholar] [CrossRef]
- Caillet-Saguy, C.; Wolff, N. PDZ-Containing Proteins Targeted by the ACE2 Receptor. Viruses 2021, 13, 2281. [Google Scholar] [CrossRef]
- Wang, X.M.; Mannan, R.; Xiao, L.; Abdulfatah, E.; Qiao, Y.; Farver, C.; Myers, J.L.; Zelenka-Wang, S.; McMurry, L.; Su, F.; et al. Characterization of SARS-CoV-2 and host entry factors distribution in a COVID-19 autopsy series. Commun. Med. (Lond.) 2021, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Renin-Angiotensin System, SARS-CoV-2 and Hypotheses about Adverse Effects Following Vaccination. EC Pharmacol. Toxicol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Lu, J.; Sun, P.D. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J. Biol. Chem. 2020, 295, 18579–18588. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.C.; Montgomery, D.; Ito, K.; Woods, R.J. Analysis of the SARS-CoV-2 spike protein glycan shield: Implications for immune recognition. BioRxiv 2020. [Google Scholar] [CrossRef]
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; Martinez de la Escalera, G.; Clapp, C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin alpha5beta1 and NF-kappaB signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef]
- Aleksova, A.; Gagno, G.; Sinagra, G.; Beltrami, A.P.; Janjusevic, M.; Ippolito, G.; Zumla, A.; Fluca, A.L.; Ferro, F. Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (ACE2) as the Virus Receptor and Homeostasis Regulator-Review. Int. J. Mol. Sci. 2021, 22, 4526. [Google Scholar] [CrossRef]
- Zamai, L. Upregulation of the Renin-Angiotensin System Pathways and SARS-CoV-2 Infection: The Rationale for the Administration of Zinc-Chelating Agents in COVID-19 Patients. Cells 2021, 10, 506. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Trapasso, M.; Zappa, M.; Spanevello, A.; Verdecchia, P. COVID-19, vaccines and deficiency of ACE2 and other angiotensinases. Closing the loop on the “Spike effect”. Eur. J. Intern. Med. 2022, 103, 23–28. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hasan, M.; Ahmed, A. Potential detrimental role of soluble ACE2 in severe COVID-19 comorbid patients. Rev. Med. Virol. 2021, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor Necrosis Factor-α Convertase (ADAM17) Mediates Regulated Ectodomain Shedding of the Severe-acute Respiratory Syndrome-Coronavirus (SARS-CoV) Receptor, Angiotensin-converting Enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef]
- Mariappan, V.; Ranganadin, P.; Shanmugam, L.; Rao, S.; Pillai, A.B. Early shedding of membrane-bounded ACE2 could be an indicator for disease severity in SARS-CoV-2. Biochimie 2022, 201, 139–147. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Letarov, A.V.; Babenko, V.V.; Kulikov, E.E. Free SARS-CoV-2 Spike Protein S1 Particles May Play a Role in the Pathogenesis of COVID-19 Infection. Biochemistry (Mosc) 2021, 86, 257–261. [Google Scholar] [CrossRef]
- Wang, S.; Guo, F.; Liu, K.; Wang, H.; Rao, S.; Yang, P.; Jiang, C. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008, 136, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.B.; Perez, L.G.; Palmeira, V.A.; Cordeiro, M.E.; Ribeiro, V.T.; Lanza, K.; Simões E Silva, A.C. Insights on SARS-CoV-2 Molecular Interactions With the Renin-Angiotensin System. Front. Cell Dev. Biol. 2020, 8, 559841. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Wilczynski, S.; Wenceslau, C.F.; Webb, R.C. A new storm on the horizon in COVID-19: Bradykinin-induced vascular complications. Vasc. Pharmacol. 2020, 137, 106826. [Google Scholar] [CrossRef]
- Garvin, M.R.; Alvarez, C.; Miller, J.I.; Prates, E.T.; Walker, A.M.; Amos, B.K.; Mast, A.E.; Justice, A.; Aronow, B.; Jacobson, D.A. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife 2020, 9, e59177. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed. Pharmacother. 2021, 136, 111193. [Google Scholar] [CrossRef]
- Sidarta-Oliveira, D.; Jara, C.P.; Ferruzzi, A.J.; Skaf, M.S.; Velander, W.H.; Araujo, E.P.; Velloso, L.A. SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cells. Sci. Rep. 2020, 10, 19522. [Google Scholar] [CrossRef]
- Roche, J.A.; Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Consolaro, E.; Suter, F.; Rubis, N.; Pedroni, S.; Moroni, C.; Pastò, E.; Paganini, M.V.; Pravettoni, G.; Cantarelli, U.; Perico, N.; et al. A Home-Treatment Algorithm Based on Anti-inflammatory Drugs to Prevent Hospitalization of Patients With Early COVID-19: A Matched-Cohort Study (COVER 2). Front. Med. 2022, 9, 785785. [Google Scholar] [CrossRef]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Fard, S.N.; Alonzi, T.; Castilletti, C.; Palmieri, F.; Gualano, G.; Vittozzi, P.; et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2020, 27, 286.e7–286.e13. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Trapasso, M.; Verdecchia, P. Hypertension after COVID-19 vaccination. G. Ital. Cardiol. (Rome) 2022, 23, 10–14. [Google Scholar] [PubMed]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Martínez, I.D.; Rodríguez-Gutiérrez, M.M.; Ospina-Rios, J.; Ortega-Sierra, M.G.; González-Herazo, M.A.; Ortiz-Roncallo, L.M.; Martínez-Imbett, R.; Llamas-Nieves, A.E.; Janjua, T.; Moscote-Salazar, L.R. Neurogenic pulmonary edema in subarachnoid hemorrhage: Relevant clinical concepts. Egypt. J. Neurosurg. 2021, 36, 27. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Horwitz, D.A.; Fahmy, T.M.; Piccirillo, C.A.; La Cava, A. Rebalancing Immune Homeostasis to Treat Autoimmune Diseases. Trends Immunol. 2019, 40, 888–908. [Google Scholar] [CrossRef]
- Jerne, N.K.; Cocteau, J. Idiotypic Networks and Other Preconceived Ideas. Immunol. Rev. 1984, 79, 5–24. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Murphy, W.J.; Longo, D.L. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N. Engl. J. Med. 2022, 386, 394–396. [Google Scholar] [CrossRef]
- Moutal, A.; Martin, L.F.; Boinon, L.; Gomez, K.; Ran, D.; Zhou, Y.; Stratton, H.J.; Cai, S.; Luo, S.; Gonzalez, K.B.; et al. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Pain 2021, 162, 243–252. [Google Scholar] [CrossRef] [PubMed]
- De Maria, A. Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N. Engl. J. Med. 2022, 386, 897–898. [Google Scholar] [PubMed]
- Tercan, H.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G.; Bekkering, S. Trained Immunity: Long-Term Adaptation in Innate Immune Responses. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Andres, J.; Netea, M.G. Long-term reprogramming of the innate immune system. J. Leukoc. Biol. 2019, 105, 329–338. [Google Scholar] [CrossRef]
- Iv, C.D.; Saaoud, F.; Shao, Y.; Sun, Y.; Xu, K.; Lu, Y.; Ni, D.; Atar, D.; Jiang, X.; Wang, H.; et al. Trained Immunity and Reactivity of Macrophages and Endothelial Cells. Arter. Thromb. Vasc. Biol. 2021, 41, 1032–1046. [Google Scholar] [CrossRef]
- van der Meer, J.W.; Joosten, L.A.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef]
- Shao, Y.; Saredy, J.; Xu, K.; Sun, Y.; Saaoud, F.; Drummer, C.I.; Lu, Y.; Luo, J.J.; Lopez-Pastrana, J.; Choi, E.T.; et al. Endothelial Immunity Trained by Coronavirus Infections, DAMP Stimulations and Regulated by Anti-Oxidant NRF2 May Contribute to Inflammations, Myelopoiesis, COVID-19 Cytokine Storms and Thromboembolism. Front. Immunol. 2021, 12, 653110. [Google Scholar] [CrossRef]
- Lacy, M.; Atzler, D.; Liu, R.; de Winther, M.; Weber, C.; Lutgens, E. Interactions between dyslipidemia and the immune system and their relevance as putative therapeutic targets in atherosclerosis. Pharmacol. Ther. 2019, 193, 50–62. [Google Scholar] [CrossRef]
- Diani, S.; Leonardi, E.; Cavezzi, A.; Ferrari, S.; Iacono, O.; Limoli, A.; Bouslenko, Z.; Natalini, D.; Conti, S.; Mantovani, M.; et al. SARS-CoV-2-The Role of Natural Immunity: A Narrative Review. J. Clin. Med. 2022, 11, 6272. [Google Scholar] [CrossRef]
- Azzarone, B.; Veneziani, I.; Moretta, L.; Maggi, E. Pathogenic Mechanisms of Vaccine-Induced Immune Thrombotic Thrombocytopenia in People Receiving Anti-COVID-19 Adenoviral-Based Vaccines: A Proposal. Front. Immunol. 2021, 12, 728513. [Google Scholar] [CrossRef] [PubMed]
- Ropa, J.; Cooper, S.; Capitano, M.L.; Van’t Hof, W.; Broxmeyer, H.E. Human Hematopoietic Stem, Progenitor, and Immune Cells Respond Ex Vivo to SARS-CoV-2 Spike Protein. Stem. Cell Rev. Rep. 2021, 17, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Kappa18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L477–L484. [Google Scholar] [CrossRef]
- Khaddaj-Mallat, R.; Aldib, N.; Bernard, M.; Paquette, A.S.; Ferreira, A.; Lecordier, S. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol. Dis. 2021, 161, 105561. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Li, J.; Venzon, D.J.; Berzofsky, J.A. SARS-CoV-2 Spike Protein Suppresses ACE2 and Type I Interferon Expression in Primary Cells From Macaque Lung Bronchoalveolar Lavage. Front. Immunol. 2021, 12, 658428. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Magro, C.; Shaffer, T.; Awad, H.; Suster, D.; Mikhail, S.; He, B.; Michaille, J.-J.; Liechty, B.; Tili, E. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann. Diagn. Pathol. 2020, 51, 151682. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Koyama, Y.; Imai, Y.; Sawada, E.; Kishimoto, N.; Seo, K. SARS-CoV-2 recombinant proteins-induced degeneration of taste buds in rat circumvallate papillae. J. Dent. Sci. 2022, 17, 1450–1453. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Jeon, M.T.; Kim, K.S.; Lee, S.; Kim, S.; Kim, D.G. Spike Proteins of SARS-CoV-2 Induce Pathological Changes in Molecular Delivery and Metabolic Function in the Brain Endothelial Cells. Viruses 2021, 13, 2021. [Google Scholar] [CrossRef] [PubMed]
- Suprewicz, L.; Tran, K.A.; Piktel, E.; Fiedoruk, K.; Janmey, P.A.; Galie, P.A.; Bucki, R. Recombinant human plasma gelsolin reverses increased permeability of the blood-brain barrier induced by the spike protein of the SARS-CoV-2 virus. J. Neuroinflammation 2022, 19, 282. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, P.R.; Hibberd, J.; Bridle, B.W. How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use. Vaccines 2021, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Heaven, M.R.; Alayash, A.I. Cell-Free Hemoglobin Does Not Attenuate the Effects of SARS-CoV-2 Spike Protein S1 Subunit in Pulmonary Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 9041. [Google Scholar] [CrossRef]
- Avolio, E.; Carrabba, M.; Milligan, R.; Kavanagh Williamson, M.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K.; et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. (Lond.) 2021, 135, 2667–2689. [Google Scholar] [CrossRef]
- Maugeri, N.; De Lorenzo, R.; Clementi, N.; Antonia Diotti, R.; Criscuolo, E.; Godino, C.; Tresoldi, C.; Angels For Covid-BioB Study Group B; Bonini, C.; Clementi, M.; et al. Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 2022, 20, 434–448. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Petsini, F.; Detopoulou, M.; Theoharides, T.C.; Demopoulos, C.A. Is there an interplay between the SARS-CoV -2 spike protein and Platelet-Activating factor? Biofactors 2022, 48, 1271–1283. [Google Scholar] [CrossRef]
- Singh, A.; Nguyen, L.; Everest, S.; Afzal, S.; Shim, A. Acute Pericarditis Post mRNA-1273 COVID Vaccine Booster. Cureus 2022, 14, e22148. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Kurtulmus, N.; Kayikci, K. Subacute Thyroiditis Following SARS-CoV-2 Vaccines: Six Cases Report and Review of the Literature. Horm. Metab. Res. 2022, 54, 556–561. [Google Scholar] [CrossRef]
- Passariello, M.; Vetrei, C.; Amato, F.; De Lorenzo, C. Interactions of Spike-RBD of SARS-CoV-2 and Platelet Factor 4: New Insights in the Etiopathogenesis of Thrombosis. Int. J. Mol. Sci. 2021, 22, 8562. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic thrombocytopenia. Trends Cardiovasc. Med. 2022, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Thrombosis and thrombocytopenia in COVID-19 and after COVID-19 vaccination. Trends Cardiovasc. Med. 2022, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Altman, N.L.; Berning, A.A.; Saxon, C.E.; Adamek, K.E.; Wagner, J.A.; Slavov, D.; Quaife, R.A.; Gill, E.A.; Minobe, W.A.; Jonas, E.R.; et al. Myocardial Injury and Altered Gene Expression Associated With SARS-CoV-2 Infection or mRNA Vaccination. JACC Basic Transl. Sci. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kuang, M.; Li, J.; Zhu, L.; Jia, Z.; Guo, X.; Hu, Y.; Kong, J.; Yin, H.; Wang, X.; et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021, 31, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.F.; Vidal-Pla, M.; Moya, M.C.; Espasa, M.; Casabella, A.; Seda, M.; Calvet, J.; Gratacós, J.; Serrano, R.M.; Peña, P. SARS-CoV-2 Spike Protein Vaccine-Induced Immune Imprinting Reduces Nucleocapsid Protein Antibody Response in SARS-CoV-2 Infection. J. Immunol. Res. 2022, 2022, 8287087. [Google Scholar] [CrossRef] [PubMed]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID -19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- Kiblboeck, D.; Klingel, K.; Genger, M.; Traxler, S.; Braunsteiner, N.; Steinwender, C.; Kellermair, J. Myocarditis following mRNA COVID-19 vaccination: Call for endomyocardial biopsy. ESC Heart Fail. 2022, 9, 1996–2002. [Google Scholar] [CrossRef]
- WHO. Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Puliyel, J.; Naik, P. Revised World Health Organization (WHO)’s causality assessment of adverse events following immunization-a critique. F1000Research 2018, 7, 243. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized vaccinology: A review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Ferraresi, A.; Isidoro, C. Will Omics Biotechnologies Save Us from Future Pandemics? Lessons from COVID-19 for Vaccinomics and Adversomics. Biomedicines 2022, 11, 52. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R. Adversomics: The Emerging Field of Vaccine Adverse Event Immunogenetics. Pediatr. Infect. Dis. J. 2009, 28, 431–432. [Google Scholar] [CrossRef]
- Whitaker, J.A.; Ovsyannikova, I.G.; Poland, G.A. Adversomics: A new paradigm for vaccine safety and design. Expert Rev. Vaccines 2015, 14, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Poland, G.A.; Ovsyannikova, I.G.; Oberg, A.L.; Asmann, Y.W.; Grill, D.E.; Vierkant, R.A.; Jacobson, R.M. Impaired innate, humoral, and cellular immunity despite a take in smallpox vaccine recipients. Vaccine 2016, 34, 3283–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, W.L.; Salk, H.M.; Ovsyannikova, I.G.; Kennedy, R.B.; Poland, G.A. Cytokine production associated with smallpox vaccine responses. Immunotherapy 2014, 6, 1097–1112. [Google Scholar] [CrossRef]
- Ovsyannikova, I.G.; Pankratz, V.S.; Salk, H.M.; Kennedy, R.B.; Poland, G.A. HLA alleles associated with the adaptive immune response to smallpox vaccine: A replication study. Hum. Genet. 2014, 133, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, I.G.; Haralambieva, I.H.; Kennedy, R.B.; O’Byrne, M.M.; Pankratz, V.S.; Poland, G.A. Genetic variation in IL18R1 and IL18 genes and Inteferon gamma ELISPOT response to smallpox vaccination: An unexpected relationship. J. Infect. Dis. 2013, 208, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Lavie, C.J.; Henry, B.M.; Sanchis-Gomar, F. Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin. Chem. Lab. Med. 2020, 58, 1415–1422. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, M.; Xu, C.; Zhang, T.; Jia, Y.; Wang, T.; Zhu, B. Diagnostic Roles of Postmortem cTn I and cTn T in Cardiac Death with Special Regard to Myocardial Infarction: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 3351. [Google Scholar] [CrossRef]
- Pandey, P.; Rane, J.S.; Chatterjee, A.; Kumar, A.; Khan, R.; Prakash, A.; Ray, S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: An in silico study for drug development. J. Biomol. Struct. Dyn. 2020, 39, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Mahdian, S.; Ebrahim-Habibi, A.; Zarrabi, M. Drug repurposing using computational methods to identify therapeutic options for COVID-19. J. Diabetes Metab. Disord. 2020, 19, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Muchtaridi, M.; Fauzi, M.; Khairul Ikram, N.K.; Mohd, G.A.; Wahab, H.A. Natural Flavonoids as Potential Angiotensin-Converting Enzyme 2 Inhibitors for Anti-SARS-CoV-2. Molecules 2020, 25, 3980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.; Sun, Y.; Sui, X.; Zhu, T.; Wang, Q. A novel screening strategy of anti-SARS-CoV-2 drugs via blocking interaction between Spike RBD and ACE2. Environ. Int. 2020, 147, 106361. [Google Scholar] [CrossRef] [PubMed]
- Prasansuklab, A.; Theerasri, A.; Rangsinth, P.; Sillapachaiyaporn, C.; Chuchawankul, S.; Tencomnao, T. Anti-COVID-19 drug candidates: A review on potential biological activities of natural products in the management of new coronavirus infection. J. Tradit. Complement. Med. 2020, 11, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Vidoni, C.; Fuzimoto, A.; Ferraresi, A.; Isidoro, C. Targeting autophagy with natural products to prevent SARS-CoV-2 infection. J. Tradit. Complement. Med. 2022, 12, 55–68. [Google Scholar] [CrossRef]


| Molecular Mechanisms | Pathogenic Mechanisms | Possible Clinical Effects | Refs. |
|---|---|---|---|
| Spike-ACE2 | Platelet hyperreactivity and aggregation | Thrombosis | [116,144] |
| Spike-ACE2 | Human endothelial cell activation and pro-inflammatory phenotype | Inflammation, thrombosis | [95] |
| Spike-ACE2 | Inhibition of hematopoietic stem cells differentiation | Immunosuppression | [145] |
| Spike (S1)-ACE2 | Intratracheal S1 subunit of Spike protein in hACE2 transgenic mice that overexpress human ACE2 | Lung vascular permeability and lung injury | [146] |
| Spike-ACE2 | Mast cell activation | Lung inflammation and injury | [144] |
| Spike-ACE2 | Oxidative stress in pericytes, activation of nuclear factor-kappa-B signaling pathways | Encephalitis | [147] |
| Spike-ACE2 | Down-regulation of endothelial ACE2 and e-NOS, mitochondrial damage | Interstitial pneumonia | [148] |
| Spike-ACE2 | Decrease of type I interferons in lung primary cells | Severity of pneumonia | [149] |
| Spike (S1)-ACE2 | S1 subunit co-localized with caspase-3, ACE2, IL6, TNFα, and C5b-9 (mice brain endothelia) | Inflammation and neuropathology | [150] |
| Spike (S1)-ACE2 | S1 subunit elicits MEK/ERK pathway cell signaling in lung vascular cells. | Pulmonary vascular wall thickening, pulmonary hypertension | [92] |
| Spike-ACE2 | Decrease of taste buds of rat circumvallate papillae | Taste disorders | [151] |
| Spike-ACE2 | Loss of integrity of the human brain-blood barrier | Pro-inflammatory response on brain | [93,152,153,154,155] |
| Spike (S1)-ACE2 | Loss of integrity of human pulmonary arterial endothelial cells | Pro-inflammatory response on lung | [156] |
| Spike-sACE2-antibodies | Soluble ACE2 internalization and clearance | Hypertensive crisis, inflammation, bradykinin storm | [104,113] |
| Spike-CD147 | Cell signaling in human cardiac pericytes, secretion of cytokines, apoptosis | Cardiac microvascular damage | [157] |
| Spike-CD147 | Cell signaling in human platelets | Thrombosis, inflammation | [158] |
| Spike-PAF | Augmentation of in vitro PAF-induced platelet aggregation and stimulation of U-937 (myeloid lineage) PAF production | Inflammatory syndromes, long COVID-19 | [159] |
| Molecular mimicry | Cross-reaction of anti-Spike antibodies with pericardium | Pericarditis | [130,160] |
| Molecular mimicry | Cross-reaction of anti-Spike antibodies with thrombopoietin and with tropomyosin | Thrombocytopenia, myocarditis | [37,161] |
| Spike-autoantibody | Thyroid inflammation | Subacute thyroiditis | [162] |
| Spike-PF4 interaction | Generation of anti-PF4 antibodies and binding to platelet ACE2 | Thrombosis with thrombocytopenia | [163] |
| Anti-PF4 antibodies | Platelet activation and aggregation | Thrombosis with thrombocytopenia | [164,165] |
| Anti-idiotype | Anti-idiotype (Ab2) would bind to ACE2 and/or to neuropilin-1 | COVID-19-like symptoms | [134,136] |
| Gene expression | Decrease of ACE2 and increase of ACE | Inflammation, myocarditis | [166] |
| Spike-TLR4 | The S protein triggers TLRs and induces inflammatory cytokines | Worsening of inflammatory reactions | [167] |
| Immune imprinting | Vaccine immune memory against S protein of the original variant inhibits the response to new epitopes of SARS-CoV-2 | Increased susceptibility to COVID-19 variants | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellavite, P.; Ferraresi, A.; Isidoro, C. Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines 2023, 11, 451. https://doi.org/10.3390/biomedicines11020451
Bellavite P, Ferraresi A, Isidoro C. Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines. 2023; 11(2):451. https://doi.org/10.3390/biomedicines11020451
Chicago/Turabian StyleBellavite, Paolo, Alessandra Ferraresi, and Ciro Isidoro. 2023. "Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines" Biomedicines 11, no. 2: 451. https://doi.org/10.3390/biomedicines11020451
APA StyleBellavite, P., Ferraresi, A., & Isidoro, C. (2023). Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines, 11(2), 451. https://doi.org/10.3390/biomedicines11020451










