Cysteine Cathepsins as Therapeutic Targets in Immune Regulation and Immune Disorders
Abstract
1. Introduction
1.1. Cathepsins in Immune Regulation
1.2. Intracellular Cathepsin Signaling
1.3. Extracellular Cathepsin Signaling
2. Targeting Cysteine Cathepsins in Different Immune Pathologies
2.1. Inflammation and Autoimmune Diseases
| Cathepsin | Autoimmune Diseases | Inflammation |
|---|---|---|
| Cathepsin B | Apoptosis, inflammation, and infiltration of macrophages and TNF-α (+) cells to muscle tissue in polymyositis [66] | Stimulates production and secretion of IL-1β in microglia [59,60] |
| CatB contributes to degradation of Aβ, thus helping maintain neuronal homeostasis [61,62], and increases accumulation of Aβ in Alzheimer’s disease [64,65] | ||
| Cathepsin C | Excessive neutrophil infiltration in GPA [45] | Mediation of uncontrolled activity of NSPs [40,41] |
| Generation of proinflammatory chemokines [48] in activated microglia [47] that promote microglia M1 polarization and aggravate neuroinflammation [49] | ||
| Neuronal damage during inflammation or traumatic brain injury through stimulation of CCL2 and CXCL2 production from injured neurons and glia [51] | ||
| Cathepsin K | Excessive cleavage of main cartilage constituent type II collagen and elastin in RA, osteoarthritis and cardiovascular diseases [74,75], and | |
| bone resorption [79] | ||
| Cathepsin L | May contribute to inflammatory responses in microglia [55] | |
| Cathepsin S | MHC class II presentation of autoantigens [30] | |
| Autoantibody production and renal deposition of immune complexes in SLE-related nephritis [31] | ||
| Increased T-cell responses towards autoantigents in Sjögren’s syndrome and increased cytokine secretion by CD14+ monocytes [35] | ||
| Infiltration of proinflammatory immune cells [39] | ||
| Cathepsin X | Secretion from microglia and astrocytes both in culture and in vivo in response to neuronal damage and inflammation [67,68,69,70] | |
| Production of reactive oxygen species and proinflammatory cytokines [68] |
2.2. Role of Cysteine Peptidases in Antitumor Immune Response and Their Targeting
3. Challenges and the Future of Cathepsins as Therapeutic Targets
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Turk, D.; Turk, B. The Future of Cysteine Cathepsins in Disease Management. Trends Pharmacol. Sci. 2017, 38, 873–898. [Google Scholar] [CrossRef]
- Rossi, A.; Deveraux, Q.; Turk, B.; Sali, A. Comprehensive search for cysteine cathepsins in the human genome. Biol. Chem. 2004, 385, 363–372. [Google Scholar] [CrossRef]
- Therrien, C.; Lachance, P.; Sulea, T.; Purisima, E.O.; Qi, H.; Ziomek, E.; Alvarez-Hernandez, A.; Roush, W.R.; Ménard, R. Cathepsins X and B Can Be Differentiated through Their Respective Mono- and Dipeptidyl Carboxypeptidase Activities. Biochemistry 2001, 40, 2702–2711. [Google Scholar] [CrossRef]
- Musil, D.; Zucic, D.; Turk, D.; Engh, R.; Mayr, I.; Huber, R.; Popovic, T.; Turk, V.; Towatari, T.; Katunuma, N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: The structural basis for its specificity. EMBO J. 1991, 10, 2321–2330. [Google Scholar] [CrossRef]
- Kos, J.; Mitrović, A.; Nanut, M.P.; Pišlar, A. Lysosomal peptidases—Intriguing roles in cancer progression and neurodegeneration. FEBS Open Bio 2022, 12, 708–738. [Google Scholar] [CrossRef] [PubMed]
- Illy, C.; Quraishi, O.; Wang, J.; Purisima, E.; Vernet, T.; Mort, J.S. Role of the Occluding Loop in Cathepsin B Activity. J. Biol. Chem. 1997, 272, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, O.; Dolinar, M.; Turk, V.; Turk, B. Recombinant Human Cathepsin H Lacking the Mini Chain Is an Endopeptidase. Biochemistry 2003, 42, 13522–13528. [Google Scholar] [CrossRef]
- Neurath, H. Proteolytic enzymes, past and future. Proc. Natl. Acad. Sci. USA 1999, 96, 10962–10963. [Google Scholar] [CrossRef]
- Turk, D.; Gunčar, G.; Podobnik, M.; Turk, B. Revised Definition of Substrate Binding Sites of Papain-Like Cysteine Proteases. Biol. Chem. 1998, 379, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.; Guncar, G. Lysosomal cysteine proteases (cathepsins): Promising drug targets. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta BBA-Proteins Proteom. 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Kirschke, H. [41] Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981, 80, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Pišlar, A.; Nanut, M.P.; Kos, J. Lysosomal cysteine peptidases—Molecules signaling tumor cell death and survival. Semin. Cancer Biol. 2015, 35, 168–179. [Google Scholar] [CrossRef]
- Bright, N.A.; Davis, L.J.; Luzio, J.P. Endolysosomes Are the Principal Intracellular Sites of Acid Hydrolase Activity. Curr. Biol. 2016, 26, 2233–2245. [Google Scholar] [CrossRef]
- Creasy, B.M.; McCoy, K.L. Cytokines regulate cysteine cathepsins during TLR responses. Cell. Immunol. 2011, 267, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bird, P.I.; Trapani, J.; Villadangos, J. Endolysosomal proteases and their inhibitors in immunity. Nat. Rev. Immunol. 2009, 9, 871–882. [Google Scholar] [CrossRef]
- Brix, K.; Linke, M.; Tepel, C.; Herzog, V. Cysteine Proteinases Mediate Extracellular Prohormone Processing in the Thyroid. Biol. Chem. 2001, 382, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Authier, F.; Kouach, M.; Briand, G. Endosomal proteolysis of insulin-like growth factor-I at its C-terminal D-domain by cathepsin B. FEBS Lett. 2005, 579, 4309–4316. [Google Scholar] [CrossRef] [PubMed]
- Nanut, M.P.; Sabotič, J.; Jewett, A.; Kos, J. Cysteine Cathepsins as Regulators of the Cytotoxicity of NK and T Cells. Front. Immunol. 2014, 5, 616. [Google Scholar] [CrossRef]
- Droga-Mazovec, G.; Bojič, L.; Petelin, A.; Ivanova, S.; Romih, R.; Repnik, U.; Salvesen, G.S.; Stoka, V.; Turk, V.; Turk, B. Cysteine Cathepsins Trigger Caspase-dependent Cell Death through Cleavage of Bid and Antiapoptotic Bcl-2 Homologues. J. Biol. Chem. 2008, 283, 19140–19150. [Google Scholar] [CrossRef]
- Soond, S.M.; Kozhevnikova, M.V.; Frolova, A.S.; Savvateeva, L.V.; Plotnikov, E.Y.; Townsend, P.A.; Han, Y.-P.; Zamyatnin, A.A. Lost or Forgotten: The nuclear cathepsin protein isoforms in cancer. Cancer Lett. 2019, 462, 43–50. [Google Scholar] [CrossRef]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Olson, O.C.; Joyce, J.A. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 2015, 15, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Turk, B.; Bieth, J.G.; Björk, I.; Dolenc, I.; Turk, D.; Cimerman, N.; Kos, J.; Čolič, A.; Stoka, V.; Turk, V. Regulation of the Activity of Lysosomal Cysteine Proteinases by pH-Induced Inactivation and/or Endogenous Protein Inhibitors, Cystatins. Biol. Chem. Hoppe-Seyler 1995, 376, 225–230. [Google Scholar] [CrossRef]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Vizovišek, M.; Fonović, M.; Turk, B. Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond. Matrix Biol. 2019, 75–76, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, J.S.; Macleod, T.; McGonagle, D.; Brakefield, R.; Baron, J.M.; Alase, A.; Wittmann, M.; Stacey, M. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36γ. Proc. Natl. Acad. Sci. USA 2017, 114, E2748–E2757. [Google Scholar] [CrossRef] [PubMed]
- Breznik, B.; Motaln, H.; Turnšek, T.L. Proteases and cytokines as mediators of interactions between cancer and stromal cells in tumours. Biol. Chem. 2017, 398, 709–719. [Google Scholar] [CrossRef]
- Thanei, S.; Theron, M.; Silva, A.P.; Reis, B.; Branco, L.; Schirmbeck, L.; Kolb, F.A.; Haap, W.; Schindler, T.; Trendelenburg, M. Cathepsin S inhibition suppresses autoimmune-triggered inflammatory responses in macrophages. Biochem. Pharmacol. 2017, 146, 151–164. [Google Scholar] [CrossRef]
- Rupanagudi, K.V.; Kulkarni, O.P.; Lichtnekert, J.; Darisipudi, M.N.; Mulay, S.R.; Schott, B.; Gruner, S.; Haap, W.; Hartmann, G.; Anders, H.-J. Cathepsin S inhibition suppresses systemic lupus erythematosus and lupus nephritis because cathepsin S is essential for MHC class II-mediated CD4 T cell and B cell priming. Ann. Rheum. Dis. 2013, 74, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Baugh, M.; Black, D.; Westwood, P.; Kinghorn, E.; McGregor, K.; Bruin, J.; Hamilton, W.; Dempster, M.; Claxton, C.; Cai, J.; et al. Therapeutic dosing of an orally active, selective cathepsin S inhibitor suppresses disease in models of autoimmunity. J. Autoimmun. 2011, 36, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hamm-Alvarez, S.F.; Janga, S.R.; Edman, M.C.; Madrigal, S.; Shah, M.; Frousiakis, S.E.; Renduchintala, K.; Zhu, J.; Bricel, S.; Silka, K.; et al. Tear Cathepsin S as a Candidate Biomarker for Sjögren’s Syndrome. Arthritis Rheumatol. 2014, 66, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Janga, S.R.; Shah, M.; Ju, Y.; Meng, Z.; Edman, M.C.; Hamm-Alvarez, S.F. Longitudinal analysis of tear cathepsin S activity levels in male non-obese diabetic mice suggests its potential as an early stage biomarker of Sjögren’s Syndrome. Biomarkers 2018, 24, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, P.; Daoudlarian, D.; Theron, M.; Kolb, F.A.; Young, M.M.; Reis, B.; Tiaden, A.; Bannert, B.; Kyburz, D.; Manigold, T. Differential effects of specific cathepsin S inhibition in biocompartments from patients with primary Sjögren syndrome. Thromb. Haemost. 2019, 21, 175. [Google Scholar] [CrossRef]
- Klinngam, W.; Janga, S.R.; Lee, C.; Ju, Y.; Yarber, F.; Shah, M.; Guo, H.; Wang, D.; MacKay, J.A.; Edman, M.C.; et al. Inhibition of Cathepsin S Reduces Lacrimal Gland Inflammation and Increases Tear Flow in a Mouse Model of Sjögren’s Syndrome. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Theron, M.; Bentley, D.; Nagel, S.; Manchester, M.; Gerg, M.; Schindler, T.; Silva, A.; Ecabert, B.; Teixeira, P.C.; Perret, C.; et al. Pharmacodynamic Monitoring of RO5459072, a Small Molecule Inhibitor of Cathepsin S. Front. Immunol. 2017, 8, 806. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, R.K.; Dastidar, S.; Ray, A. Cysteine cathepsin S as an immunomodulatory target: Present and future trends. Expert Opin. Ther. Targets 2008, 12, 291–299. [Google Scholar] [CrossRef]
- Deschamps, K.; Cromlish, W.; Weicker, S.; Lamontagne, S.; Huszar, S.L.; Gauthier, J.Y.; Mudgett, J.S.; Guimond, A.; Romand, R.; Frossard, N.; et al. Genetic and Pharmacological Evaluation of Cathepsin S in a Mouse Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 45, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil Elastase, Proteinase 3, and Cathepsin G as Therapeutic Targets in Human Diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Perera, N.C.; Schilling, O.; Kittel, H.; Back, W.; Kremmer, E.; Jenne, D.E. NSP4, an elastase-related protease in human neutrophils with arginine specificity. Proc. Natl. Acad. Sci. USA 2012, 109, 6229–6234. [Google Scholar] [CrossRef] [PubMed]
- Garwicz, D.; Lindmark, A.; Persson, A.-M.; Gullberg, U. On the Role of the Proform-Conformation for Processing and Intracellular Sorting of Human Cathepsin G. Blood 1998, 92, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.B.; Chen, X.; Zhang, Z.Y.; Wu, F.F.; Liu, X.H. Cathepsin C inhibitors as anti-inflammatory drug discovery: Challenges and opportunities. Eur. J. Med. Chem. 2021, 225, 113818. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Lesner, A.; Marchand-Adam, S.; Moss, C.; Jenne, D.E. Lung Protection by Cathepsin C Inhibition: A New Hope for COVID-19 and ARDS? J. Med. Chem. 2020, 63, 13258–13265. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Pham, C.T.; Hu, Y.; Schneider, W.; Luft, F.C.; Kettritz, R. Neutrophil Serine Proteases Promote IL-1β Generation and Injury in Necrotizing Crescentic Glomerulonephritis. J. Am. Soc. Nephrol. 2012, 23, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Lesner, A.; Letast, S.; Mahdi, Y.K.; Jourdan, M.-L.; Dallet-Choisy, S.; Marchand-Adam, S.; Kellenberger, C.; Viaud-Massuard, M.-C.; Jenne, D.E.; et al. Neutrophil proteinase 3 and dipeptidyl peptidase I (cathepsin C) as pharmacological targets in granulomatosis with polyangiitis (Wegener granulomatosis). Semin. Immunopathol. 2013, 35, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, N.; Zhang, Y.; Hou, C.; Yang, X.; Shimizu, T.; Wang, X.; Ikenaka, K.; Fan, K.; Ma, J. Disinhibition of Cathepsin C Caused by Cystatin F Deficiency Aggravates the Demyelination in a Cuprizone Model. Front. Mol. Neurosci. 2016, 9, 152. [Google Scholar] [CrossRef]
- Fan, K.; Wu, X.; Fan, B.; Li, N.; Lin, Y.; Yao, Y.; Ma, J. Up-regulation of microglial cathepsin C expression and activity in lipopolysaccharide-induced neuroinflammation. J. Neuroinflamm. 2012, 9, 96. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, S.; Liu, Y.; Yang, X.; Liu, G.; Shimizu, T.; Ikenaka, K.; Fan, K.; Ma, J. Cathepsin C Promotes Microglia M1 Polarization and Aggravates Neuroinflammation via Activation of Ca2+-Dependent PKC/P38MAPK/NF-ΚB Pathway. J. Neuroinflamm. 2019, 16, 1–18. [Google Scholar] [CrossRef]
- Shimizu, T.; Wisessmith, W.; Li, J.; Abe, M.; Sakimura, K.; Chetsawang, B.; Sahara, Y.; Tohyama, K.; Tanaka, K.F.; Ikenaka, K. The balance between cathepsin C and cystatin F controls remyelination in the brain of Plp1-overexpressing mouse, a chronic demyelinating disease model. Glia 2017, 65, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Yang, X.; Liu, Y.; Liu, G.; Fan, K.; Ma, J. Cathepsin C aggravates neuroinflammation via promoting production of CCL2 and CXCL2 in glial cells and neurons in a cryogenic brain lesion. Neurochem. Int. 2021, 148, 105107. [Google Scholar] [CrossRef]
- Schurigt, U.; Sevenich, L.; Vannier, C.; Gajda, M.; Schwinde, A.; Werner, F.; Stahl, A.; von Elverfeldt, D.; Becker, A.K.; Bogyo, M.; et al. Trial of the Cysteine Cathepsin Inhibitor JPM-OEt on Early and Advanced Mammary Cancer Stages in the MMTV-PyMT-Transgenic Mouse Model. Biol. Chem. 2008, 389, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Méthot, N.; Guay, D.; Rubin, J.; Ethier, D.; Ortega, K.; Wong, S.; Normandin, D.; Beaulieu, C.; Reddy, T.J.; Riendeau, D.; et al. In Vivo Inhibition of Serine Protease Processing Requires a High Fractional Inhibition of Cathepsin C. Mol. Pharmacol. 2008, 73, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Haworth, C.S.; Metersky, M.L.; Loebinger, M.R.; Blasi, F.; Sibila, O.; O’Donnell, A.E.; Sullivan, E.J.; Mange, K.C.; Fernandez, C.; et al. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N. Engl. J. Med. 2020, 383, 2127–2137. [Google Scholar] [CrossRef]
- Liu, J.; Hong, Z.; Ding, J.; Liu, J.; Zhang, J.; Chen, S. Predominant Release of Lysosomal Enzymes by Newborn Rat Microglia After LPS Treatment Revealed by Proteomic Studies. J. Proteome Res. 2008, 7, 2033–2049. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, H.; Yang, X.; Qian, Y.; Xiao, Q. Inhibition of cathepsin L alleviates the microglia-mediated neuroinflammatory responses through caspase-8 and NF-κB pathways. Neurobiol. Aging 2018, 62, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bouras, M.; Asehnoune, K.; Roquilly, A. Immune modulation after traumatic brain injury. Front. Med. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef]
- Chen, N.; Ou, Z.; Zhang, W.; Zhu, X.; Li, P.; Gong, J. Cathepsin B regulates non-canonical NLRP3 inflammasome pathway by modulating activation of caspase-11 in Kupffer cells. Cell Prolif. 2018, 51, e12487. [Google Scholar] [CrossRef]
- Cappellano, G.; Carecchio, M.; Fleetwood, T.; Magistrelli, L.; Cantello, R.; Dianzani, U.; Comi, C. Immunity and inflammation in neurodegenerative diseases. Am. J. Neurodegener. Dis. 2013, 2, 89–107. [Google Scholar] [PubMed]
- Comi, C.; Tondo, G. Insights into the protective role of immunity in neurodegenerative disease. Neural Regen. Res. 2017, 12, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regen. Res. 2020, 15, 25–29. [Google Scholar] [CrossRef]
- Oberstein, T.J.; Utz, J.; Spitzer, P.; Klafki, H.W.; Wiltfang, J.; Lewczuk, P.; Kornhuber, J.; Maler, J.M. The Role of Cathepsin B in the Degradation of Aβ and in the Production of Aβ Peptides Starting With Ala2 in Cultured Astrocytes. Front. Mol. Neurosci. 2021, 13, 615740. [Google Scholar] [CrossRef] [PubMed]
- Embury, C.M.; Dyavarshetty, B.; Lu, Y.; Wiederin, J.L.; Ciborowski, P.; Gendelman, H.E.; Kiyota, T. Cathepsin B Improves ß-Amyloidosis and Learning and Memory in Models of Alzheimer’s Disease. J. Neuroimmune Pharmacol. 2016, 12, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ni, L.; Wang, Q. Administration of cathepsin B inhibitor CA-074Me reduces inflammation and apoptosis in polymyositis. J. Dermatol. Sci. 2013, 72, 158–167. [Google Scholar] [CrossRef]
- Greco, T.; Seeholzer, S.H.; Mak, A.; Spruce, L.; Ischiropoulos, H. Quantitative Mass Spectrometry-based Proteomics Reveals the Dynamic Range of Primary Mouse Astrocyte Protein Secretion. J. Proteome Res. 2010, 9, 2764–2774. [Google Scholar] [CrossRef]
- Pišlar, A.; Božić, B.; Zidar, N.; Kos, J. Inhibition of cathepsin X reduces the strength of microglial-mediated neuroinflammation. Neuropharmacology 2017, 114, 88–100. [Google Scholar] [CrossRef]
- Wendt, W.; Schulten, R.; Stichel, C.C.; Luebbert, H. Intra- versus extracellular effects of microglia-derived cysteine proteases in a conditioned medium transfer model. J. Neurochem. 2009, 110, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Glanzer, J.G.; Enose, Y.; Wang, T.; Kadiu, I.; Gong, N.; Rozek, W.; Liu, J.; Schlautman, J.D.; Ciborowski, P.S.; Thomas, M.P.; et al. Genomic and proteomic microglial profiling: Pathways for neuroprotective inflammatory responses following nerve fragment clearance and activation. J. Neurochem. 2007, 102, 627–645. [Google Scholar] [CrossRef]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaissé, J.-M. The Collagenolytic Activity of Cathepsin K Is Unique among Mammalian Proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef] [PubMed]
- Aguda, A.H.; Panwar, P.; Du, X.; Nguyen, N.T.; Brayer, G.D.; Brömme, D. Structural basis of collagen fiber degradation by cathepsin K. Proc. Natl. Acad. Sci. USA 2014, 111, 17474–17479. [Google Scholar] [CrossRef] [PubMed]
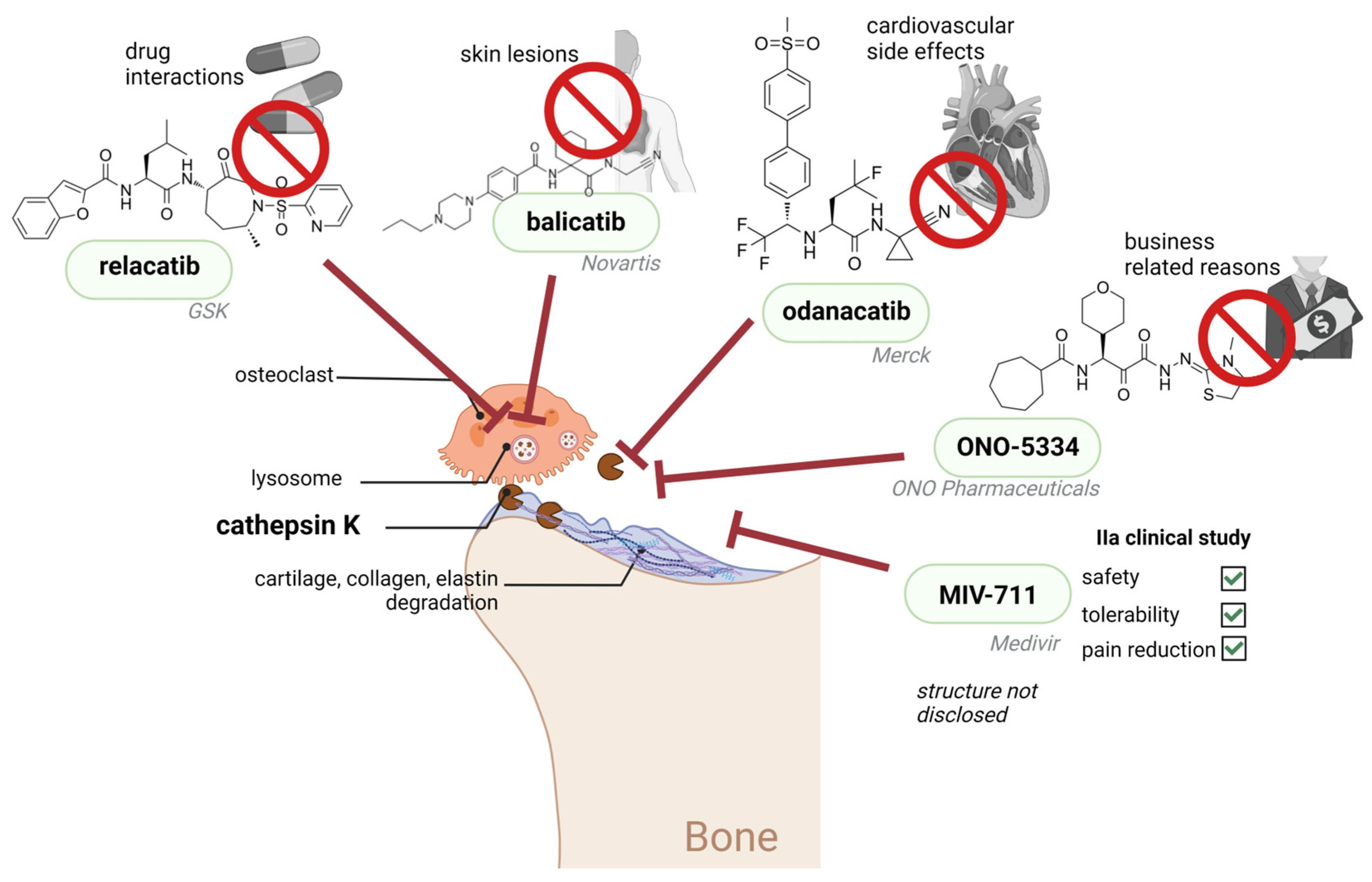
- Brömme, D.; Lecaille, F. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opin. Investig. Drugs 2009, 18, 585–600. [Google Scholar] [CrossRef]
- Sun, J.; Sukhova, G.K.; Zhang, J.; Chen, H.; Sjöberg, S.; Libby, P.; Xia, M.; Xiong, N.; Gelb, B.D.; Shi, G.-P. Cathepsin K Deficiency Reduces Elastase Perfusion–Induced Abdominal Aortic Aneurysms in Mice. Arter. Thromb. Vasc. Biol. 2012, 32, 15–23. [Google Scholar] [CrossRef]
- Sukhova, G.K.; Shi, G.P.; Simon, D.I.; Chapman, H.A.; Libby, P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J. Clin. Investig. 1998, 102, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, S.; Massé, F.; Percival, M.D. Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: A comparison of available tools. Biol. Chem. 2009, 390, 941–948. [Google Scholar] [CrossRef]
- Marquis, R.W.; Ru, Y.; Zeng, J.; Trout, R.E.L.; LoCastro, S.M.; Gribble, A.D.; Witherington, J.; Fenwick, A.E.; Garnier, B.; Tomaszek, T.; et al. Cyclic Ketone Inhibitors of the Cysteine Protease Cathepsin K. J. Med. Chem. 2001, 44, 725–736. [Google Scholar] [CrossRef]
- Stroup, G.B.; Lark, M.W.; Veber, D.F.; Bhattacharyya, A.; Blake, S.; Dare, L.C.; Erhard, K.F.; Hoffman, S.J.; James, I.E.; Marquis, R.W.; et al. Potent and Selective Inhibition of Human Cathepsin K Leads to Inhibition of Bone Resorption In Vivo in a Nonhuman Primate. J. Bone Miner. Res. 2001, 16, 1739–1746. [Google Scholar] [CrossRef]
- Bone, H.G.; McClung, M.; Roux, C.; Recker, R.R.; Eisman, J.A.; Verbruggen, N.; Hustad, C.M.; DaSilva, C.; Santora, A.C.; Ince, B.A. Odanacatib, a Cathepsin-K Inhibitor for Osteoporosis: A Two-Year Study in Postmenopausal Women With Low Bone Density. J. Bone Miner. Res. 2009, 25, 937–941. [Google Scholar] [CrossRef]
- Chapurlat, R.D. Odanacatib: A review of its potential in the management of osteoporosis in postmenopausal women. Ther. Adv. Musculoskelet. Dis. 2015, 7, 103–109. [Google Scholar] [CrossRef]
- Mullard, A. Merck & Co. drops osteoporosis drug odanacatib. Nat. Rev. Drug Discov. 2016, 15, 669. [Google Scholar] [CrossRef] [PubMed]
- Brömme, D.; Panwar, P.; Turan, S. Cathepsin K osteoporosis trials, pycnodysostosis and mouse deficiency models: Commonalities and differences. Expert Opin. Drug Discov. 2016, 11, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Godat, E.; Lecaille, F.; Desmazes, C.; Duchêne, S.; Weidauer, E.; Saftig, P.; Brömme, D.; Vandier, C.; Lalmanach, G. Cathepsin K: A cysteine protease with unique kinin-degrading properties. Biochem. J. 2004, 383, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef]
- Asagiri, M.; Hirai, T.; Kunigami, T.; Kamano, S.; Gober, H.-J.; Okamoto, K.; Nishikawa, K.; Latz, E.; Golenbock, D.T.; Aoki, K.; et al. Cathepsin K-Dependent Toll-Like Receptor 9 Signaling Revealed in Experimental Arthritis. Science 2008, 319, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Søe, K.; Guido, R.V.C.; Bueno, R.V.C.; Delaisse, J.-M.; Brömme, D. A novel approach to inhibit bone resorption: Exosite inhibitors against cathepsin K. Br. J. Pharmacol. 2015, 173, 396–410. [Google Scholar] [CrossRef]
- Sharma, V.; Panwar, P.; O’Donoghue, A.J.; Cui, H.; Guido, R.V.C.; Craik, C.S.; Brömme, D. Structural requirements for the collagenase and elastase activity of cathepsin K and its selective inhibition by an exosite inhibitor. Biochem. J. 2014, 465, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Novinec, M.; Korenč, M.; Caflisch, A.; Ranganathan, R.; Lenarčič, B.; Baici, A. A novel allosteric mechanism in the cysteine peptidase cathepsin K discovered by computational methods. Nat. Commun. 2014, 5, 3287. [Google Scholar] [CrossRef]
- Zhang, T.; Maekawa, Y.; Hanba, J.; Dainichi, T.; Nashed, B.F.; Hisaeda, H.; Sakai, T.; Asao, T.; Himeno, K.; Good, R.A.; et al. Lysosomal cathepsin B plays an important role in antigen processing, while cathepsin D is involved in degradation of the invariant chain in ovalbumin-immunized mice. Immunology 2000, 100, 13–20. [Google Scholar] [CrossRef]
- Byrne, S.M.; Aucher, A.; Alyahya, S.; Elder, M.; Olson, S.T.; Davis, D.M.; Ashton-Rickardt, P.G. Cathepsin B Controls the Persistence of Memory CD8+ T Lymphocytes. J. Immunol. 2012, 189, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, X.; Liu, W.; Chen, S.; Yang, C.; Yang, J. CTSB is a negative prognostic biomarker and therapeutic target associated with immune cells infiltration and immunosuppression in gliomas. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Komura, T.; Takabatake, H.; Harada, K.; Yamato, M.; Miyazawa, M.; Yoshida, K.; Honda, M.; Wada, T.; Kitagawa, H.; Ohta, T.; et al. Clinical features of cystatin A expression in patients with pancreatic ductal adenocarcinoma. Cancer Sci. 2017, 108, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shim, M.K.; Yang, S.; Hwang, H.S.; Cho, H.; Kim, J.; Yun, W.S.; Moon, Y.; Kim, J.; Yoon, H.Y.; et al. Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. ACS Nano 2021, 15, 12086–12098. [Google Scholar] [CrossRef]
- Yang, S.; Shim, M.K.; Kim, W.J.; Choi, J.; Nam, G.-H.; Kim, J.; Kim, J.; Moon, Y.; Kim, H.Y.; Park, J.; et al. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials 2021, 272, 120791. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Shim, M.K.; Choi, J.; Yang, S.; Kim, J.; Yun, W.S.; Cho, H.; Park, J.Y.; Kim, Y.; Seong, J.-K.; et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics 2022, 12, 1999–2014. [Google Scholar] [CrossRef]
- Zhang, C.; He, S.; Zeng, Z.; Cheng, P.; Pu, K. Smart Nano-PROTACs Reprogram Tumor Microenvironment for Activatable Photo-metabolic Cancer Immunotherapy. Angew. Chem. Int. Ed. 2021, 61, e202114957. [Google Scholar] [CrossRef]
- Dai, J.; Hu, J.; Dong, X.; Chen, B.; Dong, X.; Liu, R.; Xia, F.; Lou, X. Deep Downregulation of PD-L1 by Caged Peptide-Conjugated AIEgen/miR-140 Nanoparticles for Enhanced Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202117798. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Hostetter, D.R.; Moore, S.J.; Winter, M.B. The multifaceted roles of tumor-associated proteases and harnessing their activity for prodrug activation. Biol. Chem. 2018, 400, 965–977. [Google Scholar] [CrossRef]
- Bruchard, M.; Mignot, G.; Derangère, V.; Chalmin, F.; Chevriaux, A.; Vegran, F.; Boireau, W.; Simon, B.; Ryffel, B.; Connat, J.L.; et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2012, 19, 57–64. [Google Scholar] [CrossRef]
- Mezzasoma, L.; Costanzi, E.; Scarpelli, P.; Talesa, V.N.; Bellezza, I. Extracellular Vesicles from Human Advanced-Stage Prostate Cancer Cells Modify the Inflammatory Response of Microenvironment-Residing Cells. Cancers 2019, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.; Lipsky, P.; Thiele, D. Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J. Biol. Chem. 1993, 268, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, M.; Bird, P.I.; Peters, C.; Reinheckel, T.; Trapani, J.; Sutton, V.R. Cathepsin H Is an Additional Convertase of Pro-granzyme B. J. Biol. Chem. 2010, 285, 20514–20519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Cong, M.; Li, J.; He, D.; Wu, Q.; Tian, P.; Wang, Y.; Yang, S.; Liang, C.; Liang, Y.; et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021, 39, 423–437.e7. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, X.; Cheng, W.; Wu, Y.; Liu, M.; Liu, R.; Zhang, S.; Xia, H.; Liu, H.; Tai, X.; et al. Expression signature, prognosis value and immune characteristics of cathepsin F in non-small cell lung cancer identified by bioinformatics assessment. BMC Pulm. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Sun, Z.; Xia, W.; Lyu, Y.; Song, Y.; Wang, M.; Zhang, R.; Sui, G.; Li, Z.; Song, L.; Wu, C.; et al. Immune-related gene expression signatures in colorectal cancer. Oncol. Lett. 2021, 22, 543. [Google Scholar]
- Herroon, M.K.; Rajagurubandara, E.; Rudy, D.L.; Chalasani, A.; Hardaway, A.L.; Podgorski, I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene 2012, 32, 1580–1593. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef]
- Konjar, Š.; Sutton, V.R.; Hoves, S.; Repnik, U.; Yagita, H.; Reinheckel, T.; Peters, C.; Turk, V.; Turk, B.; Trapani, J.A.; et al. Human and mouse perforin are processed in part through cleavage by the lysosomal cysteine proteinase cathepsin L. Immunology 2010, 131, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, N.; Mills, L.; Jean, D.; Tellez, C.; Bar-Eli, M.; Frade, R. Inhibition of Tumorigenicity and Metastasis of Human Melanoma Cells by Anti-Cathepsin L Single Chain Variable Fragment. Cancer Res 2004, 64, 146–151. [Google Scholar] [CrossRef]
- Ohno, Y.; Kitamura, H.; Takahashi, N.; Ohtake, J.; Kaneumi, S.; Sumida, K.; Homma, S.; Kawamura, H.; Minagawa, N.; Shibasaki, S.; et al. IL-6 down-regulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4+ T cells. Cancer Immunol. Immunother. 2016, 65, 193–204. [Google Scholar] [CrossRef]
- Jakoš, T.; Pišlar, A.; Fonović, U.P.; Švajger, U.; Kos, J. Cysteine cathepsins L and X differentially modulate interactions between myeloid-derived suppressor cells and tumor cells. Cancer Immunol. Immunother. 2020, 69, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Shao, J.; Qin, Y.; Ji, Q.; Zhang, X.; Du, J. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol. Cancer 2014, 13, 43. [Google Scholar] [CrossRef]
- Jakoš, T.; Pišlar, A.; Jewett, A.; Kos, J. Cysteine Cathepsins in Tumor-Associated Immune Cells. Front. Immunol. 2019, 10, 2037. [Google Scholar] [CrossRef]
- Kos, J.; Sekirnik, A.; Kopitar, G.; Cimerman, N.; Kayser, K.; Stremmer, A.; Fiehn, W.; Werle, B. Cathepsin S in tumours, regional lymph nodes and sera of patients with lung cancer: Relation to prognosis. Br. J. Cancer 2001, 85, 1193–1200. [Google Scholar] [CrossRef]
- Liu, W.-L.; Liu, D.; Cheng, K.; Liu, Y.-J.; Xing, S.; Chi, P.-D.; Liu, X.-H.; Xue, N.; Lai, Y.-Z.; Guo, L.; et al. Evaluating the diagnostic and prognostic value of circulating cathepsin S in gastric cancer. Oncotarget 2016, 7, 28124–28138. [Google Scholar] [CrossRef]
- Yixuan, Y.; Kiat, L.S.; Yee, C.L.; Huiyin, L.; Yunhao, C.; Kuan, C.P.; Hassan, A.; Ting, W.T.; Manuel, S.-T.; Guan, Y.K.; et al. Cathepsin S Mediates Gastric Cancer Cell Migration and Invasion via a Putative Network of Metastasis-Associated Proteins. J. Proteome Res. 2010, 9, 4767–4778. [Google Scholar] [CrossRef]
- Bararia, D.; Hildebrand, J.A.; Stolz, S.; Haebe, S.; Alig, S.; Trevisani, C.P.; Osorio-Barrios, F.; Bartoschek, M.D.; Mentz, M.; Pastore, A.; et al. Cathepsin S Alterations Induce a Tumor-Promoting Immune Microenvironment in Follicular Lymphoma. Cell Rep. 2020, 31, 107522. [Google Scholar] [CrossRef] [PubMed]
- Bratovš, A.; Kramer, L.; Mikhaylov, G.; Vasiljeva, O.; Turk, B. Stefin A-functionalized liposomes as a system for cathepsins S and L-targeted drug delivery. Biochimie 2019, 166, 94–102. [Google Scholar] [CrossRef]
- Lecaille, F.; Chazeirat, T.; Saidi, A.; Lalmanach, G. Cathepsin V: Molecular characteristics and significance in health and disease. Mol. Asp. Med. 2022, 88, 101086. [Google Scholar] [CrossRef]
- Maher, K.; Konjar, S.; Watts, C.; Turk, B.; Kopitar-Jerala, N. Cystatin F regulates proteinase activity in IL-2-activated natural killer cells. Protein Pept. Lett. 2014, 21, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, Y.; Zhang, Y.; Jiang, S.; Li, X.; Wan, J. Identification of prognostic immune-related genes in the tumor microenvironment of endometrial cancer. Aging 2020, 12, 3371–3387. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Obermajer, N.; Doljak, B.; Turk, S.; Gobec, S.; Švajger, U.; Hailfinger, S.; Thome, M.; Kos, J. Cathepsin X cleavage of the Beta2 integrin regulates talin-binding and LFA-1 affinity in T cells. J. Leukoc. Biol. 2011, 90, 99–109. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Obermajer, N.; Kos, J. LFA-1 fine-tuning by cathepsin X. IUBMB Life 2011, 63, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Jevnikar, Z.; Obermajer, N.; Bogyo, M.; Kos, J. The role of cathepsin X in the migration and invasiveness of T lymphocytes. J. Cell Sci. 2008, 121, 2652–2661. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Obermajer, N.; Kos, J. Cysteine protease-mediated cytoskeleton interactions with LFA-1 promote T-cell morphological changes. Cell Motil. Cytoskelet. 2009, 66, 1030–1040. [Google Scholar] [CrossRef]
- Jakoš, T.; Prunk, M.; Pišlar, A.; Kos, J. Cathepsin X Activity Does Not Affect NK-Target Cell Synapse but Is Rather Distributed to Cytotoxic Granules. Int. J. Mol. Sci. 2021, 22, 13495. [Google Scholar] [CrossRef]
- Lechner, A.M.; Assfalg-Machleidt, I.; Zahler, S.; Stoeckelhuber, M.; Machleidt, W.; Jochum, M.; Nägler, D.K. RGD-dependent Binding of Procathepsin X to Integrin αvβ3 Mediates Cell-adhesive Properties. J. Biol. Chem. 2006, 281, 39588–39597. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.; Fruth, M.; Bunsen, T.; Nägler, D.K. IGF-I receptor phosphorylation is impaired in cathepsin X-deficient prostate cancer cells. Biol. Chem. 2012, 393, 1457–1462. [Google Scholar] [CrossRef]
- Obermajer, N.; Premzl, A.; Bergant, T.Z.; Turk, B.; Kos, J. Carboxypeptidase cathepsin X mediates β2-integrin-dependent adhesion of differentiated U-937 cells. Exp. Cell Res. 2006, 312, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, A.; Fonović, U.P.; Kos, J. Cysteine cathepsins B and X promote epithelial-mesenchymal transition of tumor cells. Eur. J. Cell Biol. 2017, 96, 622–631. [Google Scholar] [CrossRef]
- Obermajer, N.; Švajger, U.; Bogyo, M.; Jeras, M.; Kos, J. Maturation of dendritic cells depends on proteolytic cleavage by cathepsin X. J. Leukoc. Biol. 2008, 84, 1306–1315. [Google Scholar] [CrossRef]
- Fonovic, U.P.; Jevnikar, Z.; Rojnik, M.; Doljak, B.; Fonović, M.; Jamnik, P.; Kos, J. Profilin 1 as a Target for Cathepsin X Activity in Tumor Cells. PLoS ONE 2013, 8, e53918. [Google Scholar] [CrossRef]
- Teller, A.; Jechorek, D.; Hartig, R.; Adolf, D.; Reißig, K.; Roessner, A.; Franke, S. Dysregulation of apoptotic signaling pathways by interaction of RPLP0 and cathepsin X/Z in gastric cancer. Pathol.-Res. Pr. 2015, 211, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Balaji, K.N.; Schaschke, N.; Machleidt, W.; Catalfamo, M.; Henkart, P.A. Surface Cathepsin B Protects Cytotoxic Lymphocytes from Self-destruction after Degranulation. J. Exp. Med. 2002, 196, 493–503. [Google Scholar] [CrossRef]
- Baran, K.; Ciccone, A.; Peters, C.; Yagita, H.; Bird, P.; Villadangos, J.; Trapani, J.A. Cytotoxic T Lymphocytes from Cathepsin B-deficient Mice Survive Normally in Vitro and in Vivo after Encountering and Killing Target Cells. J. Biol. Chem. 2006, 281, 30485–30491. [Google Scholar] [CrossRef]
- Magister, Š.; Tseng, H.-C.; Bui, V.T.; Kos, J.; Jewett, A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget 2015, 6, 22310–22327. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Tseng, H.-C.; Arasteh, A.; Saadat, S.; Christensen, R.E.; Cacalano, N.A. Natural Killer Cells Preferentially Target Cancer Stem Cells; Role of Monocytes in Protection Against NK Cell Mediated Lysis of Cancer Stem Cells. Curr. Drug Deliv. 2012, 9, 5–16. [Google Scholar] [CrossRef]
- Grossenbacher, S.K.; Canter, R.J.; Murphy, W.J. Natural killer cell immunotherapy to target stem-like tumor cells. J. Immunother. Cancer 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Pallmer, K.; Oxenius, A. Recognition and Regulation of T Cells by NK Cells. Front. Immunol. 2016, 7, 251. [Google Scholar] [CrossRef]
- Jewett, A.; Kos, J.; Kaur, K.; Turnsek, T.L.; Breznik, B.; Senjor, E.; Wong, P.; Nguyen, K.Y.; Ko, M.-W. Multiple Defects of Natural Killer Cells in Cancer Patients: Anarchy, Dysregulated Systemic Immunity, and Immunosuppression in Metastatic Cancer. Crit. Rev. Immunol. 2020, 40, 93–133. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.; Christensen, I.; Nielsen, H.; Srensen, S.; Brünner, N.; Kos, J. Serum cathepsin H as a potential prognostic marker in patients with colorectal cancer. Int. J. Biol. Markers 2004, 19, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.Z.; Del Guzzo, C.A.; Shao, A.; Cho, J.; Du, R.; Cohen, A.O.; Owens, D.M. The CD200–CD200R Axis Promotes Squamous Cell Carcinoma Metastasis via Regulation of Cathepsin K. Cancer Res 2021, 81, 5021–5032. [Google Scholar] [CrossRef]
- Burden, R.E.; Gormley, J.A.; Jaquin, T.J.; Small, D.M.; Quinn, D.J.; Hegarty, S.M.; Ward, C.; Walker, B.; Johnston, J.A.; Olwill, S.A.; et al. Antibody-Mediated Inhibition of Cathepsin S Blocks Colorectal Tumor Invasion and Angiogenesis. Clin. Cancer Res. 2009, 15, 6042–6051. [Google Scholar] [CrossRef]
- Kwok, H.F.; Buick, R.J.; Kuehn, D.; Gormley, J.A.; Doherty, D.; Jaquin, T.J.; McClurg, A.; Ward, C.; Byrne, T.; Jaworski, J.; et al. Antibody targeting of Cathepsin S induces antibody-dependent cellular cytotoxicity. Mol. Cancer 2011, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wu, C.; Chen, T.; Santos, M.M.; Liu, C.-L.; Yang, C.; Zhang, L.; Ren, J.; Liao, S.; Guo, H.; et al. Cathepsin S inhibition changes regulatory T-cell activity in regulating bladder cancer and immune cell proliferation and apoptosis. Mol. Immunol. 2017, 82, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Dheilly, E.; Battistello, E.; Katanayeva, N.; Sungalee, S.; Michaux, J.; Duns, G.; Wehrle, S.; Sordet-Dessimoz, J.; Mina, M.; Racle, J.; et al. Cathepsin S Regulates Antigen Processing and T Cell Activity in Non-Hodgkin Lymphoma. Cancer Cell 2020, 37, 674–689.e12. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, N.; Meta, M.; Schuppan, D.; Nuhn, L.; Schirmeister, T. Novel Opportunities for Cathepsin S Inhibitors in Cancer Immunotherapy by Nanocarrier-Mediated Delivery. Cells 2020, 9, 2021. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, A.; Senjor, E.; Jukić, M.; Bolčina, L.; Prunk, M.; Proj, M.; Nanut, M.P.; Gobec, S.; Kos, J. New inhibitors of cathepsin V impair tumor cell proliferation and elastin degradation and increase immune cell cytotoxicity. Comput. Struct. Biotechnol. J. 2022, 20, 4667–4687. [Google Scholar] [CrossRef]
- Brown, J.; Matutes, E.; Singleton, A.; Price, C.; Molgaard, H.; Buttle, D.; Enver, T. Lymphopain, a cytotoxic T and natural killer cell-associated cysteine proteinase. Leukemia 1998, 12, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Linnevers, C.; Smeekens, S.; Brömme, D. Human cathepsin W, a putative cysteine protease predominantly expressed in CD8+T-lymphocytes. FEBS Lett. 1997, 405, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Wex, T.; Bühling, F.; Wex, H.; Günther, D.; Malfertheiner, P.; Weber, E.; Brömme, D. Human Cathepsin W, a Cysteine Protease Predominantly Expressed in NK Cells, Is Mainly Localized in the Endoplasmic Reticulum. J. Immunol. 2001, 167, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Ondr, J.K.; Pham, C.T.N. Characterization of Murine Cathepsin W and Its Role in Cell-mediated Cytotoxicity. J. Biol. Chem. 2004, 279, 27525–27533. [Google Scholar] [CrossRef] [PubMed]
- Wex, T.; Wex, H.; Hartig, R.; Wilhelmsen, S.; Malfertheiner, P. Functional involvement of cathepsin W in the cytotoxic activity of NK-92 cells. FEBS Lett. 2003, 552, 115–119. [Google Scholar] [CrossRef]
- Liu, X.-C.; Liang, H.; Tian, Z.; Ruan, Y.-S.; Zhang, L.; Chen, Y. Proteomic analysis of human NK-92 cells after NK cell-mediated cytotoxicity against K562 cells. Biochem. (Moscow) 2007, 72, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, C.; Gouttefangeas, C.; Hammer, M.; Weber, E.; Melms, A.; Tolosa, E. Cathepsin W expressed exclusively in CD8+ T cells and NK cells, is secreted during target cell killing but is not essential for cytotoxicity in human CTLs. Exp. Hematol. 2009, 37, 266–275. [Google Scholar] [CrossRef]
- Mitrović, A.; Završnik, J.; Mikhaylov, G.; Knez, D.; Fonović, U.P.; Štefin, P.M.; Butinar, M.; Gobec, S.; Turk, B.; Kos, J. Evaluation of novel cathepsin-X inhibitors in vitro and in vivo and their ability to improve cathepsin-B-directed antitumor therapy. Cell. Mol. Life Sci. 2022, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]

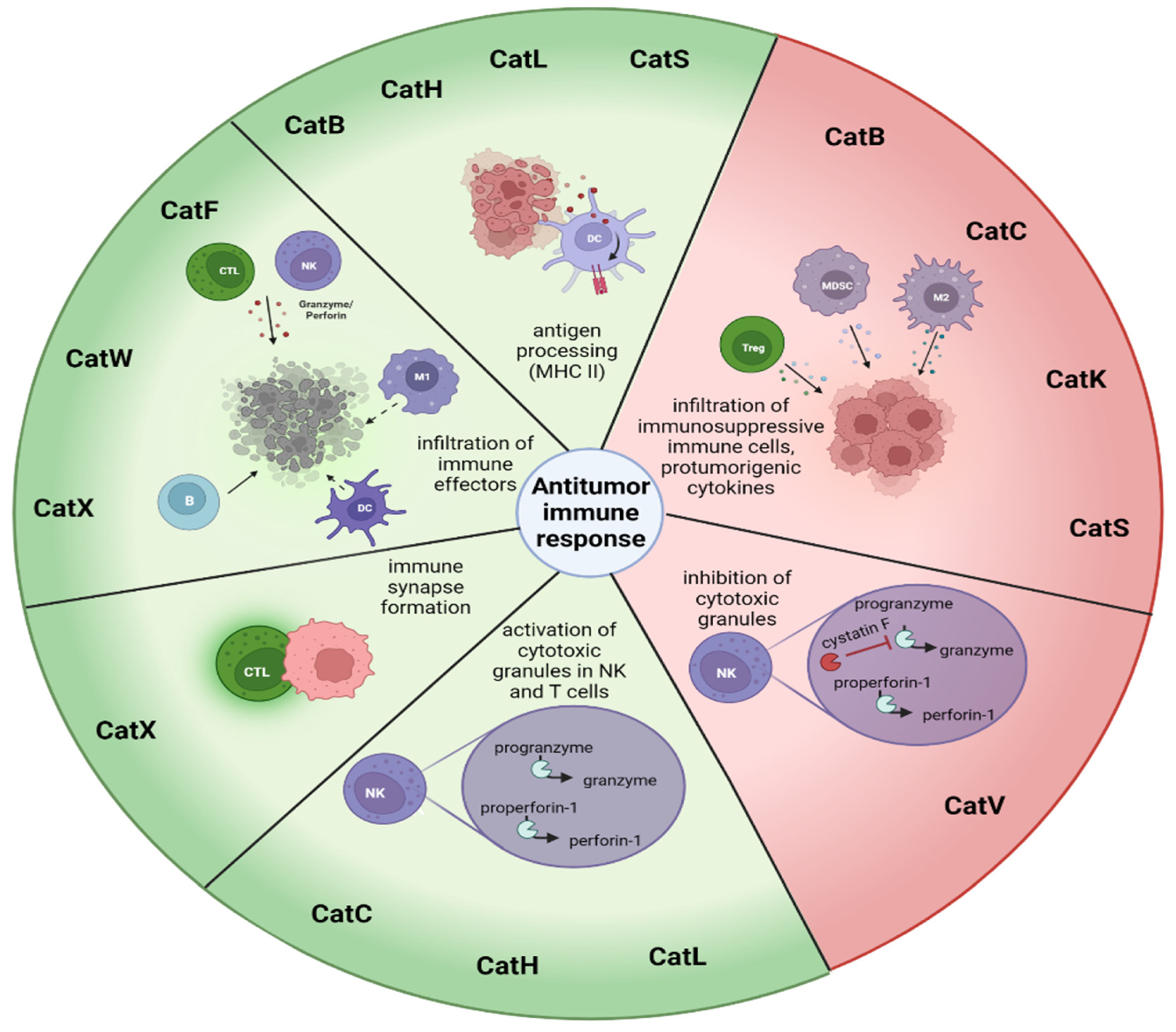
| Immune Response to Cancer | ||
|---|---|---|
| Cathepsin | Stimulating Antitumor Immune Response | Decreasing Antitumor Immune Response |
| Cathepsin B | Antigen processing [89] | Reduced persistence of CD8+ T cells [90], infiltration of immunosuppressive TAMs, MDSCs, and Tregs [91,92] |
| Target for (pro)drug delivery [2,93,94,95,96,97,98] | Activation of the inflammasome under the influence of chemotherapeutics, and IL-1β and IL-17 secretion from MDSCs [99,100] | |
| Cathepsin C | Activation of granule serine peptidases in cytotoxic immune cells [101,102] | Promotion of metastasis, by neutrophil recruitment, production of reactive oxygen species, formation of NETs and secretion of IL-1β, IL-6, and CCL3 [103] |
| Cathepsin F | Infiltration of B cells, DCs, CD8 and CD4+ T cells, and NK cells [104] | |
| Cathepsin H | Activation of granule serine peptidases in cytotoxic immune cells [101,102] | |
| MHC class II antigen presentation [105] | ||
| Cathepsin K | Bone metastasis [106] | |
| Polarization of M2 TAMs, secreting IL-10 and IL-17 [107] | ||
| Cathepsin L | Activation of perforin-1 [108] | Resistance to complement-mediated lysis [109] |
| Antigen presentation [110] | Increased in MDSCs [111] | |
| Cathepsin S | MHC II-mediated antigen presentation [112] | Polarization of APCs to M2 phenotype, supporting enhanced proliferation of MDSCs and TAMs, Tregs [112,113,114,115,116,117] |
| Target for drug delivery [118] | ||
| Cathepsin V | Cancer progression [119] | |
| Activation of CysF and decreasing cytotoxicity of NK cells and CD8+ T cells [120] | ||
| Cathepsin W | Infiltration of B cells, DC, macrophages, and CD4+ T cells to the tumor site [121] | |
| Cathepsin X | Migration of T cells and formation of immunological synapse in T cells [122,123,124,125] but not NK cells [126] | Tumor invasion [127,128] |
| Phagocytosis of macrophages [129] | Epithelial-to-mesenchymal transition [130] | |
| Adhesion-dependent maturation of DCs [131] | Cleavage of tumor suppressor profilin-1 [132] | |
| Resistance of apoptosis in several tumor types [133] | ||
| Increased in MDSCs [111] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senjor, E.; Kos, J.; Nanut, M.P. Cysteine Cathepsins as Therapeutic Targets in Immune Regulation and Immune Disorders. Biomedicines 2023, 11, 476. https://doi.org/10.3390/biomedicines11020476
Senjor E, Kos J, Nanut MP. Cysteine Cathepsins as Therapeutic Targets in Immune Regulation and Immune Disorders. Biomedicines. 2023; 11(2):476. https://doi.org/10.3390/biomedicines11020476
Chicago/Turabian StyleSenjor, Emanuela, Janko Kos, and Milica Perišić Nanut. 2023. "Cysteine Cathepsins as Therapeutic Targets in Immune Regulation and Immune Disorders" Biomedicines 11, no. 2: 476. https://doi.org/10.3390/biomedicines11020476
APA StyleSenjor, E., Kos, J., & Nanut, M. P. (2023). Cysteine Cathepsins as Therapeutic Targets in Immune Regulation and Immune Disorders. Biomedicines, 11(2), 476. https://doi.org/10.3390/biomedicines11020476






