Pimozide Increases a Delayed Rectifier K+ Conductance in Chicken Embryo Vestibular Hair Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chicken Embryo Dissection
2.2. Patch-Clamp Whole-Cell Recordings
2.3. Data Analysis and Statistical Methods
3. Results
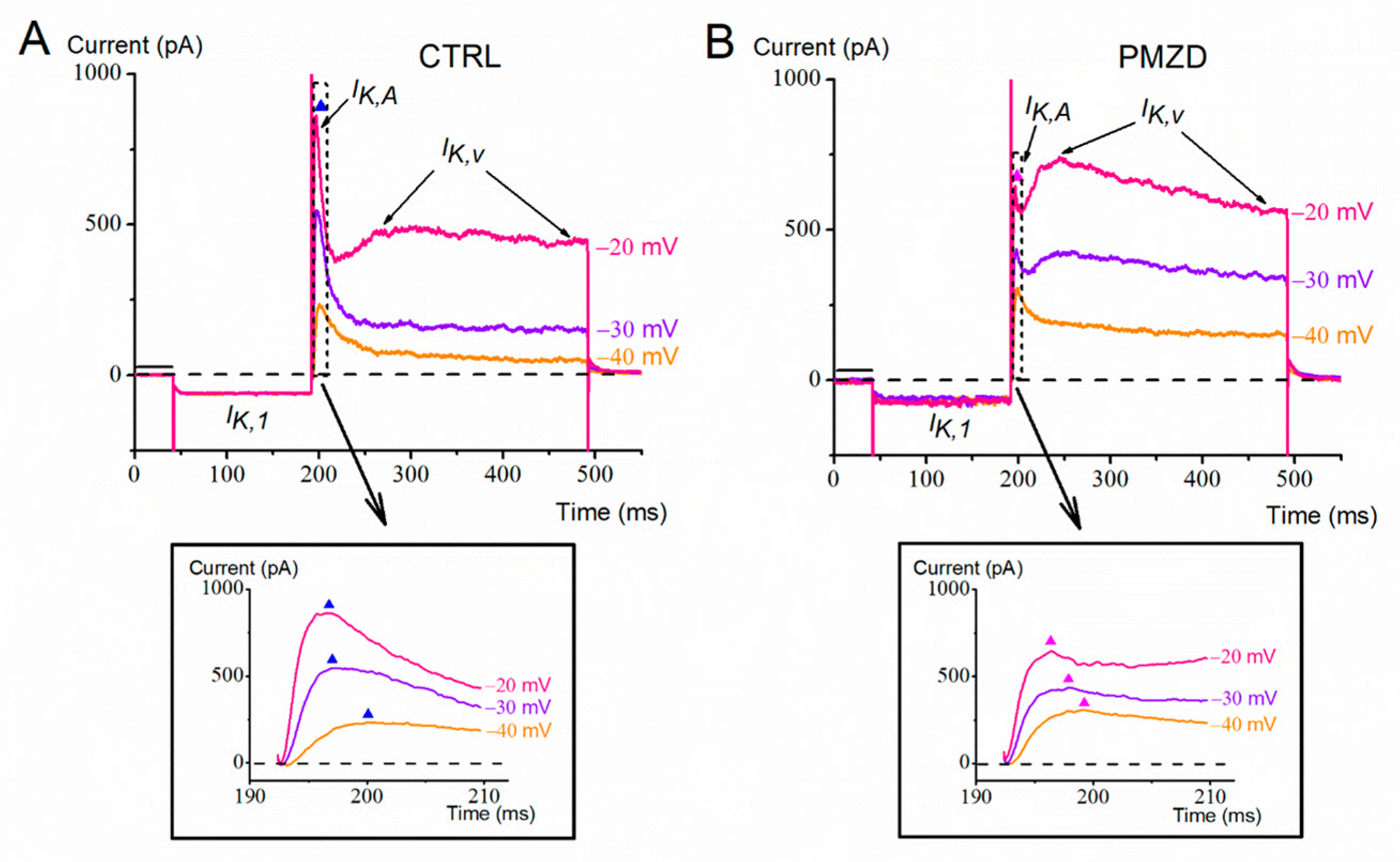
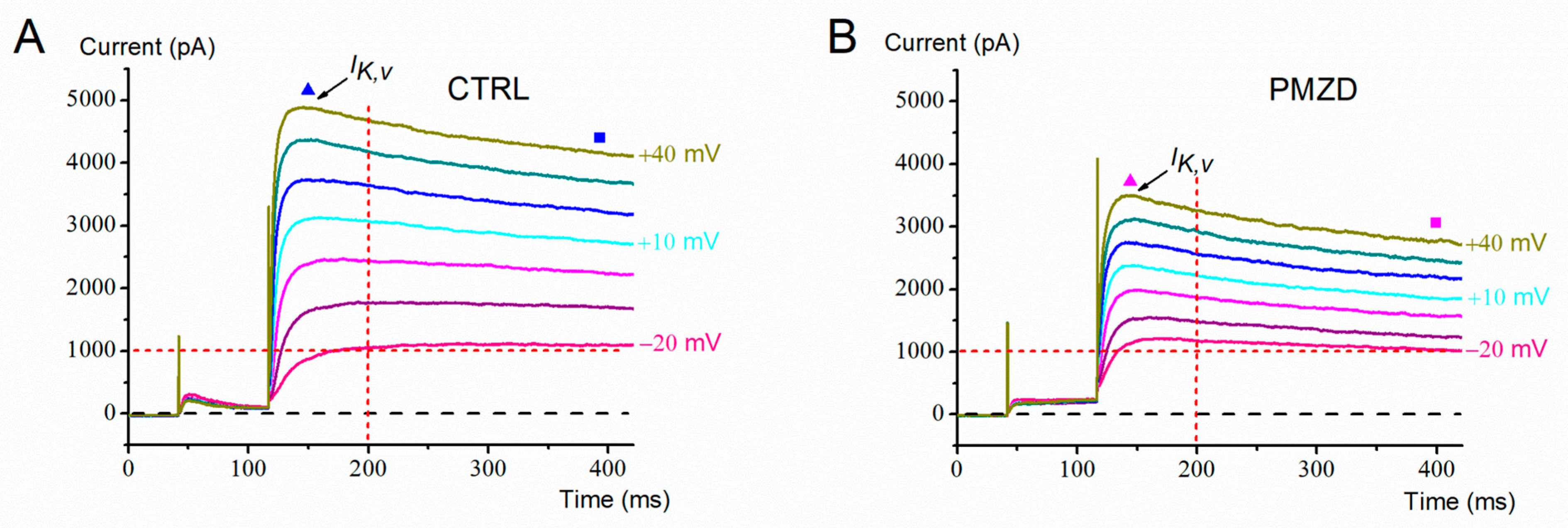
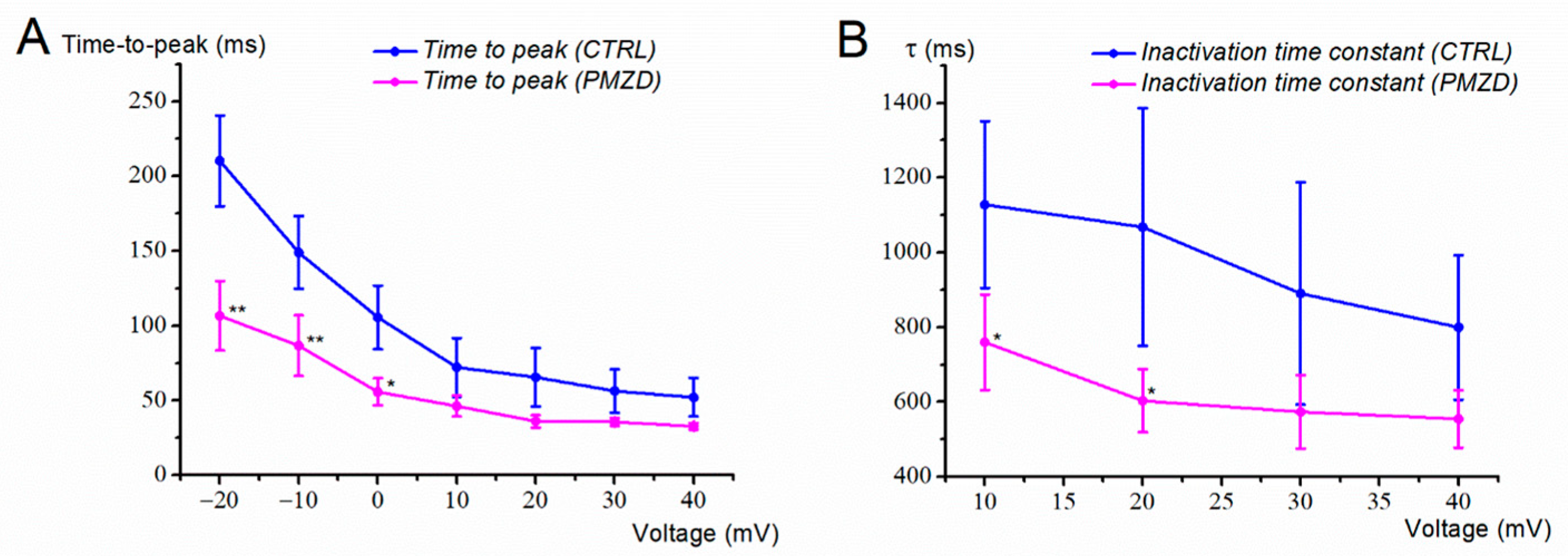
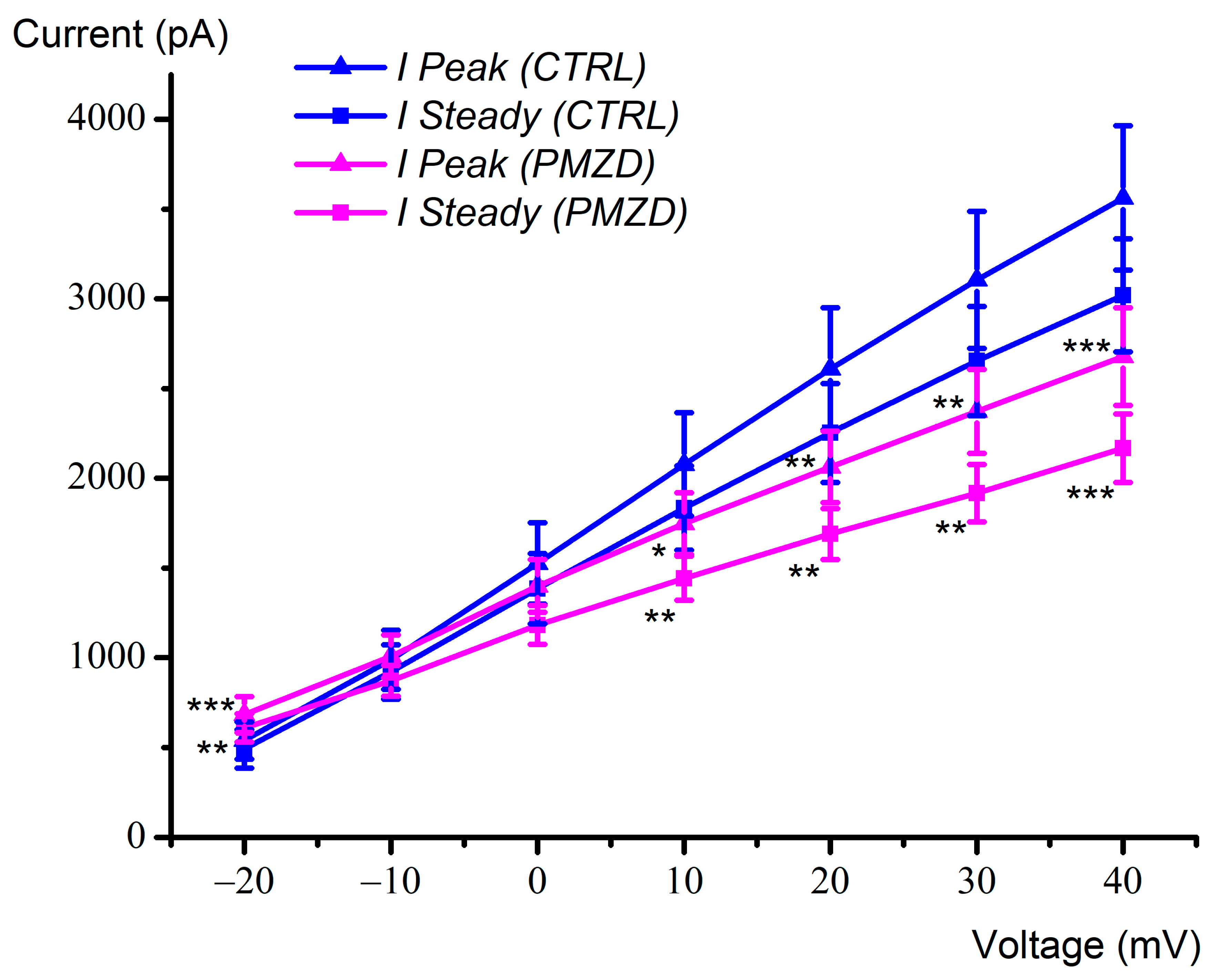
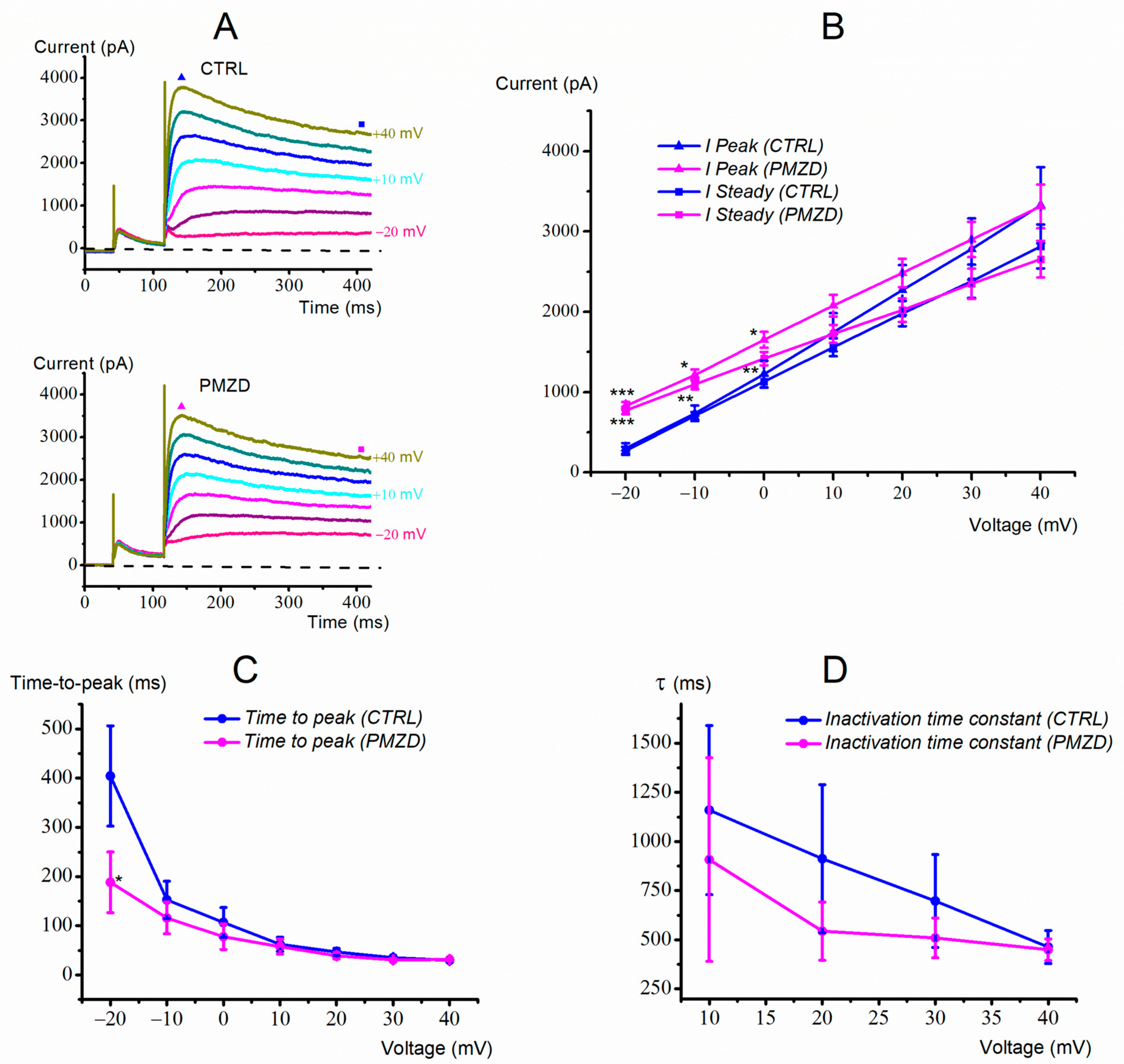
3.1. Pimozide 3 μM on Type-II Hair Cells–Voltage-Clamp Experiments
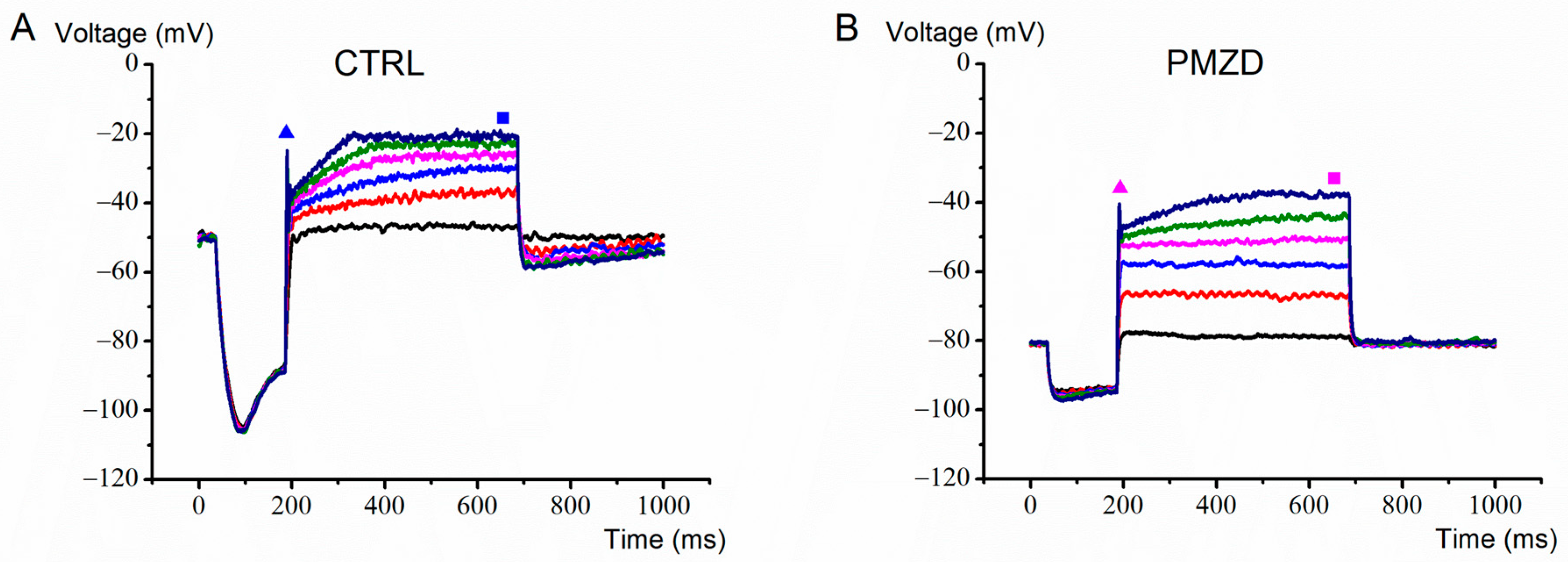
3.2. Pimozide 3 μM on Type-II Hair Cells–Current-Clamp Experiments
3.3. Pimozide 0.3 μM on Type-II Hair Cells–Voltage-Clamp Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizwan, M.; Shahid, N.U.A.; Naguit, N.; Jakkoju, R.; Laeeq, S.; Reghefaoui, T.; Zahoor, H.; Yook, J.H.; Mohammed, L. Efficacy of Behavioural Intervention, Antipsychotics, and Alpha Agonists in the Treatment of Tics Disorder in Tourette’s Syndrome. Cureus 2022, 14, e22449:10. [Google Scholar] [CrossRef]
- Roessner, V.; Eichele, H.; Stern, J.S.; Skov, L.; Rizzo, R.; Debes, N.M.; Nagy, P.; Cavanna, A.E.; Termine, C.; Ganos, C.; et al. European clinical guidelines for Tourette syndrome and other tic disorders—Version 2.0. Part III: Pharmacological treatment. Eur. Child Adolesc. Psychiatry 2021, 31, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Chau-Wong, M.; Tedesco, J.; Wong, K. Dopamine receptors in human and calf brains, using [3H]apomorphine and an antipsychotic drug. Proc. Natl. Acad. Sci. USA 1976, 73, 4354–4358. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.J.; Murphy, K.M.; Reynolds, I.J.; Snyder, S.H. Antischizophrenic drugs of the diphenylbutylpiperidine type act as calcium channel antagonists. Proc. Natl. Acad. Sci. USA 1983, 80, 5122–5125. [Google Scholar] [CrossRef] [PubMed]
- Enyeart, J.J.; Dirksen, R.T.; Sharma, V.K.; Williford, D.J.; Sheu, S.S. Antipsychotic pimozide is a potent Ca2+ channel blocker in heart. Mol. Pharmacol. 1990, 37, 752–757. [Google Scholar]
- Santi, C.M.; Cayabyab, F.S.; Sutton, K.G.; McRory, J.E.; Mezeyova, J.; Hamming, K.S.; Parker, D.; Stea, A.; Snutch, T.P. Differential Inhibition of T-Type Calcium Channels by Neuroleptics. J. Neurosci. 2002, 22, 396–403. [Google Scholar] [CrossRef]
- Kang, J.; Chen, X.-L.; Rampe, D. The Antipsychotic Drugs Sertindole and Pimozide Block erg3, a Human Brain K+ Channel. Biochem. Biophys. Res. Commun. 2001, 286, 499–504. [Google Scholar] [CrossRef]
- Drolet, B.; Rousseau, G.; Daleau, P.; Cardinal, R.; Simard, C.; Turgeon, J. Pimozide (Orap) prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current in native cardiac myocytes. J. Cardiovasc. Pharmacol. Ther. 2001, 6, 255–260. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Lee, Y.T.; Rhodes, K.; Wang, K.; Argentieri, T.M.; Wang, Q. Inhibitory effects of pimozide on cloned and native voltage-gated potassium channels. Mol. Brain Res. 2003, 115, 29–38. [Google Scholar] [CrossRef]
- Masetto, S.; Perin, P.; Malusà, A.; Zucca, G.; Valli, P. Membrane properties of chick semicircular canal hair cells in situ during embryonic development. J. Neurophysiol. 2000, 83, 2740–2756. [Google Scholar] [CrossRef]
- Wersall, J. Studies on the structure and innervation of the sensory epithelium of the cristae ampulares in the guinea pig; a light and electron microscopic investigation. Acta oto-laryngologica. Suppl. 1956, 126, 1–85. [Google Scholar]
- Gulley, R.L.; Bagger-Sjöbäck, D. Freeze-fracture studies on the synapse between the type I hair cell and the calyceal terminal in the guinea-pig vestibular system. J. Neurocytol. 1979, 8, 591–603. [Google Scholar] [CrossRef]
- Lysakowski, A.; Goldberg, J.M. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J. Comp. Neurol. 1997, 389, 419–443. [Google Scholar] [CrossRef]
- Goldberg, J.M. Afferent diversity and the organization of central vestibular pathways. Exp. Brain Res. 2000, 130, 277–297. [Google Scholar] [CrossRef]
- Lysakowski, A.; Goldberg, J.M. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J. Comp. Neurol. 2008, 511, 47–64. [Google Scholar] [CrossRef]
- Rennie, K.J.; Correia, M.J. Potassium currents in mammalian and avian isolated type I semicircular canal hair cells. J. Neurophysiol. 1994, 71, 317–329. [Google Scholar] [CrossRef]
- Rusch, A.; Eatock, R.A. A delayed rectifier conductance in type I hair cells of the mouse utricle. J. Neurophysiol. 1996, 76, 995–1004. [Google Scholar] [CrossRef]
- Rüsch, A.; Lysakowski, A.; Eatock, R.A. Postnatal Development of Type I and Type II Hair Cells in the Mouse Utricle: Acquisition of Voltage-Gated Conductances and Differentiated Morphology. J. Neurosci. 1998, 18, 7487–7501. [Google Scholar] [CrossRef]
- Haque, A.; Huss, D.; Dickman, J.D. Afferent Innervation Patterns of the Pigeon Horizontal Crista Ampullaris. J. Neurophysiol. 2006, 96, 3293–3304. [Google Scholar] [CrossRef]
- Bjørnstad, S.; Austdal, L.P.E.; Roald, B.; Glover, J.C.; Paulsen, R.E. Cracking the Egg: Potential of the Developing Chicken as a Model System for Nonclinical Safety Studies of Pharmaceuticals. Experiment 2015, 355, 386–396. [Google Scholar] [CrossRef]
- Bissonnette, J.P.; Fekete, D.M. Standard atlas of the gross anatomy of the developing inner ear of the chicken. J. Comp. Neurol. 1996, 368, 620–630. [Google Scholar] [CrossRef]
- Masetto, S.; Bosica, M.; Correia, M.J.; Ottersen, O.P.; Zucca, G.; Perin, P.; Valli, P. Na+ Currents in Vestibular Type I and Type II Hair Cells of the Embryo and Adult Chicken. J. Neurophysiol. 2003, 90, 1266–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masetto, S.; Zampini, V.; Zucca, G.; Valli, P. Ca2+ currents and voltage responses in Type I and Type II hair cells of the chick embryo semicircular canal. Pflügers Arch. 2005, 451, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Marty, A.; Neher, E. Tight-Seal Whole-Cell Recording. In Single-Channel Recording, 2nd ed.; Sakmann, B., Neher, E., Eds.; Springer: Boston, MA, USA, 1995; pp. 31–52. ISBN 978-1-4419-1229-9. [Google Scholar] [CrossRef]
- Seo, M.S.; An, J.R.; Heo, R.; Kang, M.; Park, S.; Mun, S.-Y.; Park, H.; Han, E.-T.; Han, J.-H.; Chun, W.; et al. The inhibitory effects of pimozide, an antipsychotic drug, on voltage-gated K+ channels in rabbit coronary arterial smooth muscle cells. Drug Chem. Toxicol. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, L.; Cai, F.; Rampe, D. High affinity blockade of the HERG cardiac K+ channel by the neuroleptic pimozide. Eur. J. Pharmacol. 2000, 392, 137–140. [Google Scholar] [CrossRef]
- Almanza, A.; Vega, R.; Soto, E. Calcium current in type I hair cells isolated from the semicircular canal crista ampullaris of the rat. Brain Res. 2003, 994, 175–180. [Google Scholar] [CrossRef]
- Bao, H.; Wong, W.H.; Goldberg, J.M.; Eatock, R.A. Voltage-Gated Calcium Channel Currents in Type I and Type II Hair Cells Isolated From the Rat Crista. J. Neurophysiol. 2003, 90, 155–164. [Google Scholar] [CrossRef]
- Masetto, S.; Spaiardi, P.; Johnson, S.J. Signal Transmission by Auditory and Vestibular Hair Cells. In Recent Advances in Audiological and Vestibular Research; Hatzopoulos, S., Ciorba, A., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80356-375-6. [Google Scholar]
- Spaiardi, P.; Marcotti, W.; Masetto, S.; Johnson, S.L. Signal transmission in mature mammalian vestibular hair cells. Front. Cell. Neurosci. 2022, 16, 806913:18. [Google Scholar] [CrossRef]
- Sadeghi, S.G.; Pyott, S.; Yu, Z.; Glowatzki, E. Glutamatergic Signaling at the Vestibular Hair Cell Calyx Synapse. J. Neurosci. 2014, 34, 14536–14550. [Google Scholar] [CrossRef]
- Spitzmaul, G.; Tolosa, L.; Winkelman, B.H.; Heidenreich, M.; Frens, M.A.; Chabbert, C.; de Zeeuw, C.I.; Jentsch, T.J. Vestibular Role of KCNQ4 and KCNQ5 K+ Channels Revealed by Mouse Models. J. Biol. Chem. 2013, 288, 9334–9344. [Google Scholar] [CrossRef]
- Meredith, F.L.; Rennie, K.J. Channeling your inner ear potassium: K+ channels in vestibular hair cells. Hear. Res. 2016, 338, 40–51. [Google Scholar] [CrossRef]
- Contini, D.; Holstein, G.R.; Art, J.J. Simultaneous Dual Recordings From Vestibular Hair Cells and Their Calyx Afferents Demonstrate Multiple Modes of Transmission at These Specialized Endings. Front. Neurol. 2022, 13, 891536:10. [Google Scholar] [CrossRef]
- Agrawal, Y.; Carey, J.P.; Della Santina, C.C.; Schubert, M.C.; Minor, L.B. Disorders of Balance and Vestibular Function in US Adults: Data from the National Health and Nutrition Examination Survey, 2001–2004. Arch. Intern. Med. 2009, 169, 938–944. [Google Scholar] [CrossRef]
- Tepper, D. Migraine Associated Vertigo. Headache 2015, 55, 1475–1476. [Google Scholar] [CrossRef]
- Corns, L.F.; Bardhan, T.; Houston, O.; Olt, J.; Holley, M.C.; Masetto, S.; Johnson, S.L.; Marcotti, W. Functional Development of Hair Cells in the Mammalian Inner Ear. In Development of Auditory and Vestibular Systems, 4th ed.; Romand, R., Varela-Nieto, I., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 155–188. ISBN 9780124080881. [Google Scholar] [CrossRef]
- Llorens, J.; Callejo, A.; Greguske, E.A.; Maroto, A.F.; Cutillas, B.; Martins-Lopes, V. Physiological assesment of vestibular function and toxicity in humans and animals. Neurotoxicology 2018, 66, 204–212. [Google Scholar] [CrossRef]
- Tighilet, B.; Trico, J.; Xavier, F.; Chabbert, C. What Predictability for Animal Models of Peripheral Vestibular Disorders? Biomedicines 2022, 10, 3097. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Barrese, V.; Stott, J.B.; Greenwood, I.A. KCNQ-Encoded Potassium Channels as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 625–648. [Google Scholar] [CrossRef]
- Hurley, K.M.; Gaboyard-Niay, S.; Zhong, M.; Price, S.D.; Wooltorton, J.; Lysakowski, A.; Eatock, R.A. M-Like K+ Currents in Type I Hair Cells and Calyx Afferent Endings of the Developing Rat Utricle. J. Neurosci. 2006, 26, 10253–10269. [Google Scholar] [CrossRef]
- Lipovsek, M.; Bardy, C.; Cadwell, C.R.; Hadley, K.; Kobak, D.; Tripathy, S.J. Patch-seq: Past, Present, and Future. J. Neurosci. 2021, 41, 937–946. [Google Scholar] [CrossRef]
- Patten, S.A.; Aggad, D.; Martinez, J.; Tremblay, E.; Petrillo, J.; Armstrong, G.A.; La Fontaine, A.; Maios, C.; Liao, M.; Ciura, S.; et al. Neuroleptics as therapeutic compounds stabilizing neuromuscular transmission in amyotrophic lateral sclerosis. J. Clin. Investig. 2017, 2, e97152. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Kaushik, I.; Srivastava, S.K. Pimozide Suppresses the Growth of Brain Tumors by Targeting STAT3-Mediated Autophagy. Cells 2020, 9, 2141. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giunta, R.; Cheli, G.; Spaiardi, P.; Russo, G.; Masetto, S. Pimozide Increases a Delayed Rectifier K+ Conductance in Chicken Embryo Vestibular Hair Cells. Biomedicines 2023, 11, 488. https://doi.org/10.3390/biomedicines11020488
Giunta R, Cheli G, Spaiardi P, Russo G, Masetto S. Pimozide Increases a Delayed Rectifier K+ Conductance in Chicken Embryo Vestibular Hair Cells. Biomedicines. 2023; 11(2):488. https://doi.org/10.3390/biomedicines11020488
Chicago/Turabian StyleGiunta, Roberta, Giulia Cheli, Paolo Spaiardi, Giancarlo Russo, and Sergio Masetto. 2023. "Pimozide Increases a Delayed Rectifier K+ Conductance in Chicken Embryo Vestibular Hair Cells" Biomedicines 11, no. 2: 488. https://doi.org/10.3390/biomedicines11020488





