Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol
Abstract
:1. Introduction
2. Phytochemical Overview
3. Methodology
4. Mechanisms Related to Macrophages’ and Other Immune Cells’ Activation
5. Arthritis
6. Lung Inflammation
7. Skin Inflammation
8. Neuroinflammation
9. Diabetes-Associated Inflammation
10. Cardiac Inflammation
11. Hepato- and Renal Inflammation
12. Obesity-Associated Inflammation
13. Endothelial Inflammation
14. Gut Inflammation
15. General Discussion
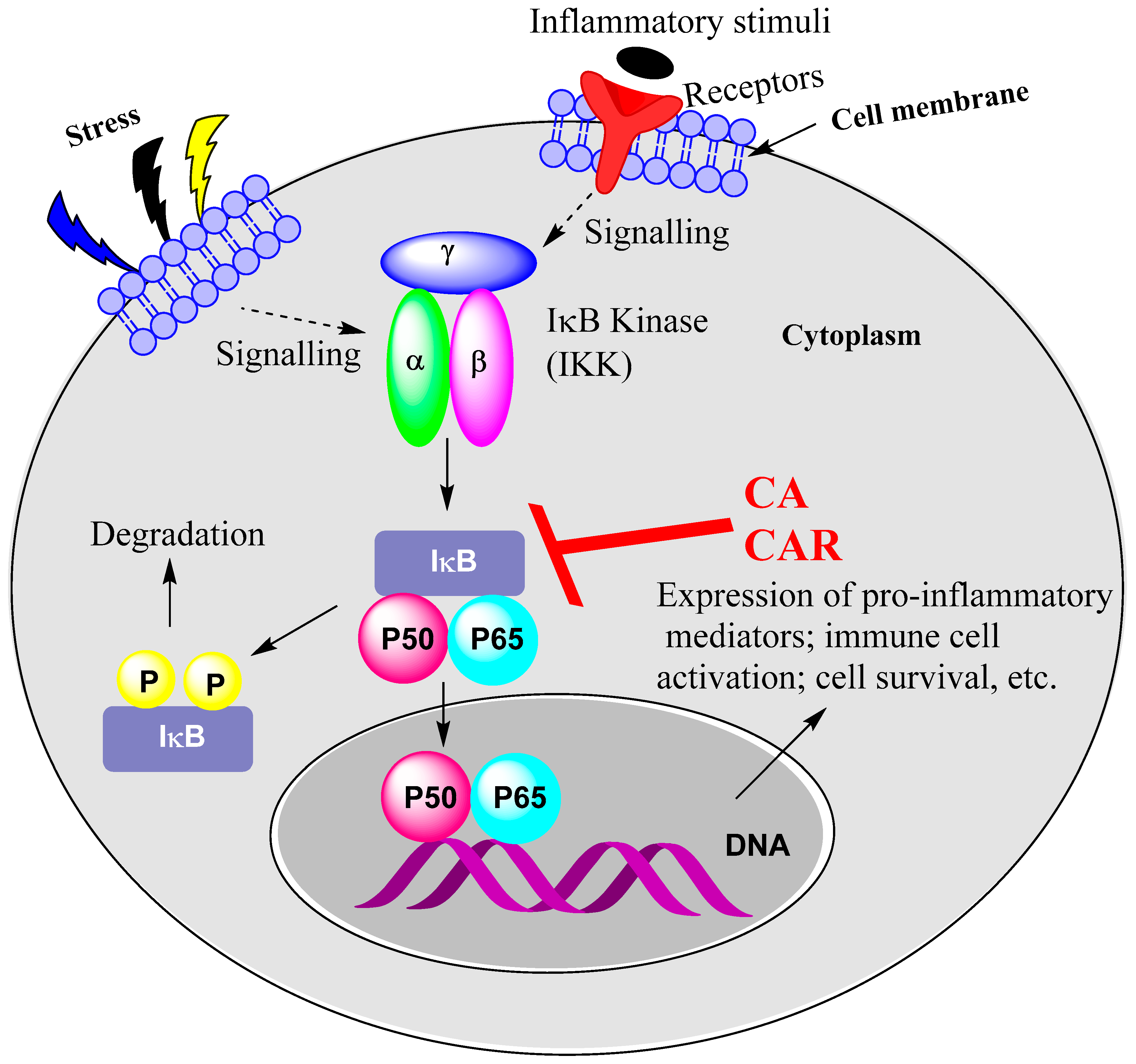
15.1. Rosemary Diterpenes Inhibit Activation of NF-κB
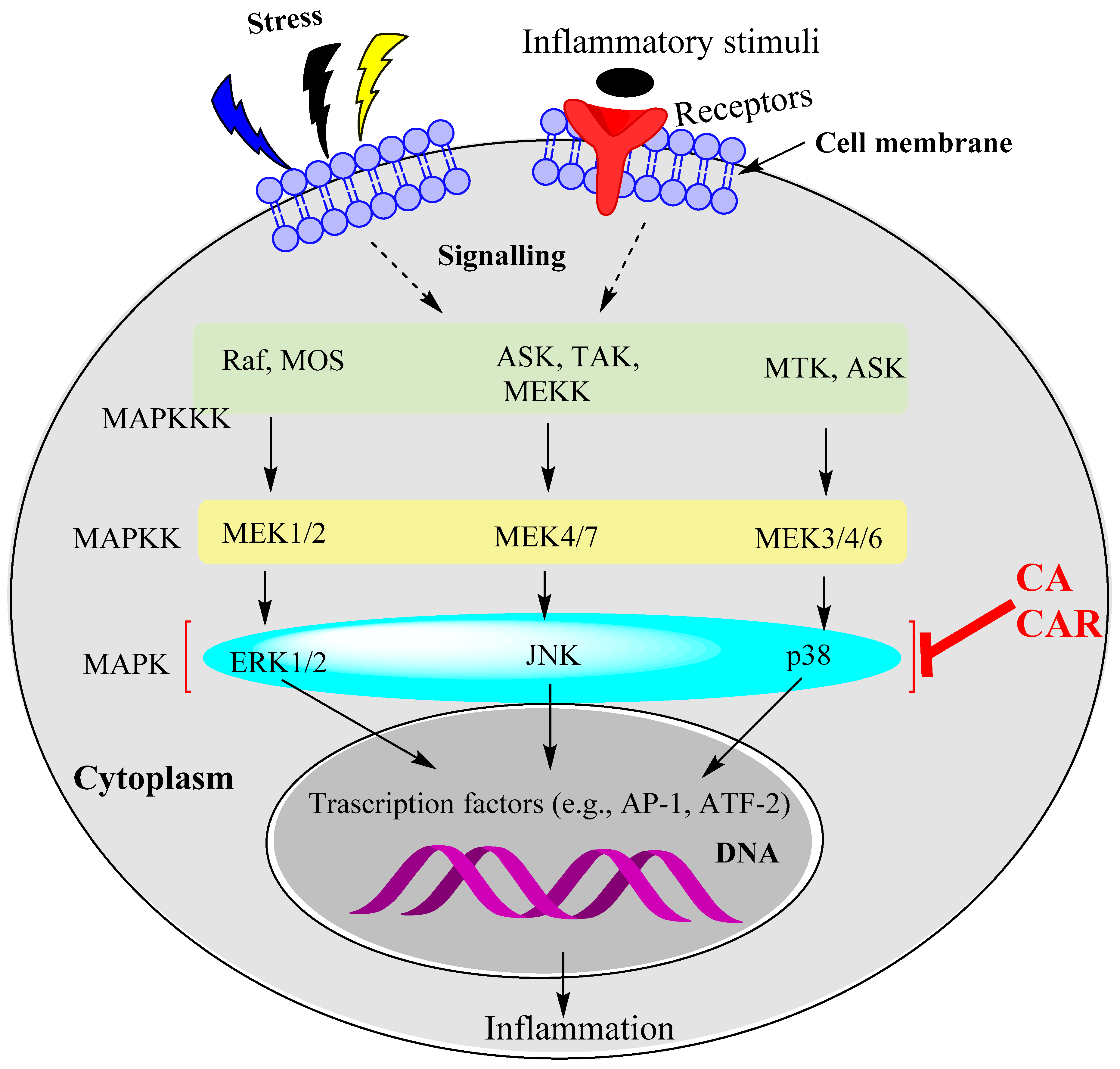
15.2. Rosemary Diterpenes Modulate the MAPK Pathways
15.3. Rosemary Diterpenes Modulate the SIRT1/SERT3 Pathways
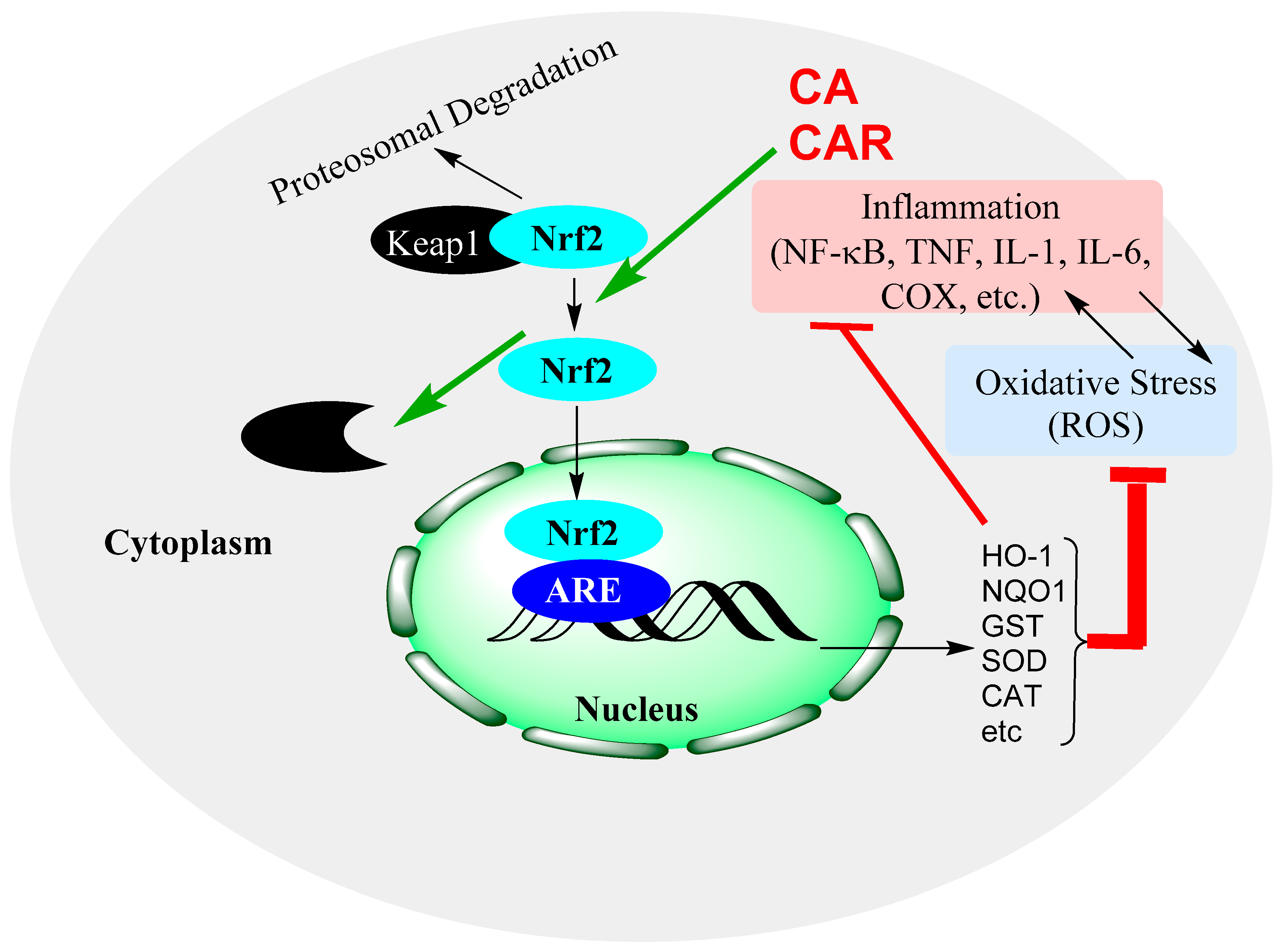
15.4. Rosemary Diterpenes Activate the Nrf2/HO-1 Pathways of Cytoprotection
15.5. Rosemary Diterpenes Suppress the NLRP3 Inflammasome
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Sig. Transduct. Target Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, C.; McKernan, D.P. Anti-Viral Pattern Recognition Receptors as Therapeutic Targets. Cells 2021, 10, 2258. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef]
- Kanneganti, T.D. The inflammasome: Firing up innate immunity. Immunol. Rev. 2015, 265, 1–5. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Andreakos, E. Therapeutic human monoclonal antibodies in inflammatory diseases. Methods Mol. Biol. 2014, 1060, 37–59. [Google Scholar]
- Lai, Y.; Dong, C. Therapeutic antibodies that target inflammatory cytokines in autoimmune diseases. Int. Immunol. 2016, 28, 181–188. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Kim, M.E.; Kim, D.H.; Lee, J.S. Transcription Factors as Targets of Natural Compounds in Age-Related Diseases and Cancer: Potential Therapeutic Applications. Int. J. Mol. Sci. 2022, 23, 13882. [Google Scholar] [CrossRef]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complement. Alternat. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef]
- Birtić, S. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Razboršek, M.I.; Ivanović, M. Stability studies and determination of carnosic acid and its oxidative degradation products by gas chromatography–mass spectrometry. Inter. J. Mass Spectr. 2016, 407, 29–39. [Google Scholar] [CrossRef]
- Buchin, Y.; Sakemi, Y.; Hamashima, R.; Morioka, Y.; Yamanaka, D.; Hakuno, F.; Takahashi, S.-I.; Shindo, K. Structures and biological activities of new carnosic acid- and carnosol-related compounds generated by heat treatment of rosemary. Phytochem. Lett. 2019, 30, 43–48. [Google Scholar] [CrossRef]
- Oh, J.; Yu, T.; Choi, S.J.; Yang, Y.; Baek, H.S.; An, S.A.; Kwon, L.K.; Kim, J.; Rho, H.S.; Shin, S.S.; et al. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediators Inflamm. 2012, 2012, 781375. [Google Scholar] [CrossRef]
- Chae, I.G.; Yu, M.H.; Im, N.K.; Jung, Y.T.; Lee, J.; Chun, K.S.; Lee, I.S. Effect of Rosemarinus officinalis L. on MMP-9, MCP-1 levels, and cell migration in RAW 264.7 and smooth muscle cells. J. Med. Food. 2012, 15, 879–886. [Google Scholar] [CrossRef]
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Wei, W.H.; Zhang, X.W.; Liu, D.; Zeng, K.W.; Tu, P.F. An Integrated Proteomics and Bioinformatics Approach Reveals the Anti-inflammatory Mechanism of Carnosic Acid. Front. Pharmacol. 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.H.; Liang, Y.C.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis 2002, 23, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Hwang, C.J.; Choi, J.Y.; Park, M.H.; Song, M.J.; Oh, K.W.; Son, D.J.; Lee, S.H.; Han, S.B.; Hong, J.T. Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3. Biomol. Ther. 2017, 25, 535–544. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules 2016, 21, 465. [Google Scholar] [CrossRef]
- Shi, W.; Xu, G.; Zhan, X.; Gao, Y.; Wang, Z.; Fu, S.; Qin, N.; Hou, X.; Ai, Y.; Wang, C.; et al. Carnosol inhibits inflammasome activation by directly targeting HSP90 to treat inflammasome-mediated diseases. Cell Death. Dis. 2020, 11, 252. [Google Scholar] [CrossRef]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz OKoeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N.; et al. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef]
- Crozier, R.W.E.; Yousef, M.; Coish, J.M.; Fajardo, V.A.; Tsiani, E.; MacNeil, A.J. Carnosic acid inhibits secretion of allergic inflammatory mediators in IgE-activated mast cells via direct regulation of Syk activation. J. Biol. Chem. 2023, 102867. [Google Scholar] [CrossRef]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; de Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.-L.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 2013, 76, 132–148. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Matsuo, T. Carnosic acid inhibits inflammatory cytokines production in human periodontal ligament cells. Immunopharmacol. Immunotoxicol. 2020, 42, 373–378. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Matsuo, T. Carnosic Acid Inhibits CXCR3 Ligands Production in IL-27-Stimulated Human Oral Epithelial Cells. Inflammation 2019, 42, 1311–1316. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, X.; Zhou, L.; Liu, Z.; Yuan, J.; Cheng, J.; Zhao, J.; Wu, L.; Li, H.; Qiu, H.; et al. Carnosic acid inhibits inflammation response and joint destruction on osteoclasts, fibroblast-like synoviocytes, and collagen-induced arthritis rats. J. Cell Physiol. 2018, 233, 6291–6303. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Yang, S.-C.; Hsu, Y.-H.; Chen, C.-Y.; Chen, P.-J.; Syu, Y.-T.; Lin, C.-H.; Hwang, T.G.-L. Carnosic acid inhibits reactive oxygen species-dependent neutrophil extracellular trap formation and ameliorates acute respiratory distress syndrome. Life Sci. 2022, 121334. [Google Scholar] [CrossRef]
- Kawamura, T.; Momozane, T.; Sanosaka, M.; Sugimura, K.; Iida, O.; Fuchino, H.; Funaki, S.; Shintani, Y.; Inoue, M.; Minami, M.; et al. Carnosol Is a Potent Lung Protective Agent: Experimental Study on Mice. Transplant. Proceed. 2015, 47, 1657–1661. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; de Souza, I.C.C.; Fürstenau, C.R. Carnosic Acid Induces Anti- Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Involving a Crosstalk Between the Nrf2/HO-1 Axis and NF-κB. Mol. Neurobiol. 2018, 55, 890–897. [Google Scholar] [CrossRef]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; de Galarreta, C.M.R.; Cuadrado, A. Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-Kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef]
- Wu, C.-R.; Tsai, C.-W.; Chang, S.-W.; Lin, C.-Y.; Huang, L.C.; Tsai, C.-W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chemico-Biol. Inter. 2015, 225, 40–46. [Google Scholar] [CrossRef]
- Hou, C.-W.; Lin, Y.-T.; Chen, Y.-L.; Wang, Y.H.; Chou, J.L.; Ping, L.Y.; Jeng, K.C. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutr. Neurosci. 2012, 15, 257–263. [Google Scholar] [CrossRef]
- Meng, P.; Yoshida, H.; Matsumiya, T.; Imaizumi, T.; Tanji, K.; Xing, F.; Hayakari, R.; Dempoya, J.; Tatsut, T.; Aizawa-Yashiro, T.; et al. Carnosic acid suppresses the production of amyloid-β 1-42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neurosci. Res. 2013, 75, 94–102. [Google Scholar] [CrossRef]
- Yoshida, H.; Meng, P.; Matsumiya, T.; Tanji, K.; Hayakari, R.; Xing, F.; Wang, L.; Tsuruga, K.; Tanaka, H.; Mimura, J.; et al. Carnosic acid suppresses the production of amyloid-β 1-42 and 1-43 by inducing an α-secretase TACE/ADAM17 in U373MG human astrocytoma cells. Neurosci. Res. 2014, 79, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Liu, K.L.; Lin, Y.R.; Kuo, W.C. The mechanisms of carnosic acid attenuates tumor necrosis factor-α-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2014, 58, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Dewanjee, S.; Joardar, S.; Chakraborty, P.; Bhattacharya, H.; Bhanja, S.; Bhattacharyya, C.; Bhowmik, M.; Bhowmick, S.; Saha, A.; et al. Carnosic acid attenuates doxorubicin-induced cardiotoxicity by decreasing oxidative stress and its concomitant pathological consequences. Food Chem. Toxicol. 2022, 166, 113205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Yang, J.J.; Zhang, H.S. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed. Pharmacother. 2019, 109, 71–83. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Momeni-Moghaddam, M.A.; Chini, M.G.; Saviano, A.; Maione, F.; Bifulco, G.; Rahmanian-Devin, P.; Jebalbarezy, A.; Askari, V.R. Carnosol Attenuates LPS- Induced Inflammation of Cardiomyoblasts by Inhibiting NF-κB: A Mechanistic in Vitroand in SilicoStudy. Evid. Based Complement. Alternat. Med. 2022, 2022, 7969422. [Google Scholar] [CrossRef]
- Gao, L.; Shan, W.; Zeng, W.; Hu, Y.; Wang, G.; Tian, X.; Zhang, N.; Shi, X.; Zhao, Y.; Ding, C.; et al. Carnosic acid alleviates chronic alcoholic liver injury by regulating the SIRT1/ChREBP and SIRT1/p66shc pathways in rats. Mol. Nutr. Food Res. 2016, 60, 1902–1911. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.J.; Chen, G.Y.; Wang, W.W.; Xie, Z.T.; Tang, G.F.; Wei, S.D. Carnosic acid nanoparticles suppress liver ischemia/reperfusion injury by inhibition of ROS, Caspases and NF-κB signaling pathway in mice. Biomed. Pharmacother. 2016, 82, 237–246. [Google Scholar] [CrossRef]
- Park, M.Y.; Mun, S.T. Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS- stimulated 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 516–620. [Google Scholar] [CrossRef]
- D’Agata, V.; D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Rossi, S.; Giunta, S. Carnosol attenuates high glucose damage in human retinal endothelial cells through regulation of ERK/Nrf2/HO-1 pathway. J. Asian Nat. Prod. Res. 2022; in press. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Hou, N.; Li, J.; Liu, M.; Peng, S.; Zhang, Y.; Luo, Y.; Zhao, B.; Wang, S.; et al. Carnosol as a Nrf2 Activator Improves Endothelial Barrier Function Through Antioxidative Mechanisms. Int. J. Mol. Sci. 2019, 20, 880. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, G.; Peng, K.; Gao, Y.; Wang, J.; Gao, C.; He, C.; Lu, F. Carnosol Maintains Intestinal Barrier Function and Mucosal Immune Homeostasis in DSS-Induced Colitis. Front. Nutr. 2022, 9, 894307. [Google Scholar] [CrossRef]
- Chrastina, M.; Poništ, S.; Tóth, J.; Czigle, S.; Pašková, Ľ.; Vyletelová, V.; Švík, K.; Bauerová, K. Combination Therapy of Carnosic Acid and Methotrexate Effectively Suppressed the Inflammatory Markers and Oxidative Stress in Experimental Arthritis. Molecules 2022, 27, 7115. [Google Scholar] [CrossRef]
- Xia, G.; Wang, X.; Sun, H.; Qin, Y.; Fu, M. Carnosic acid (CA) attenuates collagen- induced arthritis in db/db mice via inflammation suppression by regulating ROS- dependent p38 pathway. Free Radic. Biol. Med. 2017, 108, 418–432. [Google Scholar] [CrossRef]
- Li, L.; Pan, Z.; Ning, D.; Fu, Y. Rosmanol and Carnosol Synergistically Alleviate Rheumatoid Arthritis through Inhibiting TLR4/NF-κB/MAPK Pathway. Molecules 2022, 27, 78. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Sun, H.; Cao, K. Carnosic acid protects against lipopolysaccharide-induced acute lung injury in mice. Exp. Ther. Med. 2019, 18, 3707–3714. [Google Scholar] [CrossRef]
- Kalantar, H.; Sadeghi, E.; Abolnezhadian, F.; Goudarzi, M.; Hemmati, A.A.; Basir, Z.; Kalantar, M. Carnosol attenuates bleomycin-induced lung damage via suppressing fibrosis, oxidative stress and inflammation in rats. Life Sci. 2021, 287, 120059. [Google Scholar] [CrossRef]
- Lee, J.E.; Im, D.S. Suppressive Effect of Carnosol on Ovalbumin-Induced Allergic Asthma. Biomol. Ther. 2021, 29, 58–63. [Google Scholar] [CrossRef]
- da Rosa, J.S.; Facchin, B.M.; Bastos, J.; Siqueira, M.A.; Micke, G.A.; Dalmarco, E.M.; Pizzolatti, M.G.; Fröde, T.S. Systemic administration of Rosmarinus officinalis attenuates the inflammatory response induced by carrageenan in the mouse model of pleurisy. Planta Med. 2013, 79, 1605–1614. [Google Scholar] [CrossRef]
- Yeo, I.J.; Park, J.H.; Jang, J.S.; Lee, D.Y.; Park, J.E.; Choi, Y.E.; Joo, J.H.; Song, J.K.; Jeon, H.O.; Hong, J.T. Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3. Arch. Pharm. Res. 2019, 42, 274–283. [Google Scholar] [CrossRef]
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Ali, D.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. Int. 2020, 27, 11663–11670. [Google Scholar] [CrossRef]
- Maynard, M.E.; Underwood, E.L.; Redell, J.B.; Zhao, J.; Kobori, N.; Hood, K.N.; Moore, A.N.; Dash, P.K. Carnosic Acid Improves Outcome after Repetitive Mild Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Xie, Y.X.; Zhang, J.W.; Qiu, X.H.; Cheng, A.B.; Tian, L.; Ma, B.Y.; Hou, Y.B. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J. Recept. Signal Transduct. Res. 2016, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Singh, I.N.; Wang, J.A.; Hall, E.D. Nrf2–ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 2015, 264, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Fan, L.; Peng, Y.; He, X.; Chen, H.; Duan, H.; Yang, F.; Lin, D.; Lin, Z.; Li, H.; et al. Carnosic Acid Mitigates Early Brain Injury After Subarachnoid Hemorrhage: Possible Involvement of the SIRT1/p66shc Signaling Pathway. Front. Neurosci. 2019, 13, 26. [Google Scholar] [CrossRef]
- Yi-Bin, W.; Xiang, L.; Bing, Y.; Qi, Z.; Fei-Tong, J.; Minghong, W.; Xiangxiang, Z.; Le, K.; Yan, L.; Ping, S.; et al. Inhibition of the CEBPβ-NFκB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death Dis. 2022, 13, 318. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Han, J.J.; Zhang, F.; Liu, S.; Zhu, L.; Wang, Z.Z.; Zhang, G.X.; Zhang, Y. Carnosol Modulates Th17 Cell Differentiation and Microglial Switch in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 1807. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Farkhondeh, T. Evaluation of Antidiabetic Activity of Carnosol (Phenolic Diterpene in Rosemary) in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Hematol. Disord. Drug Targets 2017, 17, 11–17. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef]
- Xie, Z.; Zhong, L.; Wu, Y.; Wan, X.; Yang, H.; Xu, X.; Li, P. Carnosic acid improves diabetic nephropathy by activating Nrf2/ARE and inhibition of NF-κB pathway. Phytomedicine 2018, 47, 161–173. [Google Scholar] [CrossRef]
- Hu, M.; Li, T.; Bo, Z.; Xiang, F. The protective role of carnosic acid in ischemic/reperfusion injury through regulation of autophagy under T2DM. Exp. Biol. Med. 2019, 244, 602–611. [Google Scholar] [CrossRef]
- Song, H.M.; Li, X.; Liu, Y.Y.; Lu, W.P.; Cui, Z.H.; Zhou, L.; Yao, D.; Zhang, H.M. Carnosic acid protects mice from high-fat diet-induced NAFLD by regulating MARCKS. Int. J. Mol. Med. 2018, 42, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Liu, Z.; Wang, Y.; Xiao, H.; Wu, W.; Xiao, C.; Liu, X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2013, 53, 1–9. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, H.L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules 2021, 26, 7589. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Zheng, Y.; Zhang, N. Carnosol protects against renal ischemia-reperfusion injury in rats. Exp. Anim. 2018, 67, 545–553. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Hu, M.; Li, Y.H.; Cao, X.H. Carnosic acid alleviates brain injury through NF-κB-regulated inflammation and Caspase-3-associated apoptosis in high fat-induced mouse models. Mol. Med. Rep. 2019, 20, 495–504. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chuang, L.T.; Lien, T.J.; Liing, Y.R.; Chen, W.Y.; Tsai, P.J. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses. J. Med. Food. 2013, 16, 324–333. [Google Scholar] [CrossRef]
- Yousef, M.; Crozier, R.W.E.; Hicks, N.J.; Watson, C.J.F.; Boyd, T.; Tsiani, E.; MacNeil, A.J. Attenuation of allergen-mediated mast cell activation by rosemary extract (Rosmarinus officinalis L.). J. Leukoc. Biol. 2020, 107, 843–857. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Yang, Y.; Ding, Y.; Sun, X. Carnosol suppresses microglia cell inflammation and apoptosis through PI3K/AKT/mTOR signaling pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 656–662. [Google Scholar] [CrossRef]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef]
- Inoue, K.; Takano, H.; Shiga, A.; Fujita, Y.; Makino, H.; Yanagisawa, R.; Ichinose, T.; Kato, Y.; Yamada, T.; Yoshikawa, T. Effects of volatile constituents of a rosemary extract on allergic airway inflammation related to house dust mite allergen in mice. Int. J. Mol. Med. 2005, 16, 315–319. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Kanazawa, L.K.; das Neves, T.L.; da Silva, C.F.; Horst, H.; Pizzolatti, M.G.; Santos, A.R.; Baggio, C.H.; Werner, M.F. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol. 2012, 139, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Ali-Beig, H.; Farahbakhsh, S.; Mojabi, N.; Rastegar-Moghadam, B.; Arbabian, S.; Kazemi, M.; Tekieh, E.; Golmanesh, L.; Ranjbaran, M.; et al. Hydroalcoholic extract of Rosemary (Rosmarinus officinalis L.) and its constituent carnosol inhibit formalin-induced pain and inflammation in mice. Pak. J. Biol. Sci. 2013, 16, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bakirel, T.; Bakirel, U.; Keleş, O.U.; Ulgen, S.G.; Yardibi, H. In Vivo Assessment of Antidiabetic and Antioxidant Activities of Rosemary (Rosmarinus officinalis) in Alloxan-Diabetic Rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Eidi, M.; Eidi, A.; Zamanizadeh, H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 310–313. [Google Scholar] [CrossRef]
- Lima, C.F.; Azevedo, M.F.; Araujo, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Metformin-like effect of Salvia officinalis (common sage): Is it useful in diabetes prevention? Br. J. Nutr. 2006, 96, 326–333. [Google Scholar] [CrossRef]
- Emam, M. Comparative Evaluation of Antidiabetic Activity of Rosmarinus officinalis L. and Chamomile Recutita in Streptozotocin Induced Diabetic Rats. Agric. Biol. J. N. Am. 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Khalil, O.A.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Hypoglycemic and Hepatoprotective Activity of Rosmarinus officinalis Extract in Diabetic Rats. J. Physiol. Biochem. 2013, 69, 779–783. [Google Scholar] [CrossRef]
- Hasei, S.; Yamamotoya, T.; Nakatsu, Y.; Ohata, Y.; Itoga, S.; Nonaka, Y.; Matsunaga, Y.; Sakoda, H.; Fujishiro, M.; Kushiyama, A.; et al. Carnosic Acid and Carnosol Activate AMPK, Suppress Expressions of Gluconeogenic and Lipogenic Genes, and Inhibit Proliferation of HepG2 Cells. Int. J. Mol. Sci. 2021, 22, 4040. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Tsiani, E. Attenuation of Free Fatty Acid-Induced Muscle Insulin Resistance by Rosemary Extract. Nutrients 2018, 10, 1623. [Google Scholar] [CrossRef]
- Naimi, M.; Tsakiridis, T.; Stamatatos, T.C.; Alexandropoulos, D.I.; Tsiani, E. Increased Skeletal Muscle Glucose Uptake by Rosemary Extract through AMPK Activation. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 407–413. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Murphy, B.; Hudlicky, T.; Tsiani, E. Carnosic Acid as a Component of Rosemary Extract Stimulates Skeletal Muscle Cell Glucose Uptake via AMPK Activation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Afonso, M.S.; de O Silva, A.M.; Carvalho, E.B.; Rivelli, D.P.; Barros, S.B.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic Compounds from Rosemary (Rosmarinus officinalis L.) Attenuate Oxidative Stress and Reduce Blood Cholesterol Concentrations in Diet-Induced Hypercholesterolemic Rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef]
- Christensen, K.B.; Jørgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the nuclear receptor PPARγ by metabolites isolated from sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133. [Google Scholar] [CrossRef]
- Park, M.-Y.; Mun, S.T. Dietary Carnosic Acid Suppresses Hepatic Steatosis Formation via Regulation of Hepatic Fatty Acid Metabolism in High-Fat Diet-Fed Mice. Nutr. Res. Pract. 2013, 7, 294–301. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Selma, M.V.; Larrosa, M.; Obiol, M.; García-Villalba, R.; González-Barrio, R.; Issaly, N.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; et al. A rosemary extract rich in carnosic acid selectively modulates caecum microbiota and inhibits β-glucosidase activity, altering fiber and short chain fatty acids fecal excretion in lean and obese female rats. PLoS ONE 2014, 9, e94687. [Google Scholar] [CrossRef]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic Acid as a Major Bioactive Component in Rosemary Extract Ameliorates High-Fat-Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006, 72, 881–887. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Li, L.; Wang, W.; Tan, H.Y.; Qu, Y.; Wang, D. The involvement of gut microbiota in the anti-tumor effect of carnosic acid via IL-17 suppression in colorectal cancer. Chem. Biol. Interact. 2022, 365, 110080. [Google Scholar] [CrossRef]
- He, X.; Zhang, M.; Li, S.T.; Li, X.; Huang, Q.; Zhang, K.; Zheng, X.; Xu, X.T.; Zhao, D.G.; Ma, Y.Y. Alteration of gut microbiota in high-fat diet-induced obese mice using carnosic acid from rosemary. Food Sci. Nutr. 2022, 10, 2325–2332. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Bahjat, F.R.; Pine, P.R.; Reitsma, A.; Cassafer, G.; Baluom, M.; Grillo, S.; Chang, B.; Zhao, F.F.; Payan, D.G.; Grossbard, E.B.; et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 2008, 58, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S.; Kim, H.G.; Kim, J.H.; Yang, W.S.; Kim, E.; Jeong, D.; Park, J.G.; Aziz, N.; Kim, S.; Parameswaran, N.; et al. Syk-MyD88 Axis Is a Critical Determinant of Inflammatory-Response in Activated Macrophages. Front. Immunol. 2021, 12, 767366. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.F.; Niu, X.L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhan, L.; Liao, H.; Chen, L.; Lv, X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-κB signaling pathway. Plant. Med. 2013, 79, 102–109. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem. Pharmacol. 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sudhakaran, P.R.; Helen, A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell Immunol. 2016, 310, 131–140. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. In Vitro 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Jakus, P.B.; Kalman, N.; Antus, C.; Radnai, B.; Tucsek, Z.; Gallyas, F., Jr.; Sumegi, B.; Veres, B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by resveratrol. J. Nutr. Biochem. 2013, 24, 819–823. [Google Scholar] [CrossRef]
- Machado, T.R.; Machado, T.R.; Pascutti, P.J. The p38 MAPK Inhibitors and Their Role in Inflammatory Diseases. Chem. Eur. 2021, 6, 5729–5742. [Google Scholar] [CrossRef]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef]
- Westenberger, G.; Sellers, J.; Fernando, S.; Junkins, S.; Han, S.M.; Min, K.; Lawan, A. Function of Mitogen-Activated Protein Kinases in Hepatic Inflammation. J. Cell Signal. 2021, 2, 172–180. [Google Scholar]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Du, L. Doxorubicin-induced Toxicity Through the p38 MAPK Protein Kinase Pathway. Highlights Sci. Eng. Technol. 2022, 19, 9–15. [Google Scholar] [CrossRef]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Mo, L.Q.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, Y.; Wang, Z.; Ji, Y.; Zhang, X.; Yan, X.; Zhan, Z. Carnosic Acid Induces Antiproliferation and Anti-Metastatic Property of Esophageal Cancer Cells via MAPK Signaling Pathways. J. Oncol. 2021, 2021, 4451533. [Google Scholar] [CrossRef]
- Wang, X.; Gupta, P.; Jramne, Y.; Danilenko, M.; Liu, D.; Studzinski, G.P. Carnosic acid increases sorafenib-induced inhibition of ERK1/2 and STAT3 signaling which contributes to reduced cell proliferation and survival of hepatocellular carcinoma cells. Oncotarget 2020, 11, 3129–3143. [Google Scholar] [CrossRef]
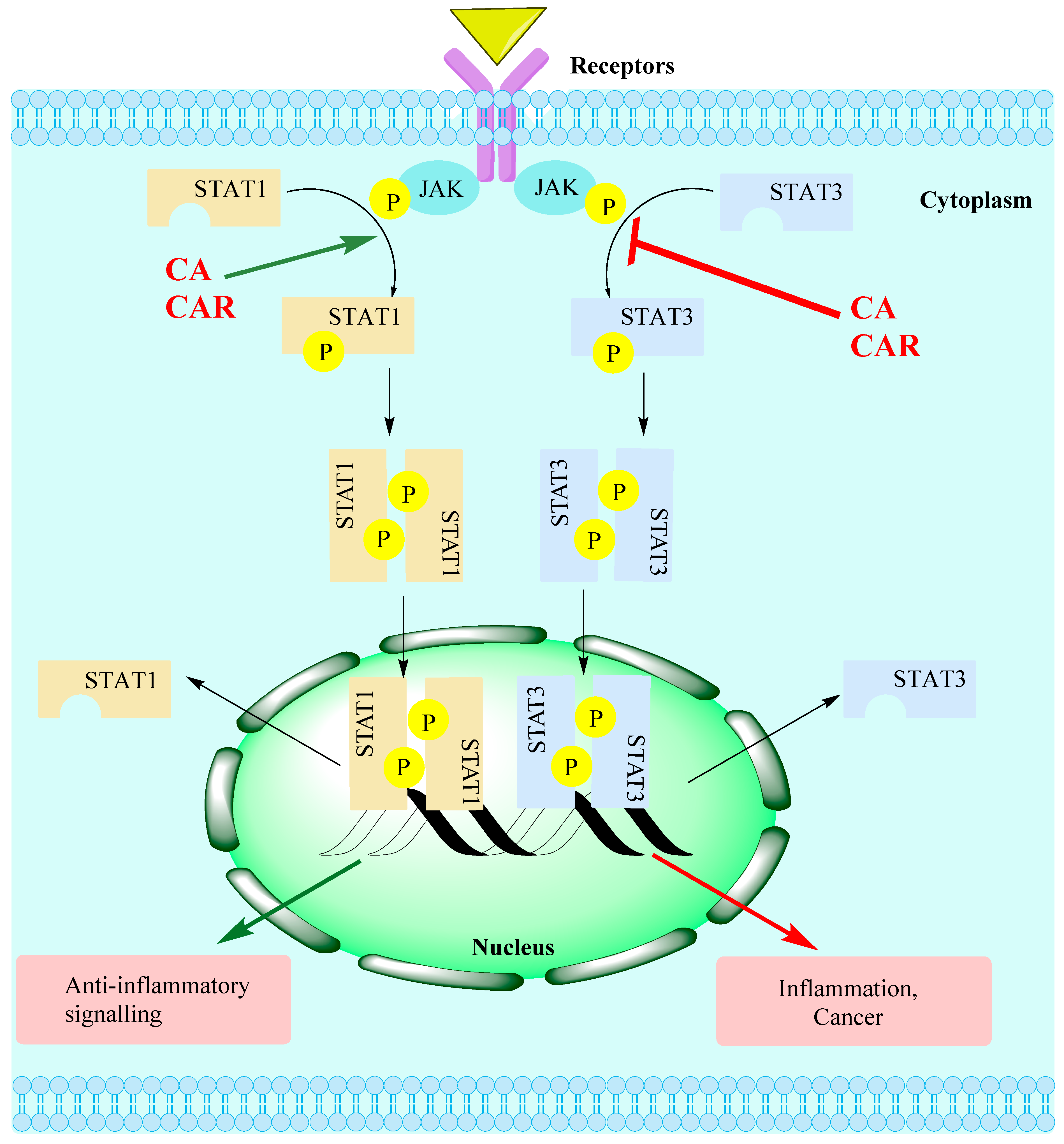
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.-Q.; Sethi, G.; Goh, B.-C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, L.; Chen, X.; Lu, Q.; Yang, Y.; Liu, J.; Ma, X. SIRT1 counteracted the activation of STAT3 and NF-κB to repress the gastric cancer growth. Int. J. Clin. Exp. Med. 2014, 7, 5050–5058. [Google Scholar] [PubMed]
- Li, L.; Sun, Q.; Li, Y.; Yang, Y.; Yang, Y.; Chang, T.; Man, M.; Zheng, L. Overexpression of SIRT1 Induced by Resveratrol and Inhibitor of miR-204 Suppresses Activation and Proliferation of Microglia. J. Mol. Neurosci. 2015, 56, 858–867. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Zhang, Q.; Lu, Y.; Liu, J.; Li, W.; Lv, S.; Zhou, M.; Zhang, X.; Hang, C. Resveratrol Attenuates Early Brain Injury after Experimental Subarachnoid Hemorrhage via Inhibition of NLRP3 Inflammasome Activation. Front. Neurosci. 2017, 11, 611. [Google Scholar] [CrossRef]
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Wang, Y.; Hou, Y.; Li, L.; Zhao, J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017, 50, 208–215. [Google Scholar] [CrossRef]
- Sadeghi, A.; Seyyed Ebrahimi, S.S.; Golestani, A.; Meshkani, R. Resveratrol ameliorates palmitate-induced inflammation in skeletal muscle cells by attenuating oxidative stress and JNK/NF-κB pathway in a SIRT1-independent mechanism. J. Cel. Biochem. 2017, 118, 2654–2663. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef]
- Li, D.; Liu, N.; Zhao, H.H.; Zhang, X.; Kawano, H.; Liu, L.; Zhao, L.; Li, H.P. Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. J. Neuroinflamm. 2017, 14, 67. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Z.T.; Li, L.; Maegele, M.; Zhou, B.Y.; Li, F.; Zhao, M.; Zhao, K.S. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol. Sin. 2017, 38, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef]
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.B.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015–7029. [Google Scholar] [CrossRef]
- Chen, D.L.; Yang, K.Y. Berberine Alleviates Oxidative Stress in Islets of Diabetic Mice by Inhibiting miR-106b Expression and Up-Regulating SIRT1. J. Cell Biochem. 2017, 118, 4349–4357. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxid. Med. Cell Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Ye, B.; Wang, Q.; Xie, X.; Shen, H. Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement. Altern. 2016, 16, 299. [Google Scholar] [CrossRef]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid. Med. Cell Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, M.A.; Rucker, L.G.; Russo, H.M.; Dubyak, G.R. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 2015, 194, 3937–3952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Bartok, E.; Rieger, A.; Franchi, L.; Nuñez, G.; Hornung, V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011, 187, 613–617. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Kong, R.; Sun, L.; Li, H.; Wang, D. The role of NLRP3 inflammasome in the pathogenesis of rheumatic disease. Autoimmunity 2022, 55, 1–7. [Google Scholar] [CrossRef]
- Lu, X.; Tan, Q.; Ma, J.; Zhang, J.; Yu, P. Emerging Role of LncRNA Regulation for NLRP3 Inflammasome in Diabetes Complications. Front. Cell. Dev. Biol. 2022, 9, 792401. [Google Scholar] [CrossRef]
- Vafaei, S.; Taheri, H.; Hajimomeni, Y.; Fakhre Yaseri, A.; Abolhasani Zadeh, F. The role of NLRP3 inflammasome in colorectal cancer: Potential therapeutic target. Clin. Transl. Oncol. 2022, 24, 1881–1889. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar]
- Lee, H.E.; Yang, G.; Kim, N.D.; Jeong, S.; Jung, Y.; Choi, J.Y.; Park, H.H.; Lee, J.Y. Targeting ASC in NLRP3 inflammasome by caffeic acid phenethyl ester: A novel strategy to treat acute gout. Sci. Rep. 2016, 6, 38622. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.P.; Ka, S.M.; Hsu, W.H.; Chen, A.; Chao, L.K.; Lin, C.C.; Hsieh, C.C.; Chen, M.C.; Chiu, H.W.; Ho, C.L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Eltutan, B.I.; Isci, K.B.; Genc, S. Resveratrol Inhibits NLRP3 Inflammasome-Induced Pyroptosis and miR-155 Expression in Microglia Through Sirt1/AMPK Pathway. Neurotox. Res. 2021, 39, 1812–1829. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Zhu, M.J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157:H7. Free Radic. Biol. Med. 2017, 108, 760–769. [Google Scholar] [CrossRef]

| Experimental Model | Compound (Dose) | Main Finding | Reference |
|---|---|---|---|
| RAW 264.7 cells activated by Gram-positive bacteria-derived peptidoglycan, pam3CSK or LPS | CA (5–20 μg/mL) | Inhibits the release of NO, TNF-α and PGE₂; inhibits NF-κB activation and the phosphorylation of Syk/Src, PI3K, Akt, IκBα, IKK and IκBα. | Oh et al. [19] |
| RAW 264.7 cells activated by LPS | CA and CAR (10 μM) | Suppresses MMP-9 and MCP-1 release. | Chae et al. [20] |
| RAW 264.7 cells activated by LPS | CA and CAR (12.5–50 μg/mL) | Suppresses NO production. | Mengoni et al. [21] |
| RAW 264.7 cells activated by LPS | CA (2.5–20 μM) | Inhibits NO, TNF-α and COX-2 expression; suppresses the transcription of inflammatory genes (Nos2, Tnfα, Cox2 and Mcp1); inhibits IKKβ/IκB-α/NF-κB, MAPKs (ERK, JNK and p38) and FoxO1/3 signalling pathways. | Wang et al. [22] |
| RAW 264.7 cells activated by LPS | CAR (IC50 9.4 μM) | Inhibits NO production and iNOS expression (mRNA and protein); inhibits NF-κB translocation and DNA binding activity; inhibits IKK activity and degradation of IκBα; inhibits MAPK (p38 and p44/42) activation. | Lo et al. [23] |
| RAW 264.7 cells activated by LPS | CAR (1, 2 and 5 μM) | Inhibits NO and expression of iNOS and COX-2; inhibits STAT3 phosphorylation and DNA binding activity. | Lee et al. [24] |
| RAW 264.7 cells activated by LPS | CA and CAR (5–15 μM) | Inhibits NO and PGE₂, cytokine (IL-1α and IL-6) and chemokine (CCL5/RANTES, CXCL10/IP-10) production, along with gene expression of iNOS; suppresses nuclear translocation of NF-κBp65. | Schwager et al. [25] |
| Primary mouse bone-marrow-derived macrophages (BMDMs) simulated by LPS | CAR (2.5–40 µM) | Inhibits NLRP3 inflammasome activation and HSP90; inhibits pro-inflammatory cytokine (pro-IL-1β, TNF-α and IL-6) expression. | Shi et al. [26] |
| Human whole-blood simulated by LPS | CA and CAR (IC50 1.9–3.5 μg/mL) | Inhibits the activity of microsomal PGE2 synthase (mPGES)-1. | Bauer et al. [27] Maione et al. [28] |
| Mouse bone-marrow-derived mast cells stimulated by anti-TNP IgE | CA (15 and 50 μM) | Inhibits ROS generation, Ca2+ mobilisation and degranulation; suppresses protein and gene expression of pro-inflammatory cytokines (IL-6, IL-13 and TNF) and chemokines (CCL2, CCL3 and CCL9); reduces phosphorylation of IKK and IκBα, Syk (Tyr352 and 525/526), TAK1 (Ser412) and Akt; decreases the level of NFKB2 mRNA and genes (c-jun, Egr1 and Egr2). | Crozier et al. [29] |
| BV2 mouse microglial cells stimulated by LPS and INF-γ | CA and CAR (5 μM) | Inhibits NO and TNF-α, and PGE2 production; induces HO-1 expression. | Foresti et al. [30] |
| IL-1β- or TNF-α-stimulated human periodontal ligament cells | CA (3.125–50 µM) | Suppresses the release of IL-6 and chemokines’ (CXCL10, CCL2 and CCL20) production; inhibits JNK, NF-κB and STAT3. | Hosokawa et al., 2020 [31] |
| Human oral epithelial cell line (TR146 cells) stimulated by IL-27 | CA (3.125–50 µM) | Suppresses chemokine (CXCL9, CXCL10 and CXCL11) production; inhibits the phosphorylation of STAT1, STAT3 and Akt. | Hosokawa et al., 2019 [32] |
| Bone marrow cells and osteoblasts stimulated by M-CSF | CA (10 or 20 μM) | Inhibits ROS production while augmenting SOD and GPx activity; inhibits the RANKL-mediated activation of NF-κB and MAPKs (JNK and p38) and expression of cytokines (TNF-α, IL-1β and IL-18) and COX2. | Liu et al. [33] |
| Chondrosarcoma cell line SW1353 and primary human chondrocytes stimulated by IL-1β | CA, and CAR (5–15 µM) | Inhibits catabolic genes such as MMP-13 and ADAMTS-4 and nuclear translocation of NF-κBp65. | Schwager et al. [25] |
| Human neutrophils stimulated by fMLF, MMK1 or PMA | CA (1–10 μM) | Suppresses the expression of integrin adhesion molecules (CD11b) and adhesion of neutrophils to endothelial (bEND 3) cells; inhibits the phosphorylation of MAPKs (ERK, JNK and p38). | Tsai et al. [34] |
| Human lung NCI-H1975 cells for H2O2-induced cell death; excised-lung organ culture ischemia model | CAR (3 μM) | Cytoprotection via upregulation of HO-1. | Kawamura et al. [35] |
| Keratinocyte HaCaT cells stimulated with SLS and RA | CA (5–20 μg/mL) | Suppresses the production of IL-6, IL-8 and MCP-1. | Oh et al. [19] |
| SH-SY5Y cells exposed to paraquat | CA (1 μM) | Inhibits NF-κB transcription and IL-1β, TNF-α and COX-2 expression; effect mediated via activation of the Nrf2 and HO-1 signalling pathway. | de Oliveira et al. [36] |
| PC12 cells subjected to serum starvation | CAR (10 µM) | Cytoprotective effect via activation of the HO-1 and Nrf2 pathway. | Martin et al. [37] |
| 6-OHDA-induced neuronal (SH-SY5Y) cell death | CA (1 µM) | Cytoprotective effect through inhibition of the MAPK pathway; inhibition of phosphorylation of JNK and p38. | Wu et al. [38] |
| PC12 cells; hypoxia-induced neuronal cell injury model | CA (1 μM) | Improves cell viability; suppresses ROS generation and lipid peroxidation; PGE2 (also COX-2 activation) NO and pro-inflammatory cytokines (IL-1 and IL-6) production; and ERK, JNK and p38 MAPK activation. | Hou et al. [39] |
| SH-SY5Y | CA (30 µM) | Inhibits Aβ (1-40 and 1-42) production by activating α-secretase, TACE. | Meng et al. [40] |
| U373MG human astrocytoma cells | CA (50 μM) | Inhibits Aβ peptides (1-40, 1-42 and 1-43) by increasing mRNA expression of α-secretase (TACE). | Yoshida et al. [41] |
| 3T3-L1 adipocytes stimulated by TNF-α | CA (1–20 µM) | Inhibits mRNA expression of inflammatory genes (IL-6 and MCP-1), the activation of ERK and JNK, the phosphorylation of IκB and IKK, the nuclear translocation of p65 and the DNA-binding activity of NF-κB and AP-1. | Tsai et al. [42] |
| Rat cardiomyocytes (H9C2 cells); DOX-induced cardiotoxicity | CA (2.4–10 µM) | Suppresses production of ROS and NO and activation or phosphorylation of p38 and JNK; inhibits NF–κB (p65) activation; upregulates Nrf2 and HO-1 levels. | Manna et al. [43] |
| H9C2 cells; DOX-induced cardiotoxicity | CA (5–20 μM) | Suppresses the level of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-18) and COX-2; inhibits NF-κB activation. | Zhang et al. [44] |
| H9C2 cells stimulated by LPS | CAR (5–20 μM) | Inhibits NF-κB activation and cytokine (TNF-α, IL-1β, IL-6) and COX-2 (as well as PGE2) expression; possible direct interaction with IKKβ (in silico study). | Baradaran Rahimi et al. [45] |
| HepG2 cells exposed to ethanol (100 mM) | CA (10 µM) | Inhibits oxidative stress, inflammation and cell death; effect mediated by activation of SIRT1 (see also in vivo effect). | Gao et al. [46] |
| LPS-treated hepatic stellate cells from mice | CA nanoparticles | Deactivates phosphorylated IKKα, IκBα and NF-κB; decreases TNF-α, IL-1β and IL-18 expression; suppresses ROS production while increasing SOD1, SOD2, HO-1 and Nrf-2 levels. | Li et al. [47] |
| 3T3-L1 adipocytes stimulated by LPS | CA (up to 20 µM) | Suppresses TNF-α, IL-6 and MCP-1 mRNA levels; downregulates NF-κB and ERK. | Park and Mun, [48] |
| Human retinal endothelial cells challenged by high glucose | CAR (2.5–20 µM) | Upregulates the expression and activity of Nrf2, HO-1 and ERK1/2; suppresses ROS production and apoptosis. | D’Agata et al. [49] |
| Human lung microvascular endothelial cells (HMVEC-L) challenged by t-BHP | CAR (10 µM) | Increases the expression of Nrf2 and HO-1 while it also interrupts the Nrf2-Keap1 protein−protein interaction; inhibits cell death. | Li et al. [50] |
| HCT-116 cells challenged by thapsigargin | CAR (10 µM) | Ameliorates the induced endoplasmic reticulum stress; suppresses the expression of pro-inflammatory mediators (TNF-α, IL-6, IFN-γ, CXCL10). | Xu et al. [51] |
| Experimental Model | Compound (Dose) | Main Finding | Reference |
|---|---|---|---|
| LPS-induced septic shock in mice | CAR (20 or 40 mg/kg, i.p.) | Prevents NLRP3 inflammasome activation; downregulates the serum levels of IL-1β and TNF-α. | Shi et al. [26] |
| Methionine- and choline-deficient (MCD) diet-fed mouse NASH model | CAR (20 or 40 mg/kg, i.p.) | Suppresses liver injury, fibrosis, NLRP3 inflammasome activation, IL-1β, TNF-α and profibrotic marker alpha-smooth muscle actin (α-SMA). | Shi et al. [26] |
| Adjuvant arthritis model in rats | Methotrexate (0.3 mg/kg) in combination with CA (100 mg/kg, p.o.) | Suppresses hind paw swelling, the levels of IL-17A, MMP-9 and MCP-1 in plasma, and GGT activity in the joint; increases mRNA expression levels of HO-1 and CAT; suppresses IL-1β level in the liver. | Chrastina et al. [52] |
| Collagen-induced arthritis-db/db mice model of rheumatoid arthritis | CA (30 and 60 mg/kg, i.p.) | Improves bone loss coupled with antidiabetic effects (e.g., OGTT and ITT). | Xia et al. [53] |
| Type II collagen-induced arthritis model in mice | CAR (40 mg/kg, p.o.) or rosmanol (40 mg/kg/d, p.o.) | Alleviates swelling, redness and synovitis; decreases the arthritis index score and the serum level of pro-inflammatory cytokines (IL-6, MCP-1 and TNF-α); blocks NF-κB and MAPK (JNK and p38 MAPK) pathways; better result in drug combination with rosmanol. | Li et al. [54] |
| ARDS in mice induced by LPS | CA (5 or 10 mg/kg, i.v.) | Improves inflammatory status (histology); reduces MPO activities, neutrophil infiltration and lipid peroxidation. | Tsai et al. [34] |
| LPS-induced acute lung injury (ALI) experimental model in mice | CA (10, 20 and 40 mg/kg, i.p.) | In addition to histological improvement, reduces the production (mRNA and protein) of IL-1β, IL-6, TNF-α, TLR4 and NF-κB expression and NF-κB phosphorylation in lung tissues. | Li et al. [55] |
| Bleomycin-induced lung damage in rats | CAR (10, 20 and 40 mg/kg, p.o.) | Reduces oxidative markers (MDA, NO, protein carbonyl), proinflammatory cytokines (TNF-α and IL-6 levels) and MPO activity in the lungs; increases GSH content and activities of CAT, GPx and SOD; reduces lung fibrosis. | Kalantar et al. [56] |
| Ovalbumin-induced allergic asthma in mice | CAR (5 mg/kg, i.p.) | Reduces eosinophils in the bronchoalveolar lavage fluids, and pro-inflammatory cytokines’ production (IL-4 and IL-13) in the bronchoalveolar lavage fluids and the lungs. | Lee and Im [57] |
| PMA-induced ear inflammation in mice | CA and CAR-EC50 values for reduction of oedema of 10.20 μg/cm2 and 10.70 μg/cm2, respectively | Reduces oedema, ulceration, leucocyte infiltration and expression levels of IL-1β, TNF-α and COX-2. | Mengoni et al. [21] |
| Carrageenan-induced oedema model in mice | CAR (1–10 mg/ kg, i.p.) | Reduces oedema; decreases MPO, NO and IL-17A; increases the level of anti-inflammatory cytokine, IL-10. | da Rosa et al. [58] |
| Atopic dermatitis in mice induced by 5% phthalic anhydride | CAR (0.05 µg/cm2) | Inhibits the expression of iNOS and COX-2 in skin tissue; inhibits STAT3 in skin tissue; reduces the serum levels of TNF-α, IL-1β and IgE. | Lee et al., 2017 [24] |
| UVB-induced skin inflammation in mice | CAR (0.05 µg/cm2) | Reduces erythema, epidermal thickness and serum levels of IgE and IL-1β; suppresses iNOS and COX-2; decreases activation of STAT3 and JAK. | Yeo et al. [59] |
| Carrageenan-induced oedema model in mice | CA (30 or 100 µg per paw) | Reduces oedema and levels of microsomal prostaglandin E synthase-1 (mPGES-1) and 5-LO-derived products. | Maione et al. [28] |
| 6-OHDA model of PD in rats | CA (20 mg/kg, p.o.) | Improves behavioural changes along with LPO, GSH and SOD. | Wu et al. [38] |
| Chlorpyrifos-induced neuronal damage in mice | CA (30 and 60 mg/kg p.o.) | Suppresses the serum level of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in cerebral and ocular tissues, reverses the decrease in AChE and antioxidant markers (GSH, GPx, SOD and CAT) and reduces pro-oxidant (MDA and NO) markers. | AlKahtane et al. [60] |
| Mild TBI in mice | CA (1 mg/kg, i.p.) | Improves motor and cognitive dysfunction, activates Nrf2 and suppresses NF-κB. | Maynard et al. [61] |
| Spinal cord injury in rats | CAR (5 mg/kg, i.p.) | Activates Nrf2; reduces ROS generation, LPO content, protein carbonyl and sulfhydryl levels; increases antioxidant status (SOD, CAT GPx, GSH, GSH-S-transferase); inhibits NF-κB and COX-2 expression; reverses the reduction in phosphor-Akt. | Wang et al. [62] |
| Traumatic brain injury in mice | CA (0.3, 1.0 or 3.0 mg/kg, i.p.) | Activates the Nrf2–ARE pathways; improves mitochondrial respiratory dysfunction, lipid peroxidation and protein nitration in brain tissues. | Miller et al. [63] |
| Subarachnoid haemorrhage brain injury model in rats | CA (3 mg/kg, i.p.) | Increases SIRT1, MnSOD and Bcl-2 in addition to improving brain oedema and neuronal structure and function. | Teng et al. [64] |
| APP/PS1 mouse model of AD | CA (10 or 30 mg/kg, p.o) | Reduces Aβ deposition, cognitive decline and levels of pro-inflammatory cytokine (IL-1β, TNFα and IL-6) production; inhibits Aβ secretion and interaction between CEBPβ and NFκB p65. | Yi-Bin et al. [65] |
| Experimental autoimmune encephalomyelitis in mice | CAR (50 mg/kg, i.p.) | Reduces demyelination and inhibits Th17 cell differentiation and STAT3 phosphorylation; blocks translocation of NF-κB; switches macrophage/microglia to non-inflammatory phenotype. | Li et al. [66] |
| STZ-induced diabetic rats | CAR (1, 5, 10 mg/kg/day, i.p. for 4 weeks) | Suppresses serum levels of glucose, IL-6, TNF-α, MDA, TG, TC, LDL-C, GST, SOD, CAT and HDL-C in a dose-dependent manner. | Samarghandian et al. [67] |
| STZ-induced diabetes in rats | CA (30 mg/kg) | Reduces glucose level in diabetic rats; reduces MDA and glycated end products, tissue damage and inflammation score; reverses change in the gut microbiota population. | Ou et al. [68] |
| STZ-induced diabetic mice db/db mice | CA (15 or 30 mg/kg, i.g.) | Nephroprotective effect coupled with activation of Nrf2 and inhibition of NF-κB. | Xie et al. [69] |
| Ischaemia/reperfusion model in diabetic mice | CA (50 mg/kg, p.o.) | Suppresses ROS and pro-inflammatory cytokine (IL-6 and TNF-α) production. | Hu et al. [70] |
| DOX-induced cardiotoxicity in rats | CA (10 mg/kg, p.o.) | Decreases the levels of ROS, NO, phospho-p38, phospho-JNK1 proteins and NF–κB (p65); reverses the downregulation of Nrf2 in the nucleus and HO-1 in the cardiomyocytes. | Manna et al. [43] |
| DOX-induced cardiotoxicity mice | CA or Carvedilol (5 mg/kg, p.o.) | Ameliorates cardiac injury and suppresses the levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-18) and COX-2, and NF-κB; reverses the reduced antioxidant level (GSH) or activity (SOD, CAT and NQO-1) and the increased oxidative stress (MDA level); increases Nrf2 in heart tissue; drug combination offers better result. | Zhang et al. [44] |
| Chronic alcoholic liver injury model in rats | (15 or 30 mg/kg, i.g.) | Activates SIRT1 and increases MnSOD; suppresses NF-κB and serum level of TNF-α. | Gao et al. [46] |
| Ischemia/reperfusion model of liver damage in rats | CA (10 and 20 mg/kg, i.p.) | Normalises the levels of SOD, CAT and GSH and GPx) and the NF-κB signalling pathway of pro-inflammatory cytokine (TNF-α and IL-1β) expression. | Li et al. [47] |
| HFD-induced NAFLD model in mice | CA (15 mg/kg, p.o.) | Improves glucose and insulin tolerance; suppresses the serum and hepatic levels of IL-1β, IL-18, TNF-α, IL-2, IL-4, IL-6, IL-12 and IFN-γ; reverses the low-level MARCKS under diabetes; ameliorates the diabetes-associated activation of PI3K/Akt, NLRP3/NF-κB and SREBP-1c signalling pathway. | Song et al. [71] |
| LPS-induced liver injury in rats | CA (30 or 60 mg/kg, p.o.) | Ameliorates liver damage (histology and biochemical markers) and suppresses inflammatory cells’ infiltration and the serum level of pro-inflammatory cytokines (TNF-α and IL-6); increases antioxidant levels (SOD, GSH and GPx) in serum and liver. | Xiang et al. [72] |
| LPS-induced liver injury in mice | CA (40 mg/kg, i.p.) | Inhibits the expression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α and MCP-1, mRNA and protein), NOX4 (mRNA and protein) immune cell (neutrophil) infiltration and NF-κB activation; increases GSH, CAT and MnSOD. | Kim et al. [73] |
| Renal ischemia-reperfusion injury in rats | CAR (3 mg/kg, i.v.) | Inhibits apoptotic tubular cell death and activation of the p38 pathway. | Zheng et al., 2018 [74] |
| NASH model in mice | CAR (20 or 40 mg/kg, i.p.) | Suppresses NLRP3 inflammasome activity via direct effect on heat-shock protein 90 (HSP90). | Shi et al. [26] |
| HFD-induced mouse obesity and metabolic syndrome model | CA (10 or 20 mg/kg, p.o.) | Downregulates the levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in serum and brain tissues, and the NF-κB signalling pathway. | Liu et al. [75] |
| Dextran sulphate sodium (DSS) experimental model of colitis mice | CAR (50 mg/kg i.p.) | Reduces inflammatory cell infiltration and pro-inflammatory cytokine (TNF-α, IL-1β, IL-6 and IFN-γ) expression. | Xu et al. [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines 2023, 11, 545. https://doi.org/10.3390/biomedicines11020545
Habtemariam S. Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines. 2023; 11(2):545. https://doi.org/10.3390/biomedicines11020545
Chicago/Turabian StyleHabtemariam, Solomon. 2023. "Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol" Biomedicines 11, no. 2: 545. https://doi.org/10.3390/biomedicines11020545
APA StyleHabtemariam, S. (2023). Anti-Inflammatory Therapeutic Mechanisms of Natural Products: Insight from Rosemary Diterpenes, Carnosic Acid and Carnosol. Biomedicines, 11(2), 545. https://doi.org/10.3390/biomedicines11020545






