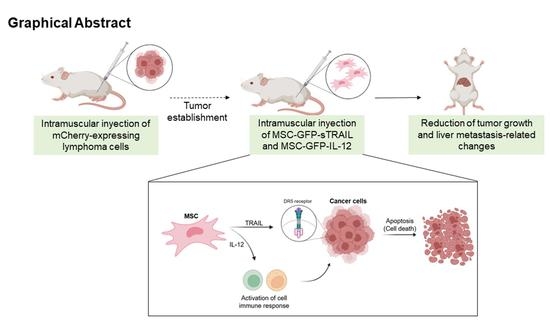
Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of MSC
2.2. Evaluation of BM-MSC Multipotency
2.3. Lentiviral Transduction of BM-MSC
2.4. Transgene Expression Validation
2.5. Quantification of Soluble TRAIL
2.6. Lymphoma Cell Gene Modification
2.7. Metastasis Analysis
2.8. Experimental Treatments
2.9. Survival Time Determination
2.10. Post-Implant Tumor Evolution and Metastasis Analysis
2.11. Statistical Analysis
2.12. Ethics
3. Results
3.1. Genetic Modified BM-MSC Are Capable of sTRAIL and IL-12 Overexpression
3.2. L5178Y Lymphoma Cell Line Develops Liver Metastasis
3.3. The L5178Y Lymphoma Cell Line Expresses TRAIL Receptor
3.4. IL-12 MSC and the Combination plus sTRAIL MSC Reduce Tumor Growth and Improve Mice Survival
3.5. IL-12 MSC and sTRAIL plus IL-12 MSC Reduce Tumor Cell Infiltration and Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorburn, A. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Pathway Signaling. J. Thorac. Oncol. 2007, 2, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spano, C.; Grisendi, G.; Golinelli, G.; Rossignoli, F.; Prapa, M.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; Miselli, F.; et al. Soluble TRAIL Armed Human MSC as Gene Therapy for Pancreatic Cancer. Sci. Rep. 2019, 9, 1788. [Google Scholar] [CrossRef] [Green Version]
- Rossignoli, F.; Spano, C.; Grisendi, G.; Foppiani, E.M.; Golinelli, G.; Mastrolia, I.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; et al. MSC-delivered soluble TRAIl and paclitaxel as novel combinatory treatment for pancreatic adenocarcinoma. Theranostics 2019, 9, 436–448. [Google Scholar] [CrossRef]
- Quiroz-Reyes, A.G.; González-Villarreal, C.A.; Martínez-Rodriguez, H.; Said-Fernández, S.; Salinas-Carmona, M.C.; Limón-Flores, A.Y.; Soto-Domínguez, A.; Padilla-Rivas, G.; De Oca-Luna, R.M.; Islas, J.F.; et al. A combined antitumor strategy of separately transduced mesenchymal stem cells with soluble TRAIL and IFNβ produces a synergistic activity in the reduction of lymphoma and mice survival enlargement. Mol. Med. Rep. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Weiss, J.M.; Subleski, J.J.; Wigginton, J.M.; Wiltrout, R.H. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin. Biol. Ther. 2007, 7, 1705–1721. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Rad, A.A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar]
- Yuan, Z.; Kolluri, K.K.; Sage, E.K.; Gowers, K.H.; Janes, S.M. Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy. Cytotherapy 2015, 17, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- National Research Council. Rodents; National Academies Press: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Cheng, S.; Nethi, S.K.; Rathi, S.; Layek, B.; Prabha, S. Engineered mesenchymal stem cells for targeting solid tumors: Therapeutic potential beyond regenerative therapy. J. Pharmacol. Exp. Ther. 2019, 370, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Elzaouk, L.; Moelling, K.; Pavlovic, J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp. Dermatol. 2006, 15, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Liu, X.; Lv, P. Expression of interleukin-12 by adipose-derived mesenchymal stem cells for treatment of lung adenocarcinoma. Thorac. Cancer 2015, 6, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hwang, H.S.; Na, K. TRAIL-secreting human mesenchymal stem cells engineered by a non-viral vector and photochemical internalization for pancreatic cancer gene therapy. Biomaterials 2018, 182, 259–268. [Google Scholar] [CrossRef]
- Ryu, C.H.; Park, S.-H.; Park, S.A.; Kim, S.M.; Lim, J.Y.; Jeong, C.H.; Yoon, W.-S.; Oh, W.-I.; Sung, Y.C.; Jeun, S.-S. Gene Therapy of Intracranial Glioma Using Interleukin-12 Secreting Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells. Hum. Gene Ther. 2011, 22, 733–743. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Chen, X.; Lin, X.; Zhao, J.; Shi, W.; Zhang, H.; Wang, Y.; Kan, B.; Du, L.; Wang, B.; Wei, Y.; et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol. Ther. 2008, 16, 749–756. [Google Scholar] [CrossRef]
- Nurieva, R.; Wang, J.; Sahoo, A. T-cell tolerance in cancer. Immunotherapy 2013, 5, 513–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Zhao, J.; Xu, J.; Wen, Y. Mesenchymal stem cells genetically modified by lentivirus-mediated interleukin-12 inhibit malignant ascites in mice. Exp. Ther. Med. 2014, 8, 1330–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Lim, J.Y.; Park, S.I.; Jeong, C.H.; Oh, J.H.; Jeong, M.; Oh, W.; Sung, Y.-C.; Jeun, S.-S. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008, 68, 9614–9623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studeny, M.; Marini, F.C.; Dembinski, J.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal Stem Cells: Potential Precursors for Tumor Stroma and Targeted-Delivery Vehicles for Anticancer Agents. J. Natl. Cancer Inst. 2004, 96, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.-Y.; Lee, E.-J.; Kim, S.J.; Yang, S.-H.; Sung, Y.C.; Seong, J. Irradiation-induced localization of IL-12 expressing mesenchymal stem cells to enhance the curative effect in murine metastatic hepatoma. Int. J. Cancer 2015, 137, 721–730. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Ding, Q.; Wu, Z.; Jiang, H.; Fang, Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010, 290, 157–166. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, K.S.; Park, S.H.; Suh, Y.S.; Kim, S.J.; Jeun, S.-S.; Sung, Y.C. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011, 18, 488. [Google Scholar] [CrossRef]
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, X.; Zhao, J.; Liu, T.; Xu, J. Therapeutic effects of bone marrow mesenchymal stem cells expressing interleukin-12 in mice bearing malignant ascites tumor. Int. J. Clin. Exp. Med. 2015, 8, 15840–15845. [Google Scholar]
- Fakiruddin, K.S.; Lim, M.N.; Nordin, N.; Rosli, R.; Zakaria, Z.; Abdullah, S. Targeting of CD133+ cancer stem cells by mesenchymal stem cell expressing TRAIL reveals a prospective role of apoptotic gene regulation in non-small cell lung cancer. Cancers 2019, 11, 1261. [Google Scholar] [CrossRef] [Green Version]
- Eom, Y.W.; Akter, R.; Li, W.; Lee, S.; Hwang, S.; Kim, J.; Cho, M.-Y. M1 macrophages promote trail expression in adipose tissue-derived stem cells, which suppresses colitis-associated colon cancer by increasing apoptosis of cd133+ cancer stem cells and decreasing m2 macrophage population. Int. J. Mol. Sci. 2020, 21, 3887. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz-Reyes, A.G.; Gonzalez-Villarreal, C.A.; Limon-Flores, A.Y.; Delgado-Gonzalez, P.; Martinez-Rodriguez, H.G.; Said-Fernandez, S.L.; Soto-Dominguez, A.; Rivas-Estilla, A.M.; Islas, J.F.; Molina-De la Garza, J.F.; et al. Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model. Biomedicines 2023, 11, 595. https://doi.org/10.3390/biomedicines11020595
Quiroz-Reyes AG, Gonzalez-Villarreal CA, Limon-Flores AY, Delgado-Gonzalez P, Martinez-Rodriguez HG, Said-Fernandez SL, Soto-Dominguez A, Rivas-Estilla AM, Islas JF, Molina-De la Garza JF, et al. Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model. Biomedicines. 2023; 11(2):595. https://doi.org/10.3390/biomedicines11020595
Chicago/Turabian StyleQuiroz-Reyes, Adriana G., Carlos A. Gonzalez-Villarreal, Alberto Y. Limon-Flores, Paulina Delgado-Gonzalez, Herminia G. Martinez-Rodriguez, Salvador L. Said-Fernandez, Adolfo Soto-Dominguez, Ana M. Rivas-Estilla, Jose F. Islas, Juan F. Molina-De la Garza, and et al. 2023. "Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model" Biomedicines 11, no. 2: 595. https://doi.org/10.3390/biomedicines11020595
APA StyleQuiroz-Reyes, A. G., Gonzalez-Villarreal, C. A., Limon-Flores, A. Y., Delgado-Gonzalez, P., Martinez-Rodriguez, H. G., Said-Fernandez, S. L., Soto-Dominguez, A., Rivas-Estilla, A. M., Islas, J. F., Molina-De la Garza, J. F., & Garza-Treviño, E. N. (2023). Mesenchymal Stem Cells Genetically Modified by Lentivirus-Express Soluble TRAIL and Interleukin-12 Inhibit Growth and Reduced Metastasis-Relate Changes in Lymphoma Mice Model. Biomedicines, 11(2), 595. https://doi.org/10.3390/biomedicines11020595