Revisiting the Pathogenesis of Type 1 Diabetes: Importance of Neural Input to Pancreatic Islets and the Therapeutic Capability of Stem Cell Educator TM Therapy to Restore Their Integrity
Abstract
1. Introduction
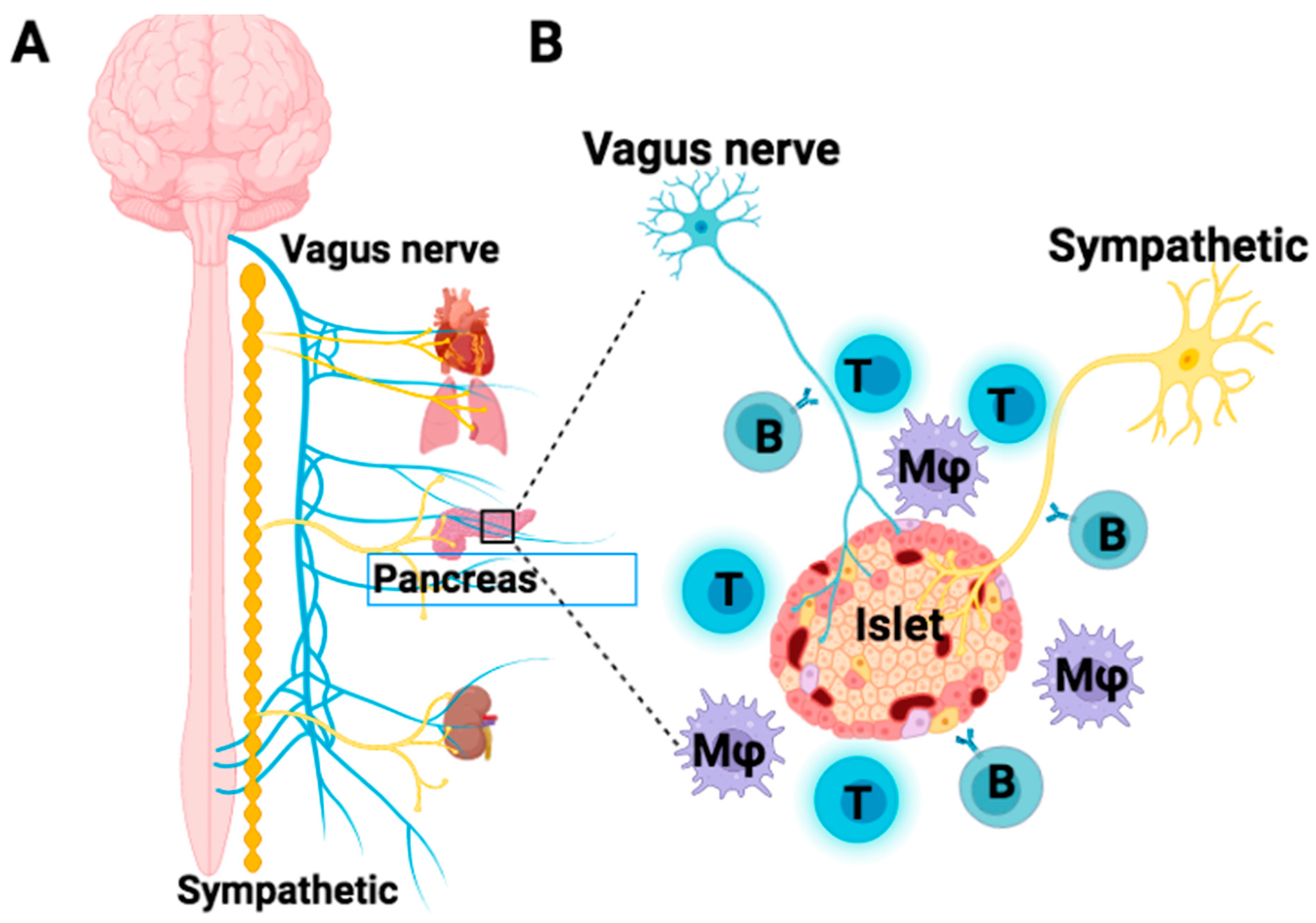
2. Pancreatic Islet Innervation Contributes to the Normalization of Islet β-Cell Function
3. Cross-Reaction of T1D Autoimmunity between Islet β-Cells and Autonomic Nerves
4. Infiltration of Autoimmune Cells against the Islet Nerves
5. Reversal of T1D by the Treatment with Stem Cell Educator TM Therapy
5.1. Correct the Autoimmunity through the Induction of Immune Tolerance by Stem Cell Educator TM Therapy
5.2. Overcome the Shortage of Islet β Cells through the Alternative Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sapede, D.; Cau, E. The pineal gland from development to function. Curr. Top. Dev. Biol. 2013, 106, 171–215. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tirgoviste, C.; Gagniuc, P.A.; Gubceac, E.; Mardare, L.; Popescu, I.; Dima, S.; Militaru, M. A 3D map of the islet routes throughout the healthy human pancreas. Sci. Rep. 2015, 5, 14634. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.O.; Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2339. [Google Scholar] [CrossRef] [PubMed]
- Brissova, M.; Fowler, M.J.; Nicholson, W.E.; Chu, A.; Hirshberg, B.; Harlan, D.M.; Powers, A.C. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 2005, 53, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Xavier, G. The Cells of the Islets of Langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; Pucino, V.; De Rosa, V.; Marone, G.; Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 2014, 5, 143. [Google Scholar] [CrossRef]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef]
- Trifunovic, S.; Stevanovic, I.; Milosevic, A.; Ristic, N.; Janjic, M.; Bjelobaba, I.; Savic, D.; Bozic, I.; Jakovljevic, M.; Tesovic, K.; et al. The Function of the Hypothalamic-Pituitary-Adrenal Axis During Experimental Autoimmune Encephalomyelitis: Involvement of Oxidative Stress Mediators. Front. Neurosci. 2021, 15, 649485. [Google Scholar] [CrossRef]
- Webster, J.I.; Tonelli, L.; Sternberg, E.M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002, 20, 125–163. [Google Scholar] [CrossRef]
- Zhao, Y.; Knight, C.M.; Jiang, Z.; Delgado, E.; Van Hoven, A.M.; Ghanny, S.; Zhou, Z.; Zhou, H.; Yu, H.; Hu, W.; et al. Stem Cell Educator therapy in type 1 diabetes: From the bench to clinical trials. Autoimmun. Rev. 2022, 21, 103058. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Z.; Zhao, T.; Ye, M.; Hu, C.; Yin, Z.; Li, H.; Zhang, Y.; Diao, Y.; Li, Y.; et al. Reversal of type 1 diabetes via islet beta cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.K.; Olaker, V.R.; Kaelber, D.C.; Xu, R.; Davis, P.B. Association of SARS-CoV-2 Infection With New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021. JAMA Netw. Open 2022, 5, e2233014. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, B.L.; Yu, J.; Tanaka, C.; Longhurst, C.A.; Kim, J.J. Incidence of New-Onset Type 1 Diabetes Among US Children During the COVID-19 Global Pandemic. JAMA Pediatr. 2022, 176, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Lehuen, A.; Diana, J.; Zaccone, P.; Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 2010, 10, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef]
- Calderon, B.; Carrero, J.A.; Ferris, S.T.; Sojka, D.K.; Moore, L.; Epelman, S.; Murphy, K.M.; Yokoyama, W.M.; Randolph, G.J.; Unanue, E.R. The pancreas anatomy conditions the origin and properties of resident macrophages. J. Exp. Med. 2015, 212, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; McCarthy, D.P.; Ferris, S.T.; Wan, X.; Hu, H.; Zinselmeyer, B.H.; Vomund, A.N.; Unanue, E.R. Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10418–E10427. [Google Scholar] [CrossRef]
- Zinselmeyer, B.H.; Vomund, A.N.; Saunders, B.T.; Johnson, M.W.; Carrero, J.A.; Unanue, E.R. The resident macrophages in murine pancreatic islets are constantly probing their local environment, capturing beta cell granules and blood particles. Diabetologia 2018, 61, 1374–1383. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Leete, P.; Willcox, A.; Krogvold, L.; Dahl-Jorgensen, K.; Foulis, A.K.; Richardson, S.J.; Morgan, N.G. Differential Insulitic Profiles Determine the Extent of beta-Cell Destruction and the Age at Onset of Type 1 Diabetes. Diabetes 2016, 65, 1362–1369. [Google Scholar] [CrossRef]
- Leete, P.; Mallone, R.; Richardson, S.J.; Sosenko, J.M.; Redondo, M.J.; Evans-Molina, C. The Effect of Age on the Progression and Severity of Type 1 Diabetes: Potential Effects on Disease Mechanisms. Curr. Diab. Rep. 2018, 18, 115. [Google Scholar] [CrossRef]
- Greenbaum, C.J.; Beam, C.A.; Boulware, D.; Gitelman, S.E.; Gottlieb, P.A.; Herold, K.C.; Lachin, J.M.; McGee, P.; Palmer, J.P.; Pescovitz, M.D.; et al. Fall in C-peptide during first 2 years from diagnosis: Evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012, 61, 2066–2073. [Google Scholar] [CrossRef]
- Mundinger, T.O.; Mei, Q.; Foulis, A.K.; Fligner, C.L.; Hull, R.L.; Taborsky, G.J., Jr. Human Type 1 Diabetes Is Characterized by an Early, Marked, Sustained, and Islet-Selective Loss of Sympathetic Nerves. Diabetes 2016, 65, 2322–2330. [Google Scholar] [CrossRef]
- Carrillo, J.; Puertas, M.C.; Alba, A.; Ampudia, R.M.; Pastor, X.; Planas, R.; Riutort, N.; Alonso, N.; Pujol-Borrell, R.; Santamaria, P.; et al. Islet-infiltrating B-cells in nonobese diabetic mice predominantly target nervous system elements. Diabetes 2005, 54, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Mundinger, T.O.; Lernmark, A.; Taborsky, G.J., Jr. Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 2002, 51, 2997–3002. [Google Scholar] [CrossRef] [PubMed]
- Taborsky, G.J., Jr.; Mei, Q.; Hackney, D.J.; Figlewicz, D.P.; LeBoeuf, R.; Mundinger, T.O. Loss of islet sympathetic nerves and impairment of glucagon secretion in the NOD mouse: Relationship to invasive insulitis. Diabetologia 2009, 52, 2602–2611. [Google Scholar] [CrossRef]
- Kiba, T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: Recent developments. Pancreas 2004, 29, e51–e58. [Google Scholar] [CrossRef]
- Hampton, R.F.; Jimenez-Gonzalez, M.; Stanley, S.A. Unravelling innervation of pancreatic islets. Diabetologia 2022, 65, 1069–1084. [Google Scholar] [CrossRef]
- Brunicardi, F.C.; Shavelle, D.M.; Andersen, D.K. Neural regulation of the endocrine pancreas. Int. J. Pancreatol. 1995, 18, 177–195. [Google Scholar] [CrossRef]
- Lausier, J.; Diaz, W.C.; Roskens, V.; LaRock, K.; Herzer, K.; Fong, C.G.; Latour, M.G.; Peshavaria, M.; Jetton, T.L. Vagal control of pancreatic ss-cell proliferation. Am. J. Physiol Endocrinol. Metab. 2010, 299, E786–E793. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Speier, S.; Molano, R.D.; Formoso, A.; Gans, I.; Abdulreda, M.H.; Cabrera, O.; Molina, J.; Fachado, A.; Ricordi, C.; et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc. Natl. Acad. Sci. USA 2012, 109, 21456–21461. [Google Scholar] [CrossRef] [PubMed]
- Lkhagvasuren, B.; Mee-Inta, O.; Zhao, Z.W.; Hiramoto, T.; Boldbaatar, D.; Kuo, Y.M. Pancreas-Brain Crosstalk. Front. Neuroanat. 2021, 15, 691777. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.C.; Almarghalani, D.A.; Gibson, H.M.; Meqdad, M.A.; Antypas, R.B.; Lingireddy, A.; AbouAlaiwi, W.A. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol. Genom. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Rodriguez-Diaz, R.; Fachado, A.; Jacques-Silva, M.C.; Berggren, P.O.; Caicedo, A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014, 63, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Dando, R.; Jacques-Silva, M.C.; Fachado, A.; Molina, J.; Abdulreda, M.H.; Ricordi, C.; Roper, S.D.; Berggren, P.O.; Caicedo, A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011, 17, 888–892. [Google Scholar] [CrossRef]
- Delgado, E.; Perez-Basterrechea, M.; Suarez-Alvarez, B.; Zhou, H.; Revuelta, E.M.; Garcia-Gala, J.M.; Perez, S.; Alvarez-Viejo, M.; Menendez, E.; Lopez-Larrea, C.; et al. Modulation of Autoimmune T-Cell Memory by Stem Cell Educator Therapy: Phase 1/2 Clinical Trial. EBioMedicine 2015, 2, 2024–2036. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Abdulreda, M.H.; Formoso, A.L.; Gans, I.; Ricordi, C.; Berggren, P.O.; Caicedo, A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011, 14, 45–54. [Google Scholar] [CrossRef]
- Sorenson, R.L.; Garry, D.G.; Brelje, T.C. Structural and functional considerations of GABA in islets of Langerhans. Beta-cells and nerves. Diabetes 1991, 40, 1365–1374. [Google Scholar] [CrossRef]
- Saravia-Fernandez, F.; Faveeuw, C.; Blasquez-Bulant, C.; Tappaz, M.; Throsby, M.; Pelletier, G.; Vaudry, H.; Dardenne, M.; Homo-Delarche, F. Localization of gamma-aminobutyric acid and glutamic acid decarboxylase in the pancreas of the nonobese diabetic mouse. Endocrinology 1996, 137, 3497–3506. [Google Scholar] [CrossRef]
- Burton, A.R.; Baquet, Z.; Eisenbarth, G.S.; Tisch, R.; Smeyne, R.; Workman, C.J.; Vignali, D.A. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J. Immunol. 2010, 184, 4863–4870. [Google Scholar] [CrossRef]
- Lampasona, V.; Liberati, D. Islet Autoantibodies. Curr. Diab. Rep. 2016, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, F.; Devergnas, S.; Pattou, F.; Schuit, F.; Garcia-Cuenca, R.; Vandewalle, B.; Kerr-Conte, J.; Van Lommel, L.; Grunwald, D.; Favier, A.; et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006, 119, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Tsui, H.; Lau, A.; Song, A.; Li, X.; Cheung, R.K.; Sampson, A.; Afifiyan, F.; Elford, A.; Jackowski, G.; et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat. Med. 2003, 9, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Shen, C.N.; Lin, P.Y.; Peng, S.J.; Chien, H.J.; Chou, Y.H.; Chamberlain, C.E.; Pasricha, P.J. Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology. Diabetologia 2018, 61, 158–167. [Google Scholar] [CrossRef]
- Brode, S.; Raine, T.; Zaccone, P.; Cooke, A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 2006, 177, 6603–6612. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, C.; Hwang, D.; Lin, B.; Dingeldein, M.; Mihailescu, D.; Sam, S.; Sidhwani, S.; Zhang, Y.; Jain, S.; et al. Selective destruction of mouse islet beta cells by human T lymphocytes in a newly-established humanized type 1 diabetic model. Biochem. Biophys. Res. Commun. 2010, 399, 629–636. [Google Scholar] [CrossRef]
- Tomita, T. PGP 9.5 immunocytochemical staining for pancreatic endocrine tumors. Islets 2013, 5, 122–128. [Google Scholar] [CrossRef]
- Tamariz, E.; Varela-Echavarria, A. The discovery of the growth cone and its influence on the study of axon guidance. Front. Neuroanat. 2015, 9, 51. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, B.; Darflinger, R.; Zhang, Y.; Holterman, M.J.; Skidgel, R.A. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS ONE 2009, 4, e4226. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Z.; Delgado, E.; Li, H.; Zhou, H.; Hu, W.; Perez-Basterrechea, M.; Janostakova, A.; Tan, Q.; Wang, J.; et al. Platelet-Derived Mitochondria Display Embryonic Stem Cell Markers and Improve Pancreatic Islet beta-cell Function in Humans. Stem Cells Transl. Med. 2017, 6, 1684–1697. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Z.; Zhao, T.; Ye, M.; Hu, C.; Zhou, H.; Yin, Z.; Chen, Y.; Zhang, Y.; Wang, S.; et al. Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: Phase I/II clinical trial. BMC Med. 2013, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, B.; Wang, H.; Li, H.; Li, Q.; Zhao, D.; Chen, Y.; Zhang, Y.; Li, W.; Zhang, J.; et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, X.; Yu, H.; Sun, J.; Zhao, Y. Released Exosomes Contribute to the Immune Modulation of Cord Blood-Derived Stem Cells. Front. Immunol. 2020, 11, 165. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Qi, M.; Lazzarini, P.; Mazzone, T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol. Lett. 2007, 108, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Hu, W.; Song, X.; Zhao, Y. Immune Modulation of Platelet-Derived Mitochondria on Memory CD4+ T Cells in Humans. Int. J. Mol. Sci. 2020, 21, 6295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Stem Cell Educator Therapy and Induction of Immune Balance. Curr. Diab. Rep. 2012, 12, 517–523. [Google Scholar] [CrossRef]
- Hu, W.; Song, X.; Yu, H.; Sun, J.; Zhao, Y. Differentiation of Monocytes into Phenotypically Distinct Macrophages After Treatment with Human Cord Blood Stem Cell (CB-SC)-Derived Exosomes. J. Vis. Exp. 2020, 165, e61562. [Google Scholar] [CrossRef]
- Hu, W.; Song, X.; Yu, H.; Fan, S.; Shi, A.; Sun, J.; Wang, H.; Zhao, L.; Zhao, Y. Suppression of B-Cell Activation by Human Cord Blood-Derived Stem Cells (CB-SC) through the Galectin-9-Dependent Cell Contact Mechanism. BioRxiv 2021. [Google Scholar] [CrossRef]
- Richardson, T.M.; Saunders, D.C.; Haliyur, R.; Shrestha, S.; Cartailler, J.P.; Reinert, R.B.; Petronglo, J.; Bottino, R.; Aramandla, R.; Bradley, A.M.; et al. Human pancreatic capillaries and nerve fibers persist in type 1 diabetes despite beta cell loss. Am. J. Physiol. Endocrinol. Metab. 2023. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.; Butterworth, E.A.; Boatwright, J.L.; Nair, M.A.; Nasif, L.H.; Nasif, K.; Revell, A.Y.; Riva, A.; Mathews, C.E.; Gerling, I.C.; et al. Islet sympathetic innervation and islet neuropathology in patients with type 1 diabetes. Sci. Rep. 2021, 11, 6562. [Google Scholar] [CrossRef]
- Lundberg, M.; Lindqvist, A.; Wierup, N.; Krogvold, L.; Dahl-Jorgensen, K.; Skog, O. The density of parasympathetic axons is reduced in the exocrine pancreas of individuals recently diagnosed with type 1 diabetes. PLoS ONE 2017, 12, e0179911. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Van, D.A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Agulnick, A.D.; Ambruzs, D.M.; Moorman, M.A.; Bhoumik, A.; Cesario, R.M.; Payne, J.K.; Kelly, J.R.; Haakmeester, C.; Srijemac, R.; Wilson, A.Z.; et al. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl. Med. 2015, 4, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.G.; Chan, M.Y.; Martinson, L.A.; Kadoya, K.; Ostertag, T.M.; Ross, K.G.; Richardson, M.; Carpenter, M.K.; D’Amour, K.A.; Kroon, E.; et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 2011, 29, 750–756. [Google Scholar] [CrossRef]
- Sneddon, J.B.; Tang, Q.; Stock, P.; Bluestone, J.A.; Roy, S.; Desai, T.; Hebrok, M. Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges. Cell Stem Cell 2018, 22, 810–823. [Google Scholar] [CrossRef]
- Naqvi, R.A.; Naqvi, A.R.; Singh, A.; Priyadarshini, M.; Balamurugan, A.N.; Layden, B.T. The future treatment for type 1 diabetes: Pig islet- or stem cell-derived beta cells? Front. Endocrinol. 2022, 13, 1001041. [Google Scholar] [CrossRef]
- Vegas, A.J.; Veiseh, O.; Gurtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.W.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061 e2045. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Mazzone, T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp. Cell Res. 2006, 312, 2454–2464. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Lazzarini, P.; Wang, Y.; Di, A.; Chen, M. A unique human blood-derived cell population displays high potential for producing insulin. Biochem. Biophys. Res. Commun. 2007, 360, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hu, W.; Song, X.; Zhao, Y. Generation of Multipotent Stem Cells from Adult Human Peripheral Blood Following the Treatment with Platelet-Derived Mitochondria. Cells 2020, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hu, W.; Song, X.; Descalzi-Montoya, D.; Yang, Z.; Korngold, R.; Zhao, Y. Generation of Hematopoietic-Like Stem Cells from Adult Human Peripheral Blood Following Treatment with Platelet-Derived Mitochondria. Int. J. Mol. Sci. 2020, 21, 4249. [Google Scholar] [CrossRef] [PubMed]

| List of Products | Company/ Hospital | ClinicalTrials.gov | Target the Autoimmunity | Restoration of β-Cell Function | |||
|---|---|---|---|---|---|---|---|
| Immune Modulation | Immune Suppression | Improve the Endogenous β-Cell Regeneration | Transplant Exogenous β-Cell Surrogates | Rejection and Need Immune Suppression | |||
| Stem Cell Educator TM therapy (Gleukocell TM) | Throne | NCT04011020 Phase 2/3 | Yes | No | Yes | No | No |
| VX-880 (ES cell-derived insulin-producing cells) | Vertex | NCT04786262 Phase 1/2 | No | N/A | No | Yes | Yes |
| VC02-101 (ES cell-derived β-cell progenitors) | ViaCyte | NCT03163511 Phase 1/2 | No | N/A | No | Yes | Yes |
| PIpepTolDC (vaccine therapy) | City of Hope | NCT04590872 Phase 1 | Yes | N/A | N/A | No | No |
| TZIELD (teplizumab-mzwv) | Prevention Bio | FDA approved | No | Yes | N/A | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Veysman, B. Revisiting the Pathogenesis of Type 1 Diabetes: Importance of Neural Input to Pancreatic Islets and the Therapeutic Capability of Stem Cell Educator TM Therapy to Restore Their Integrity. Biomedicines 2023, 11, 594. https://doi.org/10.3390/biomedicines11020594
Zhao Y, Veysman B. Revisiting the Pathogenesis of Type 1 Diabetes: Importance of Neural Input to Pancreatic Islets and the Therapeutic Capability of Stem Cell Educator TM Therapy to Restore Their Integrity. Biomedicines. 2023; 11(2):594. https://doi.org/10.3390/biomedicines11020594
Chicago/Turabian StyleZhao, Yong, and Boris Veysman. 2023. "Revisiting the Pathogenesis of Type 1 Diabetes: Importance of Neural Input to Pancreatic Islets and the Therapeutic Capability of Stem Cell Educator TM Therapy to Restore Their Integrity" Biomedicines 11, no. 2: 594. https://doi.org/10.3390/biomedicines11020594
APA StyleZhao, Y., & Veysman, B. (2023). Revisiting the Pathogenesis of Type 1 Diabetes: Importance of Neural Input to Pancreatic Islets and the Therapeutic Capability of Stem Cell Educator TM Therapy to Restore Their Integrity. Biomedicines, 11(2), 594. https://doi.org/10.3390/biomedicines11020594








