High-Dose Intravenous Steroid Treatment Seems to Have No Long-Term Negative Effect on Bone Mineral Density of Young and Newly Diagnosed Multiple Sclerosis Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Bone Mineral Densitometry
2.3. Biochemistry
2.4. Power Analysis
3. Statistical Analysis
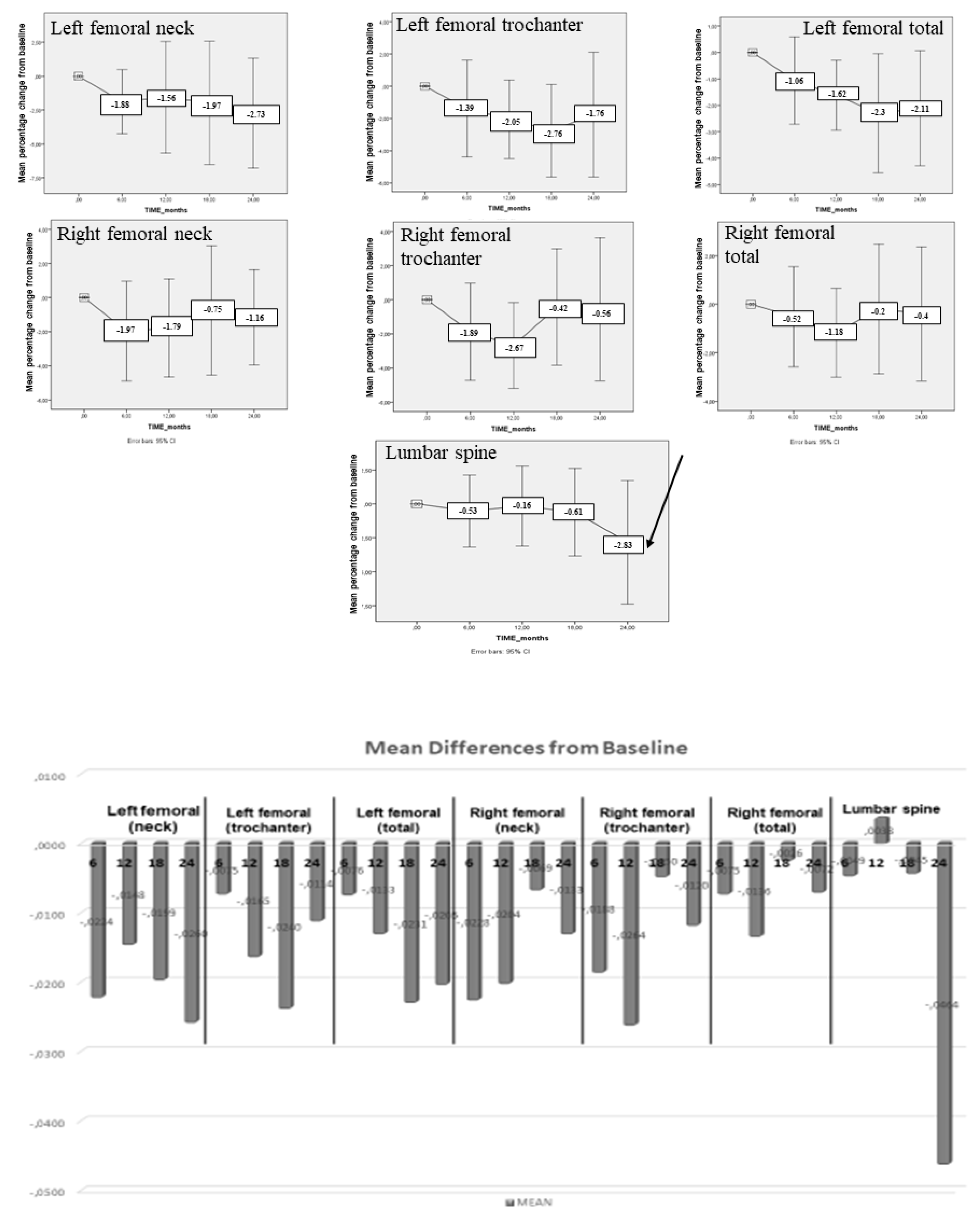
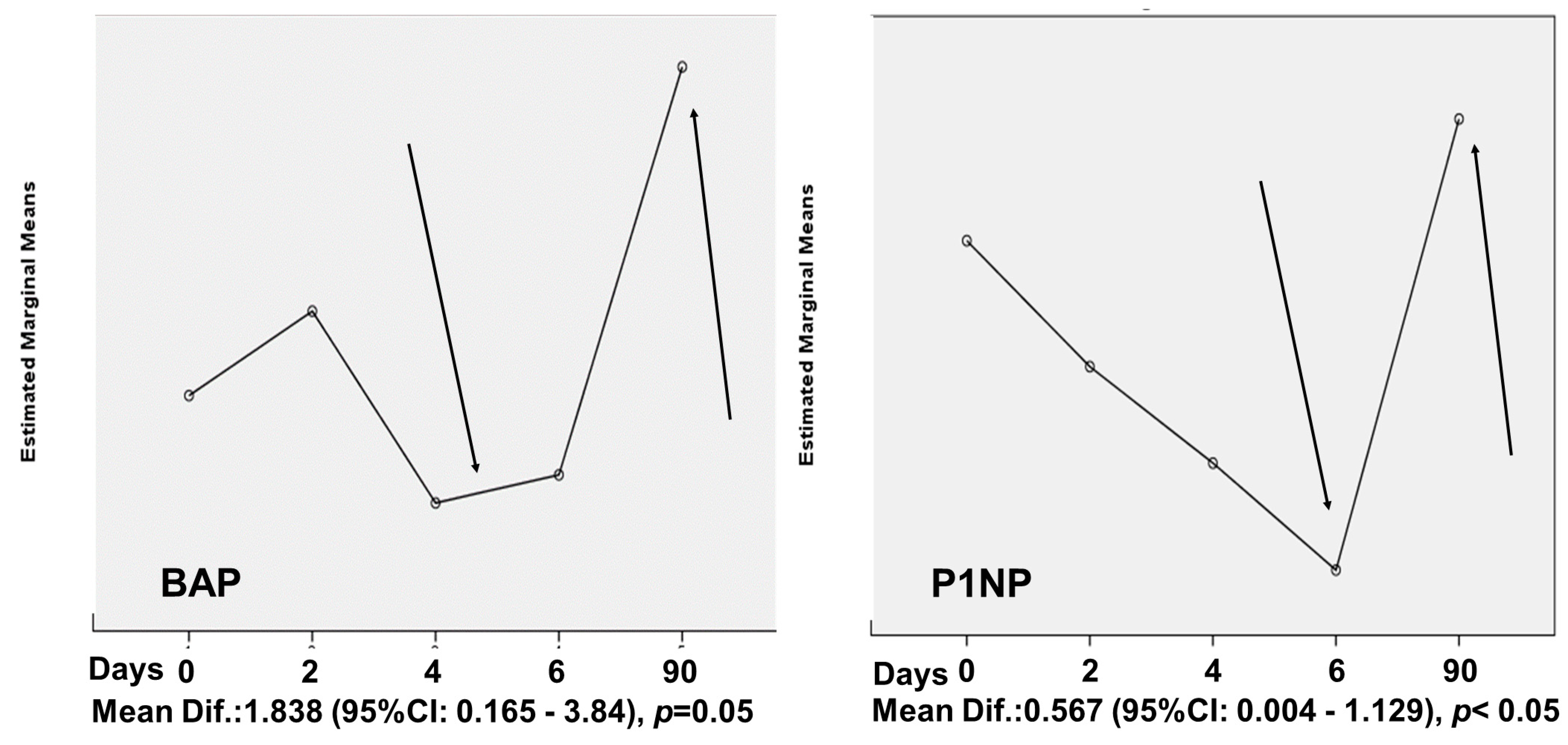
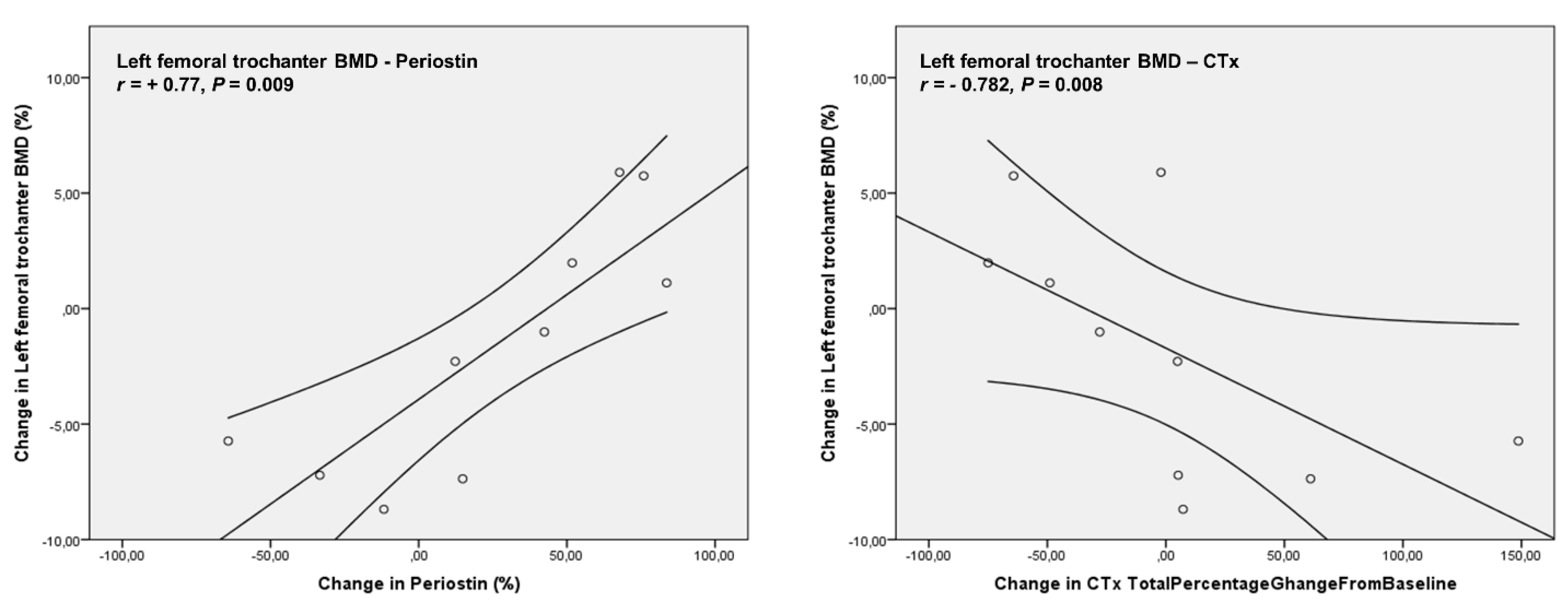
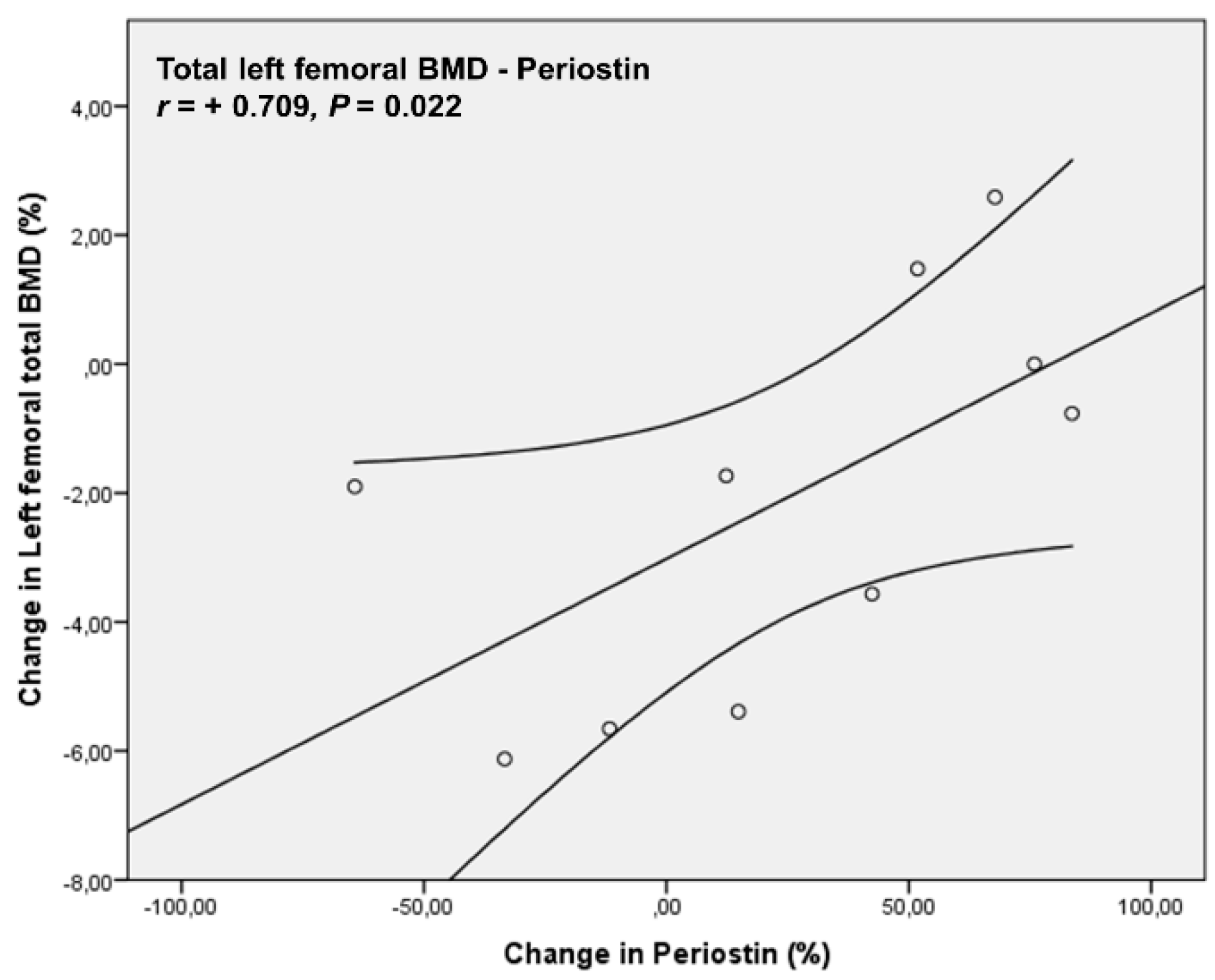
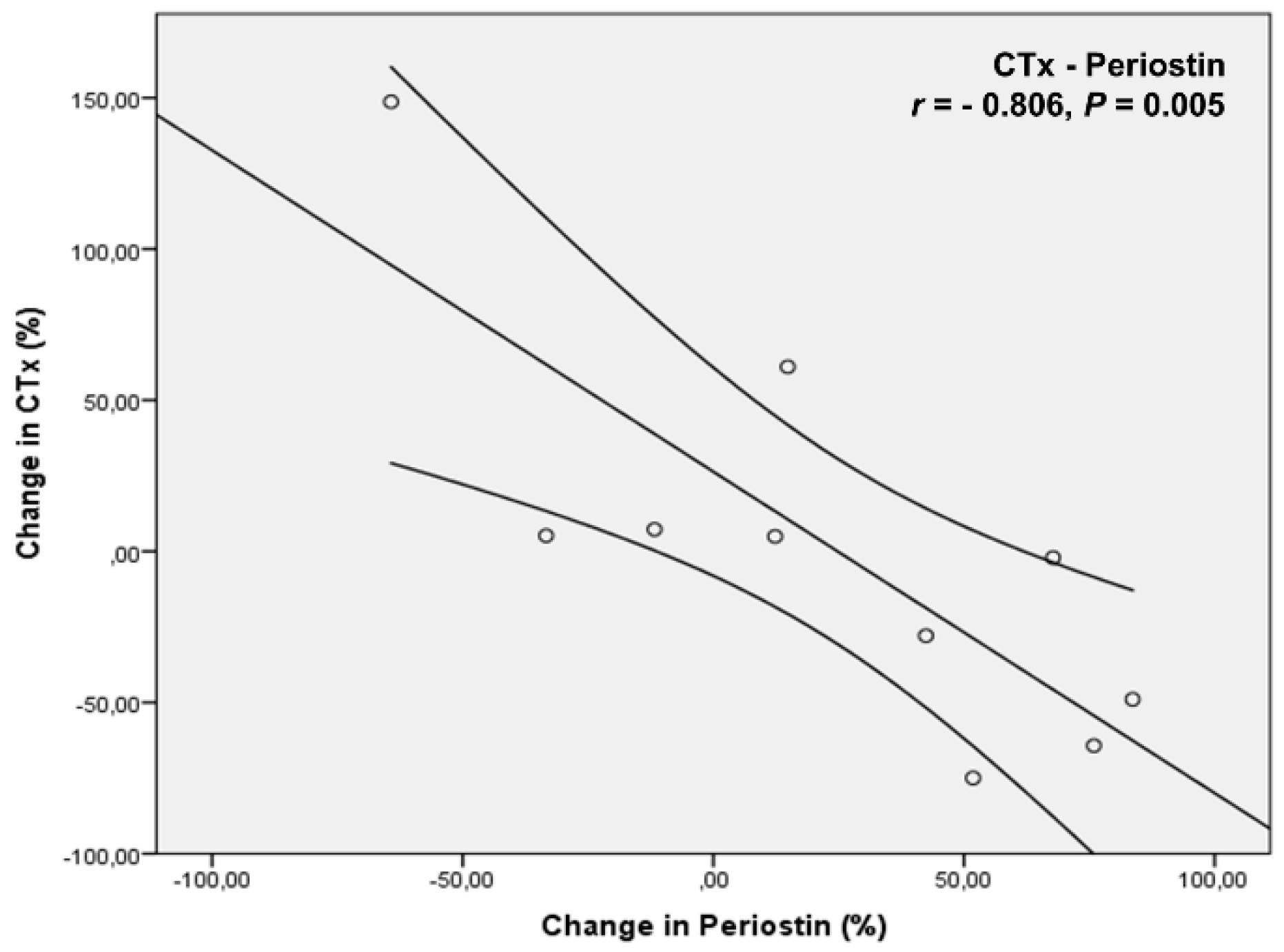
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDIST | High dose intravenous steroid treatment |
| MS | Multiple sclerosis |
| GC | Glucocorticoid |
| NDMSP | Newly diagnosed MS patients |
| BMD | Bone mineral density |
| CNS | Central nervous system |
| EDSS | Extended disability status scale |
| Th1 | Type 1 T helper |
| MHC | Major histocompatibility complex |
| RANKL | Nuclear factor kappa-Β ligand |
| OPG | Osteoprotegerin |
| BTMs | Bone turnover markers |
| IL | Interleukin |
| BM | Bone mass |
| DMTs | Disease modifying treatments |
| MRI | Magnetic resonance imaging |
| DXA | Dual-energy X-ray absorptiometry |
| 25OHD | Total 25-hydroxyvitamin D |
| iPTH | intact parathormone |
| TH | Thyroid hormones |
| FSH | Follicle stimulation hormone |
| LH | Luteinizing hormone |
| PRL | Prolactin |
| E2 | Estradiol |
| Testo | Total testosterone |
| CV | Coefficient of variations |
| DKK-1 | Dickkopf-1 |
| P1NP | N-terminal-propeptide-procollagen-type-1 |
| CTx | C-terminal-peptide-type-of-collagen |
| BAP | Bone-specific alkaline phosphatase |
| BMI | Body mass index |
| FFM | Fat free mass |
| FMI | Fat mass index |
| LMI | Lean mass index |
References
- Kamm, C.P.; Uitdehaag, B.M.; Polman, C.H. Multiple Sclerosis: Current Knowledge and Future Outlook. Eur. Neurol. 2014, 72, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J.; the MSCOI Study Group; the European Multiple Sclerosis Platform. New Insights into the Burden and Costs of Multiple Sclerosis in Europe. Mult. Scler. 2017, 23, 1123–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The Prevalence of MS in the United States: A Population-Based Estimate Using Health Claims Data. Neurology 2019, 92, e1029–e1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Multiple Sclerosis International Federation, Atlas of MS, 3rd ed.; 2020; Available online: https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (accessed on 20 January 2023).
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [Green Version]
- Anagnostouli, M.; Markoglou, N.; Chrousos, G. Psycho-Neuro-Endocrino-Immunologic Issues in Multiple Sclerosis: A Critical Review of Clinical and Therapeutic Implications. Hormones 2020, 19, 485–496. [Google Scholar] [CrossRef]
- Fraser, L.-A.; Adachi, J.D. Glucocorticoid-Induced Osteoporosis: Treatment Update and Review. Ther. Adv. Musculoskelet. Dis. 2009, 1, 71–85. [Google Scholar] [CrossRef]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef] [Green Version]
- Adami, G.; Saag, K.G. Glucocorticoid-Induced Osteoporosis: 2019 Concise Clinical Review. Osteoporos. Int. 2019, 30, 1145–1156. [Google Scholar] [CrossRef]
- van Staa, T.P.; Leufkens, H.G.; Abenhaim, L.; Zhang, B.; Cooper, C. Oral Corticosteroids and Fracture Risk: Relationship to Daily and Cumulative Doses. Rheumatology 2000, 39, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- van Staa, T.P.; Leufkens, H.G.M.; Cooper, C. The Epidemiology of Corticosteroid-Induced Osteoporosis: A Meta-Analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef] [Green Version]
- De Vries, F.; Bracke, M.; Leufkens, H.G.M.; Lammers, J.-W.J.; Cooper, C.; Van Staa, T.P. Fracture Risk with Intermittent High-Dose Oral Glucocorticoid Therapy. Arthritis Rheum. 2007, 56, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.V.; Coulombe, J.; Ernst, P.; Suissa, S. Long-Term Use of Inhaled Corticosteroids in COPD and the Risk of Fracture. Chest 2018, 153, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Chotiyarnwong, P.; McCloskey, E.V. Pathogenesis of Glucocorticoid-Induced Osteoporosis and Options for Treatment. Nat. Rev. Endocrinol. 2020, 16, 437–447. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells Is Associated with Active Disease in Multiple Sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Kim, T.-S.; Choi, Y.; Lorenzo, J. Osteoimmunology: Cytokines and the Skeletal System. BMB Rep. 2008, 41, 495–510. [Google Scholar] [CrossRef] [Green Version]
- Moen, S.M.; Celius, E.G.; Sandvik, L.; Nordsletten, L.; Eriksen, E.F.; Holmøy, T. Low Bone Mass in Newly Diagnosed Multiple Sclerosis and Clinically Isolated Syndrome. Neurology 2011, 77, 151–157. [Google Scholar] [CrossRef]
- Bazelier, M.T.; van Staa, T.-P.; Uitdehaag, B.M.J.; Cooper, C.; Leufkens, H.G.M.; Vestergaard, P.; Herings, R.M.C.; de Vries, F. Risk of Fractures in Patients with Multiple Sclerosis: A Population-Based Cohort Study. Neurology 2012, 78, 1967–1973. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, R.K.; Vaishnav, N.; Dubinsky, R.M. Is There an Increased Risk of Hip Fracture in Multiple Sclerosis? Analysis of the Nationwide Inpatient Sample. J. Multidiscip. Healthc. 2014, 7, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Hearn, A.P.; Silber, E. Osteoporosis in Multiple Sclerosis. Mult. Scler. 2010, 16, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Azadvari, M.; Mirmosayyeb, O.; Hosseini, M.; Vaheb, S.; Razavi, S.Z.E. The Prevalence of Osteoporosis/Osteopenia in Patients with Multiple Sclerosis (MS): A Systematic Review and Meta-Analysis. Neurol. Sci. 2022, 43, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Kusunoki, N.; Hasunuma, T.; Kawai, S. Changes of Serum Soluble Receptor Activator for Nuclear Factor-ΚB Ligand after Glucocorticoid Therapy Reflect Regulation of Its Expression by Osteoblasts. J. Clin. Endocrinol. Metab. 2012, 97, E1909–E1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devogelaer, J.-P.; Durnez, A.; Gruson, D.; Manicourt, D.H. Bone Turnover Markers and Glucocorticoid Treatments. In Biomarkers in Bone Disease; Patel, V.B., Preedy, V.R., Eds.; Biomarkers in Disease: Methods, Discoveries and Applications; Springer: Dordrecht, The Netherlands, 2017; pp. 905–932. ISBN 978-94-007-7692-0. [Google Scholar]
- Corrado, A.; Rotondo, C.; Mele, A.; Cici, D.; Maruotti, N.; Sanpaolo, E.; Colia, R.; Cantatore, F.P. Influence of Glucocorticoid Treatment on Trabecular Bone Score and Bone Remodeling Regulators in Early Rheumatoid Arthritis. Arthritis. Res. Ther. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of Bone Turnover for the Prediction of Fracture Risk and Monitoring of Osteoporosis Treatment: A Need for International Reference Standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Dovio, A.; Perazzolo, L.; Osella, G.; Ventura, M.; Termine, A.; Milano, E.; Bertolotto, A.; Angeli, A. Immediate Fall of Bone Formation and Transient Increase of Bone Resorption in the Course of High-Dose, Short-Term Glucocorticoid Therapy in Young Patients with Multiple Sclerosis. J. Clin. Endocrinol. Metab. 2004, 89, 4923–4928. [Google Scholar] [CrossRef]
- Olsson, A.; Oturai, D.B.; Sørensen, P.S.; Oturai, P.S.; Oturai, A.B. Short-Term, High-Dose Glucocorticoid Treatment Does Not Contribute to Reduced Bone Mineral Density in Patients with Multiple Sclerosis. Mult. Scler. 2015, 21, 1557–1565. [Google Scholar] [CrossRef]
- Zengin Karahan, S.; Boz, C.; Kilic, S.; Can Usta, N.; Ozmenoglu, M.; Altunayoglu Cakmak, V.; Gazioglu, S. Lack of Association between Pulse Steroid Therapy and Bone Mineral Density in Patients with Multiple Sclerosis. Mult. Scler. Int. 2016, 2016, 5794910. [Google Scholar] [CrossRef] [Green Version]
- Ozgocmen, S.; Bulut, S.; Ilhan, N.; Gulkesen, A.; Ardicoglu, O.; Ozkan, Y. Vitamin D Deficiency and Reduced Bone Mineral Density in Multiple Sclerosis: Effect of Ambulatory Status and Functional Capacity. J. Bone Miner. Metab. 2005, 23, 309–313. [Google Scholar] [CrossRef]
- Tyblova, M.; Kalincik, T.; Zikan, V.; Havrdova, E. Impaired Ambulation and Steroid Therapy Impact Negatively on Bone Health in Multiple Sclerosis. Eur. J. Neurol. 2015, 22, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Johannesdottir, F.; Thrall, E.; Muller, J.; Keaveny, T.M.; Kopperdahl, D.L.; Bouxsein, M.L. Comparison of Non-Invasive Assessments of Strength of the Proximal Femur. Bone 2017, 105, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Brabnikova Maresova, K.; Pavelka, K.; Stepan, J.J. Acute Effects of Glucocorticoids on Serum Markers of Osteoclasts, Osteoblasts, and Osteocytes. Calcif. Tissue Int. 2013, 92, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, M.; van Raalte, D.H.; Heijboer, A.C.; den Heijer, M.; de Jongh, R.T. Short-Term Glucocorticoid Treatment Reduces Circulating Sclerostin Concentrations in Healthy Young Men: A Randomized, Placebo-Controlled, Double-Blind Study. JBMR Plus 2020, 4, e10341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, J.C.; Sornay-Rendu, E.; Bertholon, C.; Chapurlat, R.; Garnero, P. Serum Periostin Is Associated with Fracture Risk in Postmenopausal Women: A 7-Year Prospective Analysis of the OFELY Study. J. Clin. Endocrinol. Metab. 2014, 99, 2533–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-J.; Rhee, Y.; Kim, C.H.; Baek, K.H.; Min, Y.-K.; Kim, D.-Y.; Ahn, S.H.; Kim, H.; Lee, S.H.; Lee, S.-Y.; et al. Plasma Periostin Associates Significantly with Non-Vertebral but Not Vertebral Fractures in Postmenopausal Women: Clinical Evidence for the Different Effects of Periostin Depending on the Skeletal Site. Bone 2015, 81, 435–441. [Google Scholar] [CrossRef]
- Bonnet, N.; Biver, E.; Durosier, C.; Chevalley, T.; Rizzoli, R.; Ferrari, S. Additive Genetic Effects on Circulating Periostin Contribute to the Heritability of Bone Microstructure. J. Clin. Endocrinol. Metab. 2015, 100, E1014–E1021. [Google Scholar] [CrossRef] [Green Version]
- Gossiel, F.; Scott, J.R.; Paggiosi, M.A.; Naylor, K.E.; McCloskey, E.V.; Peel, N.F.A.; Walsh, J.S.; Eastell, R. Effect of Teriparatide Treatment on Circulating Periostin and Its Relationship to Regulators of Bone Formation and BMD in Postmenopausal Women with Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 1302–1309. [Google Scholar] [CrossRef] [Green Version]
- Bonadonna, S.; Burattin, A.; Nuzzo, M.; Bugari, G.; Rosei, E.A.; Valle, D.; Iori, N.; Bilezikian, J.P.; Veldhuis, J.D.; Giustina, A. Chronic Glucocorticoid Treatment Alters Spontaneous Pulsatile Parathyroid Hormone Secretory Dynamics in Human Subjects. Eur. J. Endocrinol. 2005, 152, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Idolazzi, L.; Ridolo, E.; Fassio, A.; Gatti, D.; Montagni, M.; Caminati, M.; Martignago, I.; Incorvaia, C.; Senna, G. Periostin: The Bone and Beyond. Eur. J. Intern. Med. 2017, 38, 12–16. [Google Scholar] [CrossRef]
- Edelstein, S.L.; Barrett-Connor, E. Relation between Body Size and Bone Mineral Density in Elderly Men and Women. Am. J. Epidemiol. 1993, 138, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ravn, P.; Cizza, G.; Bjarnason, N.H.; Thompson, D.; Daley, M.; Wasnich, R.D.; McClung, M.; Hosking, D.; Yates, A.J.; Christiansen, C. Low Body Mass Index Is an Important Risk Factor for Low Bone Mass and Increased Bone Loss in Early Postmenopausal Women. Early Postmenopausal Intervention Cohort (EPIC) Study Group. J. Bone Miner. Res. 1999, 14, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Bunout, D.; Gattás, V.; de la Maza, M.P.; Leiva, L.; Hirsch, S. A High Body Mass Index Protects against Femoral Neck Osteoporosis in Healthy Elderly Subjects. Nutrition 2004, 20, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Salamat, M.R.; Salamat, A.H.; Abedi, I.; Janghorbani, M. Relationship between Weight, Body Mass Index, and Bone Mineral Density in Men Referred for Dual-Energy X-Ray Absorptiometry Scan in Isfahan, Iran. J. Osteoporos. 2013, 2013, 205963. [Google Scholar] [CrossRef] [Green Version]
- Gnudi, S.; Sitta, E.; Lisi, L. Relationship of Body Mass Index with Main Limb Fragility Fractures in Postmenopausal Women. J. Bone Miner. Metab. 2009, 27, 479–484. [Google Scholar] [CrossRef]
- Greco, E.A.; Fornari, R.; Rossi, F.; Santiemma, V.; Prossomariti, G.; Annoscia, C.; Aversa, A.; Brama, M.; Marini, M.; Donini, L.M.; et al. Is Obesity Protective for Osteoporosis? Evaluation of Bone Mineral Density in Individuals with High Body Mass Index. Int. J. Clin. Pract. 2010, 64, 817–820. [Google Scholar] [CrossRef]
- Li, Y. Association between Obesity and Bone Mineral Density in Middle-Aged Adults. J. Orthop. Surg. Res. 2022, 17, 268. [Google Scholar] [CrossRef]
- Bierhals, I.O.; Dos Santos Vaz, J.; Bielemann, R.M.; de Mola, C.L.; Barros, F.C.; Gonçalves, H.; Wehrmeister, F.C.; Assunção, M.C.F. Associations between Body Mass Index, Body Composition and Bone Density in Young Adults: Findings from a Southern Brazilian Cohort. BMC Musculoskelet. Disord. 2019, 20, 322. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Rodríguez, A.; Catalán, V.; Frühbeck, G. The Bone-Adipose Axis in Obesity and Weight Loss. Obes. Surg. 2008, 18, 1134–1143. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musella, A.; Fresegna, D.; Rizzo, F.R.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bruno, A.; Dolcetti, E.; Buttari, F.; et al. “Prototypical” Proinflammatory Cytokine (IL-1) in Multiple Sclerosis: Role in Pathogenesis and Therapeutic Targeting. Expert Opin. Ther. Targets 2020, 24, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Baseline Mean ± SD | 24-Month f-up Mean ± SD | p | |
|---|---|---|---|
| Weight (kg) | 85.61 ± 13.08 | 87.86 ± 13.42 | 0.120 |
| BMI (kg/m2) | 26.72 ± 2.76 | 27.43 ± 2.76 | 0.105 |
| FFM (kg) | 56.23 ± 13.27 | 56.03 ± 12.50 | 0.770 |
| FAT (kg) | 29.37 ± 9.17 | 31.82 ± 8.96 | 0.099 |
| TSH (mIU/mL) | 1.97 ± 0.49 | 1.92 ± 0.45 | 0.837 |
| FT4 (pmol/L) | 16.74 ± 2.30 | 16.94 ± 2.13 | 0.811 |
| FSH (mIU/mL) | 4.35 ± 1.89 | 7.28 ± 6.66 | 0.251 |
| LH (mIU/mL) | 4.65 ± 1.21 | 4.70 ± 2.06 | 0.959 |
| PRL (ng/mL) | 8.95 ± 7.16 | 5.75 ± 2.86 | 0.72 |
| E2 (pmol/L) | 272.45 ± 269.01 | 157.33 ± 124.17 | 0.447 |
| Testo (ng/mL) | 3.45 ± 4.03 | 3.82 ± 4.82 | 0.852 |
| IL1β (pg/mL) | 1.50 ± 0.71 | 1.56 ± 0.69 | 0.831 |
| IL6 (pg/mL) | 2.48 ± 1.05 | 2.54 ± 1.20 | 0.900 |
| IL17 (pg/mL) | 6.14 ± 2.51 | 6.57 ± 3.32 | 0.696 |
| Ca (mg/dL) | 9.19 ± 0.31 | 8.86 ± 0.37 | 0.060 |
| Alb (g/dL) | 4.30± 0.28 | 4.46 ± 0.19 | 0.261 |
| p (mg/dL) | 3.31 ± 0.54 | 3.49 ± 0.70 | 0.408 |
| Mg (mg/dL) | 2.09 ± 0.31 | 2.24 ± 0.13 | 0.223 |
| Cr (mg/dL) | 0.86 ± 0.17 | 0.91 ± 0.20 | 0.179 |
| 25OHD (ng/mL) | 30.00 ± 5.60 | 33.07 ± 6.60 | 0.137 |
| iPTH (pg/mL) | 37.20 ± 15.13 | 28.90 ± 13.02 | 0.021 |
| CTx (ng/mL) | 0.58 ± 0.40 | 0.42 ± 0.15 | 0.245 |
| BAP (μg/mL) | 7.24 ± 3.62 | 6.81 ± 2.13 | 0.718 |
| P1NP (ng/mL) | 1.34 ± 0.86 | 1.31 ± 0.81 | 0.895 |
| RANKL (pmol/L) | 0.23 ± 0.20 | 0.19 ± 0.16 | 0.566 |
| OPG (pmol/L) | 3.14 ± 1.12 | 3.32 ± 1.39 | 0.512 |
| SCL (pmol/L) | 37.71 ± 12.53 | 32.97 ± 9.50 | 0.438 |
| DKK1 (pmol/L) | 19.15 ± 14.24 | 17.89 ± 11.84 | 0.756 |
| Periostin (ng/mL) | 1695.63 ± 633.03 | 1906.90 ± 667.80 | 0.460 |
| BMD | Baseline Mean ± SD (g/cm²) | 24-month f-up Mean ± SD (g/cm²) | p |
|---|---|---|---|
| Left femoral (neck) | 0.95 ± 0.08 | 0.92 ± 0.08 | 0.148 |
| Left femoral (trochanter) | 0.82 ± 0.10 | 0.80 ± 0.10 | 0.327 |
| Left femoral (total) | 0.99 ± 0.08 | 0.97 ± 0.09 | 0.064 |
| Right femoral (neck) | 0.96 ± 0.10 | 0.95 ± 0.09 | 0.369 |
| Right femoral (trochanter) | 0.82 ± 0.11 | 0.81 ± 0.12 | 0.726 |
| Right femoral (total) | 0.98 ± 0.10 | 0.98 ± 0.10 | 0.738 |
| Lumbar spine | 1.24 ± 0.14 | 1.21 ± 0.16 | 0.204 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeakis, G.; Anagnostouli, M.; Fakas, N.; Koutsikos, J.; Papatheodorou, A.; Chanopoulos, K.; Athanasiou, K.; Papatheodorou, G.; Zapanti, E.; Alevizaki, M.; et al. High-Dose Intravenous Steroid Treatment Seems to Have No Long-Term Negative Effect on Bone Mineral Density of Young and Newly Diagnosed Multiple Sclerosis Patients: A Pilot Study. Biomedicines 2023, 11, 603. https://doi.org/10.3390/biomedicines11020603
Simeakis G, Anagnostouli M, Fakas N, Koutsikos J, Papatheodorou A, Chanopoulos K, Athanasiou K, Papatheodorou G, Zapanti E, Alevizaki M, et al. High-Dose Intravenous Steroid Treatment Seems to Have No Long-Term Negative Effect on Bone Mineral Density of Young and Newly Diagnosed Multiple Sclerosis Patients: A Pilot Study. Biomedicines. 2023; 11(2):603. https://doi.org/10.3390/biomedicines11020603
Chicago/Turabian StyleSimeakis, George, Maria Anagnostouli, Nikolaos Fakas, John Koutsikos, Athanasios Papatheodorou, Konstantinos Chanopoulos, Kwnstantinos Athanasiou, George Papatheodorou, Evangelia Zapanti, Maria Alevizaki, and et al. 2023. "High-Dose Intravenous Steroid Treatment Seems to Have No Long-Term Negative Effect on Bone Mineral Density of Young and Newly Diagnosed Multiple Sclerosis Patients: A Pilot Study" Biomedicines 11, no. 2: 603. https://doi.org/10.3390/biomedicines11020603
APA StyleSimeakis, G., Anagnostouli, M., Fakas, N., Koutsikos, J., Papatheodorou, A., Chanopoulos, K., Athanasiou, K., Papatheodorou, G., Zapanti, E., Alevizaki, M., Kaltsas, G., & Terpos, E. (2023). High-Dose Intravenous Steroid Treatment Seems to Have No Long-Term Negative Effect on Bone Mineral Density of Young and Newly Diagnosed Multiple Sclerosis Patients: A Pilot Study. Biomedicines, 11(2), 603. https://doi.org/10.3390/biomedicines11020603









