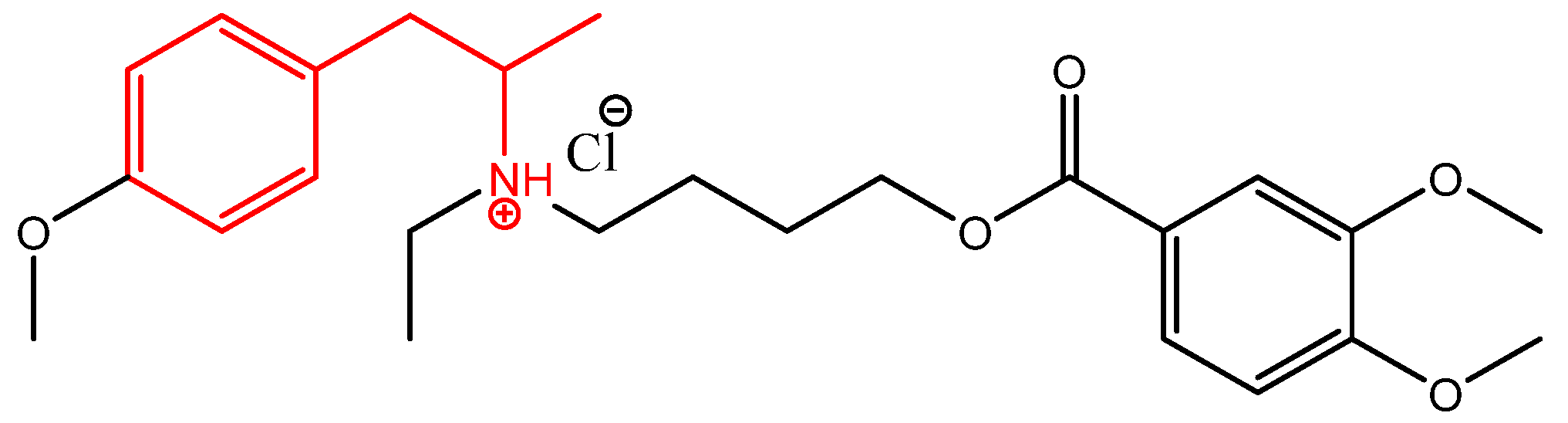
In Silico, In Vitro, and Ex Vivo Biological Activity of Some Novel Mebeverine Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthetic Methods Experimental Protocols and Spectral Data
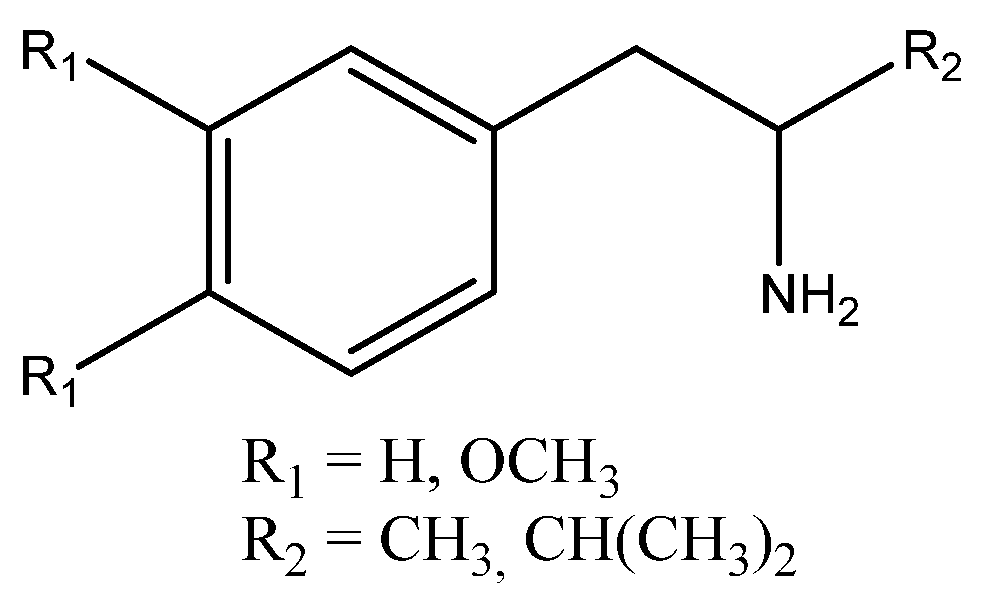
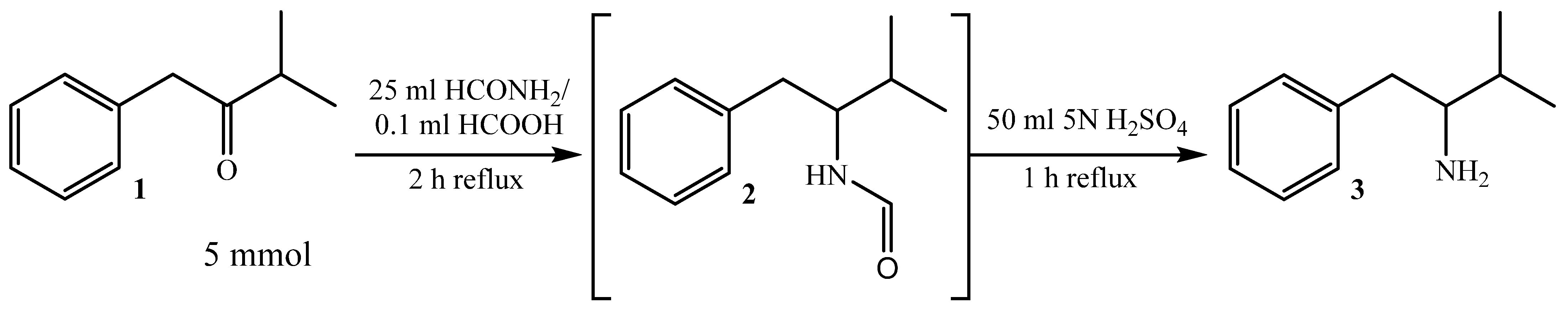
2.2.1. Synthesis of 3-Methyl-1-phenylbutan-2-amine 3
2.2.2. Preparation of 3-Methyl-1-phenylbutan-2-amides 4; Typical Procedure
2.3. In Silico Pharmacokinetic Profiling and Toxicity Analysis
2.3.1. Theoretical Prediction of Pharmacokinetic Parameters (ADME)
2.3.2. Theoretical Prediction of Toxicity
2.3.3. PASS Online Predictions
2.4. Microbiological Tests
2.4.1. Tested Microorganisms
2.4.2. Culture Media
- Luria-Bertani agar medium supplemented with glucose (LBG agar)
- Malt extract agar (MEA)
2.4.3. Antimicrobial Activity Assay
2.5. Cytotoxic Activity
Cell Viability Assay
2.6. Bioelectrical Activity
2.7. Immunohistochemical Analysis
2.7.1. Histology
2.7.2. Hematoxylin-Eosin Staining
2.7.3. Immunohistochemistry
2.7.4. Quantitative Analysis of Immunohistochemical Reactions
2.8. Statistical Analysis
3. Results and Discussion
3.1. In Silico Predictions and Synthesis
3.2. Antimicrobial Activity
3.3. Cytotoxic Activity
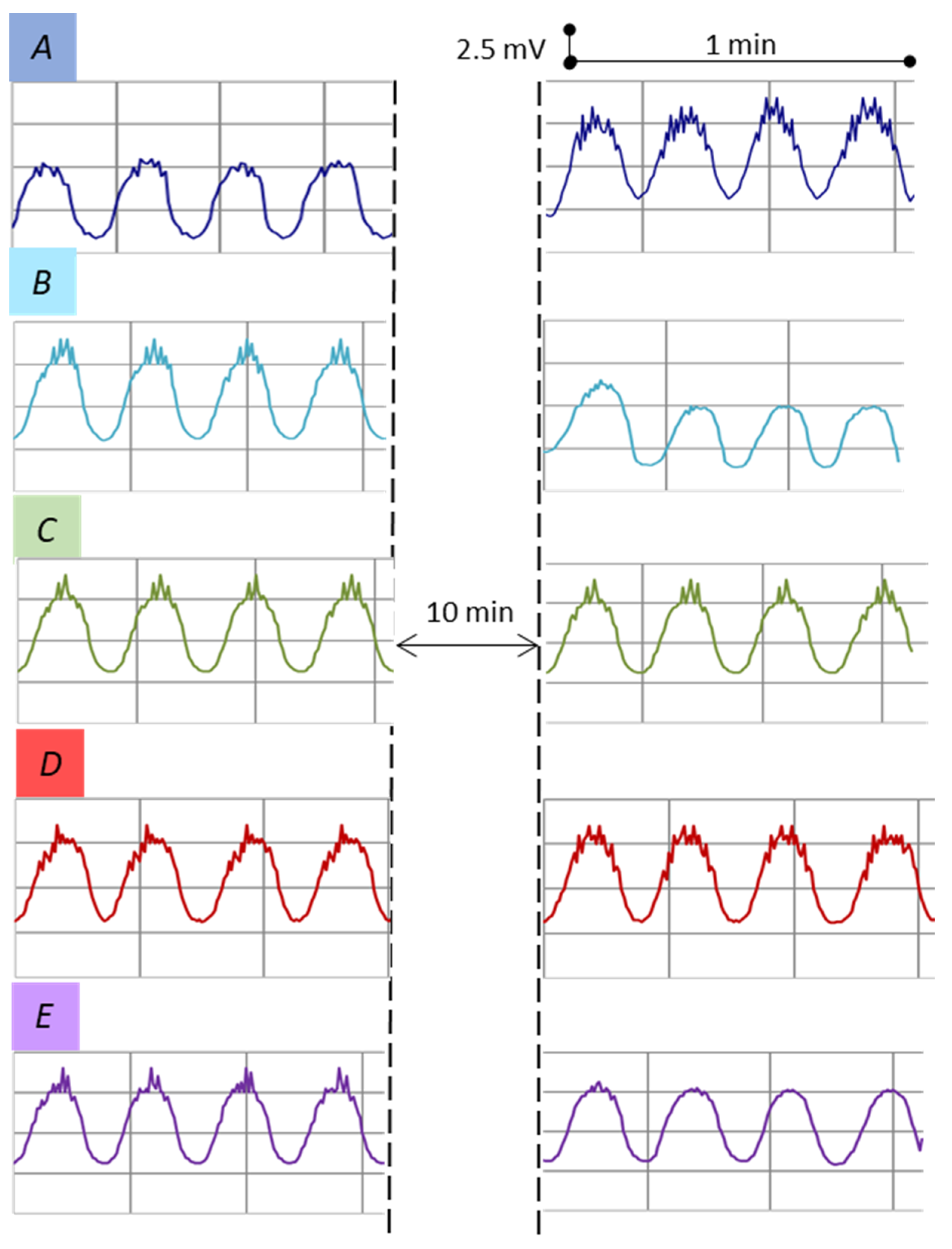
3.4. Ex Vivo Effects of 3-Methyl-1-phenylbutan-2-amine 3 and Its Amides 4a–d on the Spontaneous Bioelectric Activity
3.5. Immunoreactivity for the 5-HT3 Receptor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Salari, P.; Abdollahi, M. Current opinion in the pharmaceutical management of irritable and inflammatory bowel diseases: Role of ATP. Recent Pat. Endocr. Metab. Immune Drug Discov. 2009, 3, 69–75. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: A meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin. Ther. 2008, 30, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. Drug therapy options for patients with irritable bowel syndrome. Am. J. Manag. Care 2001, 7, S261–S267. [Google Scholar] [PubMed]
- Mahnaz, D.-D.; Nikfar, S.; Abdollahi, M. A systematic review of efficacy and tolerability of mebeverine in irritable bowel syndrome. World J. Gastroenterol. 2010, 16, 547–553. [Google Scholar]
- Szymaszkiewicz, A.; Zielińska, M. Irritable bowel syndrome: Current therapies and future perspectives. In A Comprehensive Overview of Irritable Bowel Syndrome; Fichna, J., Ed.; Elsevier: London, UK, 2020; pp. 129–144. ISBN 978-0-12-821324-7. [Google Scholar] [CrossRef]
- Milusheva, M.; Gledacheva, V.; Batmazyan, M.; Nikolova, S.; Stefanova, I.; Dimitrova, D.; Saracheva, K.; Tomov, D.; Chaova-Gizdakova, V. Ex Vivo and In Vivo Study of Some Isoquinoline Precursors. Sci. Pharm. 2022, 90, 37. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2001, 18, 148. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, H.; Ma, C.; Zhou, M.; Wu, Y.; Wang, L.; Guo, S.; Chen, T.; Shaw, C. Ex Vivo Smooth Muscle Pharmacological Effects of a Novel Bradykinin-Related Peptide, and Its Analogue, from Chinese Large Odorous Frog, Odorrana livida Skin Secretions. Toxins 2016, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.; Sharma, P.; Ryu, M.H.; Rissé, P.A.; Ngo, M.; Maarsingh, H.; Koziol-White, C.; Jha, A.; Halayko, A.J.; West, A.R. Models to study airway smooth muscle contraction in vivo, ex vivo and in vitro: Implications in understanding asthma. Pulm. Pharmacol. Ther. 2013, 26, 24–36. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Z. Vascular Smooth Muscle Cells Mechanosensitive Regulators and Vascular Remodeling. J. Vasc. Res. 2022, 59, 90–113. [Google Scholar] [CrossRef]
- Hashitani, H.; Brading, A.F.; Suzuki, H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br. J. Pharmacol. 2004, 141, 183–193. [Google Scholar] [CrossRef]
- Durnin, L.; Kwok, B.; Kukadia, P.; McAvera, R.; Corrigan, R.D.; Ward, S.M.; Zhang, Y.; Chen, Q.; Koh, S.D.; Sanders, K.M.; et al. An ex vivo bladder model with detrusor smooth muscle removed to analyze biologically active mediators released from the suburothelium. J. Physiol. 2019, 597, 1467–1485. [Google Scholar] [CrossRef]
- Tarhan, F.; Duman, N.Ç.; Özkulab, S.; Karaalp, A.; Cangüven, Ö. In vitro contractile responses of human detrusor smooth muscle to oxytocin: Does it really have effect? Aging Male 2020, 23, 1141–1145. [Google Scholar] [CrossRef]
- Durdle, N.G.; Kingma, Y.J.; Bowes, K.L.; Chambers, M.M. Origin of Slow Waves in the Canine Colon. Gastroenterology 1983, 84, 375–382. [Google Scholar] [CrossRef]
- Van Helden, D.F.; Laver, D.R.; Holdsworth, J.; Imtiaz, M.S. Generation and propagation of gastric slow waves. Clin. Exp. Phamacol. Physiol. 2010, 37, 516–524. [Google Scholar] [CrossRef]
- Thomsen, L.; Robinson, T.L.; Lee, J.C.; Farraway, L.A.; Hughes, M.J.; Andrews, D.W.; Huizinga, J.D. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat. Med. 1998, 4, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef]
- Liu, H.N.; Hirata, H.; Okuno, Y.; Okabe, M.; Furukawa, K. Dopamine and Serotonin Receptors Cooperatively Modulate Pacemaker Activity of Intestinal Cells of Cajal. Chin. J. Physiol. 2018, 61, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Lummis, S.C. 5-HT(3) receptors. J. Biol. Chem. 2012, 28, 40239–40245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galligan, J.J. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol. Motil. 2002, 14, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Trognon, J.; Vera, G.; Rima, M.; Stigliani, J.-L.; Amielet, L.; El Hage, S.; Lajoie, B.; Roques, C.; El Garah, F. Investigation of Direct and Retro Chromone-2-Carboxamides Based Analogs of Pseudomonas aeruginosa Quorum Sensing Signal as New Anti-Biofilm Agents. Pharmaceuticals 2022, 15, 417. [Google Scholar] [CrossRef]
- Isyaku, Y.; Uzairu, A.; Uba, S. Computational studies of a series of 2-substituted phenyl-2-oxo-, 2-hydroxyl- and 2-acylloxyethylsulfonamides as potent anti-fungal agents. Heliyon 2020, 6, e03724. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazumder, K.; Hossain, E.; Aktar, A.; Mohiuddin, M.; Sarkar, K.K.; Biswas, B.; Aziz, A.; Abid, A.; Fukase, K. In Silico Analysis and Experimental Evaluation of Ester Prodrugs of Ketoprofen for Oral Delivery: With a View to Reduce Toxicity. Processes 2021, 9, 2221. [Google Scholar] [CrossRef]
- Anzali, S.; Barnickel, G.; Cezanne, B.; Krug, M.; Filimonov, D.; Poroikov, V. Discriminating between Drugs and Nondrugs by Prediction of Activity Spectra for Substances (PASS). J. Med. Chem. 2001, 44, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Synthesis and PASS-assisted in silico approach of some novel 2-substituted benzim-idazole bearing a pyrimidine-2,4,6 (trione) system as mucomembranous protector. J. Pharm. Bioallied. Sci. 2013, 5, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Olechno, J.; Williams, A.J. Dispensing Processes Impact Apparent Biological Activity as Determined by Computa-tional and Statistical Analyses. PLoS ONE 2013, 8, e62325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumbarski, Y.; Deseva, I.; Mihaylova, D.; Stoyanova, M.; Krastev, L.; Nikolova, R.; Yanakieva, V.; Ivanov, I. Isolation, Characterization and Amino Acid Composition of a Bacteriocin Produced by Bacillus methylotrophicus Strain BM47. Food Technol. Biotechnol. 2018, 56, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Nikolova, S.; Aladjov, D.; Stefanova, I.; Zagorchev, P. Synthesis and Contractile Activity of Substituted 1,2,3,4-Tetrahydroisoquinolines. Molecules 2011, 16, 7019–7042. [Google Scholar] [CrossRef]
- Stefanova, I.; Argirova, M.; Krustev, A. Influence of model melanoidins on calciumdependent transport mechanisms in smooth muscle tissue. Mol. Nutr. Food Res. 2007, 51, 468–472. [Google Scholar] [CrossRef]
- Argirova, M.; Stefanova, I.; Krustev, A. New biological properties of coffee melanoidins. Food Funct. 2013, 4, 1204–1208. [Google Scholar] [CrossRef]
- Sagorchev, P.; Lukanov, J.; Beer, A.-M. Effects of 1.8-cineole (eucalyptol) on the spontaneous contractile activity of smooth muscles fibre. J. Med. Plants Res. 2015, 9, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Beer, A.M.; Lukanov, J.; Sagorchev, P. Effect of Thymol on the spontaneous contractile activity of the smooth muscles. Phytomedicine 2007, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. Swiss ADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Seavill, P.W.; Wilden, J.D. The preparation and applications of amides using electrosynthesis. Green Chem. 2020, 22, 7737–7759. [Google Scholar] [CrossRef]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef]
- Wang, X. Challenges and outlook for catalytic direct amidation reactions. Nat. Catal. 2019, 2, 98–102. [Google Scholar] [CrossRef]
- Bray, B.L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003, 2, 587–593. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Pathan, N.; Shende, P. Tailoring of P-glycoprotein for effective transportation of actives across blood-brain-barrier. J. Control. Release 2021, 335, 398–407. [Google Scholar] [CrossRef]
- Testa, B.; Kraemer, S.D. The biochemistry of drug metabolism—An introduction: Part 4. reactions of conjugation and their enzymes. Chem. Biodivers. 2008, 5, 2171–2336. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Jones, B.C.; Rollison, H.; Johansson, S.; Kanebratt, K.P.; Lambert, C.; Vishwanathan, K.; Andersson, T.B. Managing the Risk of CYP3A Induction in Drug Development: A Strategic Approach. Drug Metab. Dispos. 2017, 45, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Kandasamy, R. Nonantibiotics Enhance The Antibacterial Activity Of Ceftriaxone Against Methicillin-Resistant Staphylococcus Aureus. Asian J. Pharm. Clin. Res. 2018, 11, 134. [Google Scholar] [CrossRef]
- Krishnan, D.; Kandasamy, R. Antibacterial potentiality of antiulcer and antispasmodic drugs with selected antibiotics against methicillin resistant Staphylococcus aureus: In vitro and in silico studies. Bangladesh J. Pharmacol. 2015, 10, 875. [Google Scholar] [CrossRef] [Green Version]
- Nandini, P.; Deepnandan, D. Synthetic Process Study and Pharmacological Evaluation of Antispasmodic Drug as Potential Antimicrobial Agent. Asian J. Res. Chem. 2009, 2, 494. [Google Scholar] [CrossRef]
- Zheng, H.; Drumm, B.; Zhu, M.H.; Xie, Y.; O’Driscoll, K.; Baker, S.; Perrino, B.; Koh, S.; Sanders, K. Na+/Ca2+ Exchange and Pacemaker Activity of Interstitial Cells of Cajal. Front. Physiol. 2020, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Garland, C.J.; Bagher, P.; Powell, C.; Ye, X.; Lemmey, H.A.L.; Borysova, L.; Dora, K. Voltage-dependent Ca2+ entry into SM during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci. Signal. 2017, 10, eaal3806. [Google Scholar] [CrossRef] [Green Version]
- Jackson, W.F.; Boerman, E.M. Voltage-gated Ca2+ channel activity modulates SM cell calcium waves in hamster cremaster arterioles. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H871–H878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickens, E.J.; Hirst, G.D.S.; Tomita, T. Identification of rhythmically active cells in guinea-pig stomach. J. Physiol. 1999, 514, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.D.; Mangel, A.W. Depolarization-Stimulated Contractility of Gastrointestinal Smooth Muscle in Calcium-Free Solution: A Review. Int. Sch. Res. Not. 2010, 2011, 692528. [Google Scholar] [CrossRef] [PubMed]
- Taira, N. Nicorandil as a hybrid between nitrates and potassium channel activators. Am. J. Cardiol. 1989, 63, 18J–24J. [Google Scholar] [CrossRef]
- Kito, Y.; Suzuki, H. Modulation of slow waves by hyperpolarization with potassium channel openers in antral smooth muscle of the guinea-pig stomach. J. Physiol. 2003, 548, 175–189. [Google Scholar] [CrossRef]
- Mert, T. Sucrose-Gap Technique: Advantages and Limitations. Neurophysiology 2007, 39, 237–241. [Google Scholar] [CrossRef]

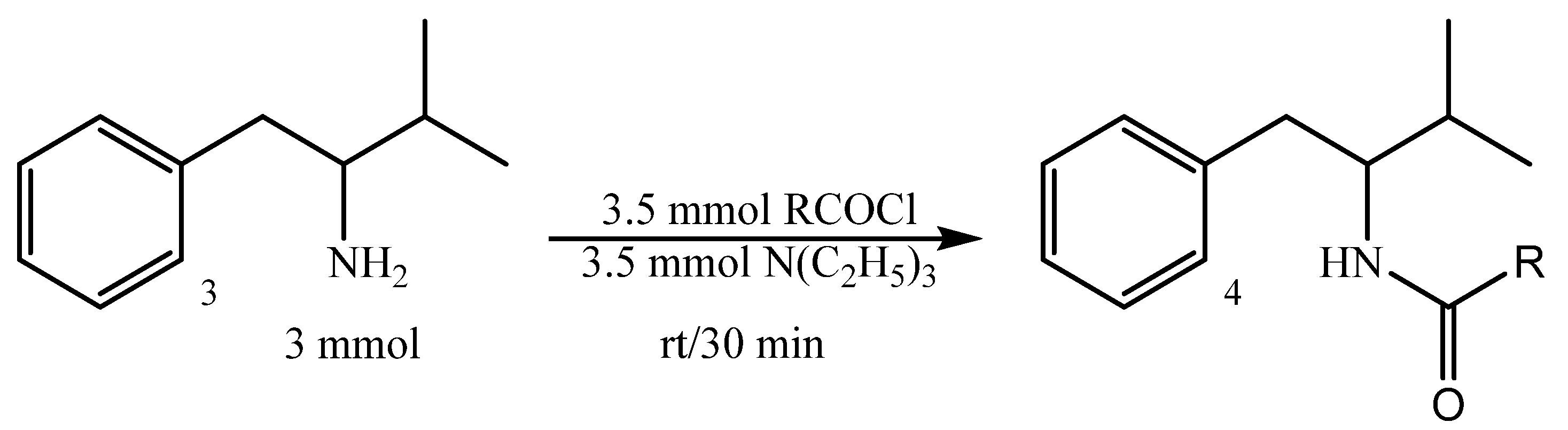

| 4 | R | Yield, % | mp, °C |
|---|---|---|---|
| a | CH3 | 83 | 97–99 |
| b | C6H5 | 79 | 144–145 |
| c | 2-Cl-C6H4 | 79 | 82–83 |
| d | CH2-C6H5 | 69 | 86–87 |
| Compound | MW, g/mol | XLOGP3 | TPSA, Å2 | ESOL Log S | Fraction Csp3 | RB | BA Score | SA Score | LD50, mg/kg |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 163.26 | 2.59 | 26.02 | −2.66 | 0.45 | 3 | 0.55 | 1.48 | 241 |
| 4a | 205.30 | 2.89 | 29.10 | −2.90 | 0.46 | 5 | 0.55 | 1.74 | 899 |
| 4b | 267.37 | 4.55 | 29.10 | −4.41 | 0.28 | 6 | 0.55 | 2.08 | 2000 |
| 4c | 301.81 | 5.18 | 29.10 | −5.00 | 0.28 | 6 | 0.55 | 2.38 | 2500 |
| 4d | 281.39 | 4.49 | 29.10 | −4.37 | 0.32 | 7 | 0.55 | 2.29 | 825 |
| Inhibition Zones, mm | |||||||
|---|---|---|---|---|---|---|---|
| Tested Microorganism/Compound | 3 | 4a | 4b | 4c | 4d | Mebeverine | Methanol |
| Klebsiella sp. | 8 | - | - | - | - | - | - |
| Escherichia coli ATCC 25922 | - | 8 | - | - | - | - | - |
| Pseudomonas aeruginosa ATCC 9027 | 9 | - | - | - | - | - | - |
| Saccharomyces cerevisiae | 8 | - | - | - | - | - | - |
| Compound/Cell Line | LAMA-84 a | K-562 b | CCL-1 c |
|---|---|---|---|
| 3 | 239.4 ± 18.2 | 249.9 ± 10.5 | >300 |
| 4a | 359.8 ± 11.0 | 276.3 ± 19.7 | >300 |
| 4b | 265.4 ± 20.2 | 328.8 ± 20.4 | >300 |
| 4c | 265.9 ± 16.8 | 366.6 ± 8.4 | >300 |
| 4d | 328.3 ± 15.9 | 243.7 ± 18.5 | >300 |
| Mebeverine hydrochloride | 72 ± 6.2 | 85.4 ± 8.3 | >300 |
| Bioelectric Activity Parameters | Synthesized Mebeverine Precursors 3 and 4a–d | |||||
|---|---|---|---|---|---|---|
| 3 | 4a | 4b | 4c | 4d | ||
| Amplitude of slow waves, mV | Autocontrol value | 5.15 ± 0.07 | 4.92 ± 0.18 | 5.05 ± 0.26 | 5.23 ± 0.17 | 5.13 ± 0.28 |
| Measured value | 5.28 ± 0.33 | 4.32 ± 0.38 | 5.09 ± 0.10 | 5.34 ± 0.20 | 4.97 ± 0.29 | |
| Number of slow waves, min−1 | Autocontrol value | 4.33 ± 0.11 | 4.20 ± 0.09 | 4.03 ± 0.08 | 4.12 ± 0.10 | 3.93 ± 0.07 |
| Measured value | 4.90 ± 0.09 | 4.72 ± 0.55 | 4.50 ± 0.49 | 4.42 ± 0.41 | 4.82 ± 0.24 | |
| Amplitude of spike potentials, mV | Autocontrol value | 0.95 ± 0.36 | 1.3 ± 0.25 | 1.1 ± 0.20 | 1.0 ± 0.12 | 1.2 ± 0.16 |
| Measured value | 2.61 ± 0.16 * | 0 * | 1.40 ± 0.12 | 1.56 ± 0.17 | 0 * | |
| Number of spike potentials min−1 | Autocontrol value | 21.43 ± 0.20 | 27.12 ± 0.23 | 20.17 ± 0.50 | 25.44 ± 0.14 | 26.79 ± 0.55 |
| Measured value | 48.22 ± 0.39 * | 0 * | 22.28 ± 0.18 | 26.16 ± 0.26 | 0 * | |
| Changes in the membrane potential, % | - | 55 ↑ | 41 ↓ | 0 | 0 | 0 |
| Compound | 5-HT | Response to Compound |
|---|---|---|
| 3 | ++ | +++ |
| 4a | ++ | − |
| 4b | ++ | − |
| 4c | ++ | − |
| 4d | ++ | + |
| MH | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milusheva, M.; Gledacheva, V.; Stefanova, I.; Pencheva, M.; Mihaylova, R.; Tumbarski, Y.; Nedialkov, P.; Cherneva, E.; Todorova, M.; Nikolova, S. In Silico, In Vitro, and Ex Vivo Biological Activity of Some Novel Mebeverine Precursors. Biomedicines 2023, 11, 605. https://doi.org/10.3390/biomedicines11020605
Milusheva M, Gledacheva V, Stefanova I, Pencheva M, Mihaylova R, Tumbarski Y, Nedialkov P, Cherneva E, Todorova M, Nikolova S. In Silico, In Vitro, and Ex Vivo Biological Activity of Some Novel Mebeverine Precursors. Biomedicines. 2023; 11(2):605. https://doi.org/10.3390/biomedicines11020605
Chicago/Turabian StyleMilusheva, Miglena, Vera Gledacheva, Iliyana Stefanova, Mina Pencheva, Rositsa Mihaylova, Yulian Tumbarski, Paraskev Nedialkov, Emiliya Cherneva, Mina Todorova, and Stoyanka Nikolova. 2023. "In Silico, In Vitro, and Ex Vivo Biological Activity of Some Novel Mebeverine Precursors" Biomedicines 11, no. 2: 605. https://doi.org/10.3390/biomedicines11020605
APA StyleMilusheva, M., Gledacheva, V., Stefanova, I., Pencheva, M., Mihaylova, R., Tumbarski, Y., Nedialkov, P., Cherneva, E., Todorova, M., & Nikolova, S. (2023). In Silico, In Vitro, and Ex Vivo Biological Activity of Some Novel Mebeverine Precursors. Biomedicines, 11(2), 605. https://doi.org/10.3390/biomedicines11020605










