Serum Neurofilaments and OCT Metrics Predict EDSS-Plus Score Progression in Early Relapse-Remitting Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Processing Protocol
2.2. Statistical Analysis
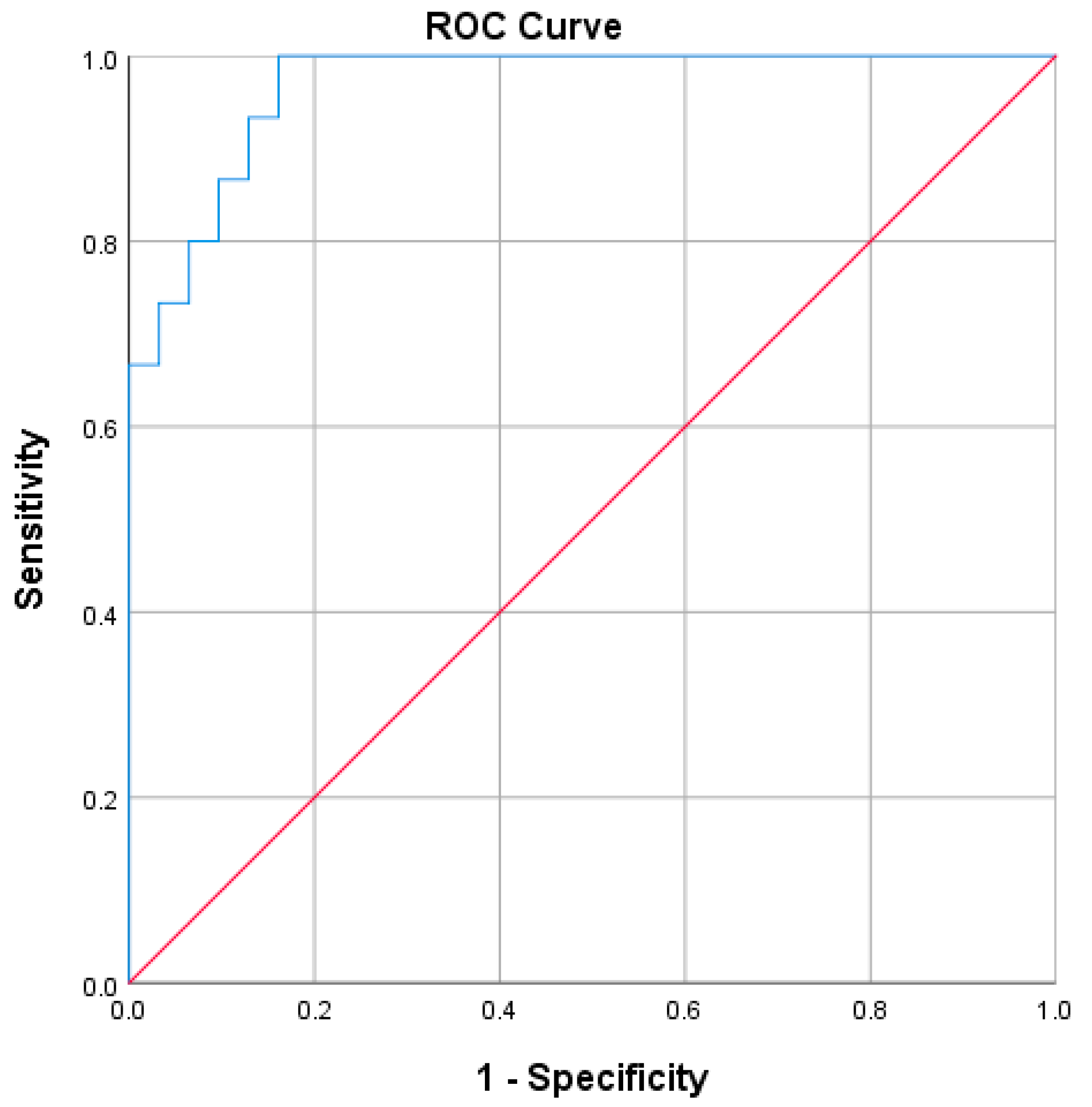
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovannoni, G.; Popescu, V.; Wuerfel, J.; Hellwig, K.; Iacobaeus, E.; Jensen, M.B.; García-Domínguez, J.M.; Sousa, L.; De Rossi, N.; Hupperts, R.; et al. Smouldering multiple sclerosis: The ‘real MS’. Ther. Adv. Neurol. Disord. 2022, 15, 17562864211066751. [Google Scholar] [CrossRef]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Trojano, M. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382–5394. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Danesi, R.; Derfuss, T.; Duddy, M.; Gallo, P.; Gold, R.; Havrdová, E.K.; Kornek, B.; Saccà, F.; Tintoré, M.; et al. Early and unrestricted access to high-efficacy disease-modifying therapies: A consensus to optimize benefits for people living with multiple sclerosis. J. Neurol. 2021, 269, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; di Cola, F.S.; Scarioni, M.; Fenoglio, C.; Spanò, B.; Arighi, A.; Cioffi, S.M.; Oldoni, E.; A De Riz, M.; Basilico, P.; et al. CSF β-amyloid as a putative biomarker of disease progression in multiple sclerosis. Mult. Scler. J. 2016, 23, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; Caprioli, M.; Carandini, T.; Scarioni, M.; Ghezzi, L.; Arighi, A.; Cioffi, S.; Cinnante, C.; Fenoglio, C.; Oldoni, E.; et al. CSF β-amyloid predicts prognosis in patients with multiple sclerosis. Mult. Scler. J. 2018, 25, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Tiu, V.E.; Popescu, B.O.; Enache, I.I.; Tiu, C.; Terecoasa, E.; Panea, C.A. Serum and CSF Biomarkers Predict Active Early Cognitive Decline Rather Than Established Cognitive Impairment at the Moment of RRMS Diagnosis. Diagnostics 2022, 12, 2571. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [Google Scholar] [CrossRef]
- Thebault, S.; Reaume, M.; Marrie, R.A.; Marriott, J.J.; Furlan, R.; Laroni, A.; A Booth, R.; Uccelli, A.; Freedman, M.S. High or increasing serum NfL is predictive of impending multiple sclerosis relapses. Mult. Scler. Relat. Disord. 2022, 59, 103535. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Tiu, V.E.; Enache, I.; Panea, C.A.; Tiu, C.; Popescu, B.O. Predictive MRI Biomarkers in MS—A Critical Review. Medicina 2022, 58, 377. [Google Scholar] [CrossRef]
- Iacobaeus, E.; Arrambide, G.; Amato, M.P.; Derfuss, T.; Vukusic, S.; Hemmer, B.; Tintoré, M.; Brundin, L. Aggressive multiple sclerosis (1): Towards a definition of the phenotype. Mult. Scler. J. 2020, 26, 1031–1044. [Google Scholar] [CrossRef]
- Wiendl, H.; Gold, R.; Berger, T.; Derfuss, T.; Linker, R.; Mäurer, M.; Aktas, O.; Baum, K.; Berghoff, M.; Bittner, S.; et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): Position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther. Adv. Neurol. Disord. 2021, 14, 17562864211039648. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Gonzalez-Moron, D.; Garcea, O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: A review. Mult. Scler. Relat. Disord. 2018, 22, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Costello, F.; Burton, J.M. Retinal imaging with optical coherence tomography: A biomarker in multiple sclerosis? Eye Brain 2018, 10, 47–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, R.; Pillay, G.; Ganger, A.; Singh, D.; Bhatia, R.; Sharma, P.; Menon, V. Retinal nerve fiber layer and ganglion cell layer changes on optical coherence tomography in early multiple sclerosis and optic neuritis cases. Indian J. Ophthalmol. 2018, 66, 114–119. [Google Scholar] [CrossRef]
- Cordano, C.; Nourbakhsh, B.; Devereux, M.; Damotte, V.; Bennett, D.; Hauser, S.L.; Cree, B.A.C.; Gelfand, J.M.; Green, A.J. pRNFL as a marker of disability worsening in the medium/long term in patients with MS. Neurol.-Neuroimmunol. Neuroinflamm. 2018, 6, e533. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lapiscina, E.H.; Arnow, S.; A Wilson, J.; Saidha, S.; Preiningerova, J.L.; Oberwahrenbrock, T.; Brandt, A.U.; E Pablo, L.; Guerrieri, S.; Gonzalez, I.; et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol. 2016, 15, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, R.; Montomoli, C.; Mallucci, G.; Lugaresi, A.; Izquierdo, G.; Grand’Maison, F.; Duquette, P.; Shaygannejad, V.; Alroughani, R.; Grammond, P.; et al. BREMSO: A simple score to predict early the natural course of multiple sclerosis. Eur. J. Neurol. 2015, 22, 981–989. [Google Scholar] [CrossRef]
- Gasperini, C.; Prosperini, L.; Rovira, À.; Tintoré, M.; Sastre-Garriga, J.; Tortorella, C.; Haggiag, S.; Galgani, S.; Capra, R.; Pozzilli, C.; et al. Scoring the 10-year risk of ambulatory disability in multiple sclerosis: The RoAD score. Eur. J. Neurol. 2021, 28, 2533–2542. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Kurtzke, J.F. On the origin of EDSS. Mult. Scler. Relat. Disord. 2015, 4, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Dalla-Costa, G.; Radaelli, M.; Maida, S.; Sangalli, F.; Colombo, B.; Moiola, L.; Comi, G.; Martinelli, V. Smart watch, smarter EDSS: Improving disability assessment in multiple sclerosis clinical practice. J. Neurol. Sci. 2017, 383, 166–168. [Google Scholar] [CrossRef]
- Cinar, B.P.; Yorgun, Y.G. What We Learned from The History of Multiple Sclerosis Measurement: Expanded Disease Status Scale. Arch. Neuropsychiatry 2018, 55, S69–S75. [Google Scholar] [CrossRef]
- Mikol, D.; Cohen, J.; Freedman, M.; Goldman, M.; Hartung, H.P.; Havrdova, E.; Jeffery, D.; Kapoor, R.; Miller, A.; Sellebjerg, F.; et al. Rationale for EDSS-Plus, the Primary Composite Endpoint of Disability Progression in the ASCEND Phase 3 Study of Natalizumab for Secondary Progressive Multiple Sclerosis: A Post Hoc Analysis of IMPACT Study Data (P7.240). Neurology 2015, 84 (Suppl. S14), 240. [Google Scholar]
- Cadavid, D.; A Cohen, J.; Freedman, M.S.; Goldman, M.D.; Hartung, H.-P.; Havrdova, E.K.; Jeffery, D.; Kapoor, R.; Miller, A.; Sellebjerg, F.; et al. The EDSS-Plus, an improved endpoint for disability progression in secondary progressive multiple sclerosis. Mult. Scler. J. 2016, 23, 94–105. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kalincik, T.; Butzkueven, H. The MSBase registry: Informing clinical practice. Mult. Scler. J. 2019, 25, 1828–1834. [Google Scholar] [CrossRef]
- Tur, C.; Carbonell-Mirabent, P.; Cobo-Calvo, Á.; Otero-Romero, S.; Arrambide, G.; Midaglia, L.; Castilló, J.; Vidal-Jordana, Á.; Rodríguez-Acevedo, B.; Zabalza, A.; et al. Association of Early Progression Independent of Relapse Activity with Long-term Disability After a First Demyelinating Event in Multiple Sclerosis. JAMA Neurol. 2022, 80, 151–160. [Google Scholar] [CrossRef]
- Cohen, M.; Bresch, S.; Rocchi, O.T.; Morain, E.; Benoit, J.; Levraut, M.; Fakir, S.; Landes, C.; Lebrun-Frénay, C. Should we still only rely on EDSS to evaluate disability in multiple sclerosis patients? A study of inter and intra rater reliability. Mult. Scler. Relat. Disord. 2021, 54, 103144. [Google Scholar] [CrossRef]
- Kapica-Topczewska, K.; Collin, F.; Tarasiuk, J.; Czarnowska, A.; Chorąży, M.; Mirończuk, A.; Kochanowicz, J.; Kułakowska, A. Assessment of Disability Progression Independent of Relapse and Brain MRI Activity in Patients with Multiple Sclerosis in Poland. J. Clin. Med. 2021, 10, 868. [Google Scholar] [CrossRef]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Oh, J.; Havrdová, E.K.; Tintoré, M.; Zipp, F. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain 2021, 144, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Valdeolivas, I.; Andorrà, M.; Gómez-Andrés, D.; Nakamura, K.; Alba-Arbalat, S.; Lampert, E.J.; Zubizarreta, I.; Llufriu, S.; Martinez-Heras, E.; Solana, E.; et al. Retinal and brain damage during multiple sclerosis course: Inflammatory activity is a key factor in the first 5 years. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cordano, C.; Nourbakhsh, B.; Yiu, H.H.; Papinutto, N.; Caverzasi, E.; Abdelhak, A.C.; Oertel, F.C.C.; Beaudry-Richard, A.; Santaniello, A.; Sacco, S.; et al. Differences in Age-related Retinal and Cortical Atrophy Rates in Multiple Sclerosis. Neurology 2022, 99, e1685–e1693. [Google Scholar] [CrossRef]
- Lie, I.A.; Kaçar, S.; Wesnes, K.; Brouwer, I.; Kvistad, S.S.; Wergeland, S.; Holmøy, T.; Midgard, R.; Bru, A.; Edland, A.; et al. Serum neurofilament as a predictor of 10-year grey matter atrophy and clinical disability in multiple sclerosis: A longitudinal study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 849–857. [Google Scholar] [CrossRef] [PubMed]
| Median (Minimum and Maximum Values) | |
|---|---|
| Age | 29 (18; 52) |
| Sex | 37 F (71.2%)/15 M (28.8%) |
| Smoker status (active/non-smoker) | 15 (28.8%)/37 (71.2%) |
| Lifestyle (active/sedentary) | 37 (71.2%)/15 (28.8%) |
| Urban/rural | 42 (80.8%)/10 (19.2%) |
| Clinical data | |
| EDSS—baseline | 2 (0; 6) |
| EDSS—1-year follow-up | 1.5 (0; 6.5) |
| Type of DMT started after study inclusion High-efficacy DMT Moderate-efficacy DMT | 8 (15.4%) 44 (84.6%) |
| Relapses in the first year (no. of) Zero relapses One relapse Two relapses | 32 (61.5%) 18 (34.6%) 2 (3.8%) |
| CSF immunological analysis | No. of patients (%) |
| Positive oligoclonal bands | 37 (71.2%) |
| Baseline MRI characteristics | |
| Twenty or more T2/FLAIR hyperintense lesions | 37 (71.2%) |
| Two or more gadolinium-enhancing lesions (GdE) | 12 (23.1%) |
| 1-year follow-up MRI characteristics | |
| Three or more new T2/FLAIR hyperintense lesions | 26 (50%) |
| One or more new GdE | 4 (7.7%) |
| Baseline OCT characteristics | Mean (minimum and maximum values) |
| RNFL | 95.2 µm (67; 118) |
| GCL + IPL | 79.0 µm (56; 92.5) |
| Neurofilaments * | |
| Baseline CSF NfL raw values | 1114 pg/mL (201; 4210) |
| Baseline sNfL-adjusted z-score | 2.14 (−1.64; 3.81) |
| Three-month follow-up sNfL-adjusted z-score | 1.34 (−0.99; 3.29) |
| Six-month follow-up sNfL-adjusted z-score | 0.98 (−1.8; 2.95) |
| Twelve-month follow-up sNfL-adjusted z-score | 0.81 (−1.85; 2.65) |
| CSF Beta-amyloid | 650 (280; 1211) |
| Predictive scores | |
| BREMSO | 0.44 (−0.65; 2,39) |
| RoAD | 3 (0; 7) |
| EDSS-Plus Progressor | EDSS-Plus Non-Progressors | p Value | |
|---|---|---|---|
| Age (mean) | 32.1 years | 29.5 years | 0.32 |
| Active smoker | 5 (26.3%) | 10 (30.3%) | 0.76 |
| Masculine sex | 5 (26.3%) | 10 (30.3%) | 0.76 |
| Rural environment | 6 (31.6%) | 4 (12.1%) | 0.14 |
| Sedentary lifestyle | 6 (31.6%) | 9 (27.3%) | 0.74 |
| Positive OCBs | 14 (73.7%) | 23 (69.7%) | 0.76 |
| EDSS baseline score (mean) | 2.1 | 2.0 | 0.85 |
| Baseline MRI Twenty or more T2/FLAIR hyperintense lesions | 15 (78.9%) | 22 (66.7%) | 0.34 |
| Two or more GdE lesions | 6 (31.6%) | 76 (18.2%) | 0.31 |
| One-year follow-up MRI Three or more new T2/FLAIR hyperintense lesions | 10 (52.6%) | 16 (48.5%) | 0.77 |
| One or more new GdE | 1 (1.9%) | 3 (5.8%) | 1.0 |
| Moderate efficacy DMT type | 18 (94.7%) | 26 (78.8%) | 0.232 |
| Relapses during the first year (no. of) 0 1 2 | 9 (47.4%) 9 (47.4%) 1 (5.3%) | 23 (69.7%) 9 (27.3%) 1 (1.9%) | 0.28 |
| EDSS-Plus Progressor | EDSS-Plus Non-Progressors | p Value | |
|---|---|---|---|
| BREMSO | 0.56 | 0.42 | 0.583 |
| Baseline RoAD score | 2.37 | 2.48 | 0.752 |
| RoAD final score | 4.05 | 3.0 | 0.023 |
| Baseline sNfL-adjusted z-score | 2.01 | 1.77 | 0.474 |
| Three-month sNfL-adjusted z-score | 1.57 | 1.35 | 0.502 |
| Six-month sNfL-adjusted z-score | 1.44 | 0.71 | 0.028 |
| Twelve-month sNfL-adjusted z-score | 1.32 | 0.49 | 0.010 |
| Baseline CSF NfL (pg/mL) | 1339 | 1319 | 0.946 |
| CSF Aβ42 (pg/mL) | 667 | 685 | 0.769 |
| OCT RNFL mean thickness | 90 µm | 97 µm | 0.024 |
| OCT GCL-IPL mean thickness | 73 µm | 80 µm | 0.003 |
| B | SE | Wald | df | p | Odds Ratio | 95% CI for OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| BREMSO | −0.801 | 0.830 | 0.929 | 1 | 0.335 | 0.449 | 0.088 | 2.287 |
| Baseline sNfL-adjusted z-score | −1.681 | 0.911 | 3.409 | 1 | 0.065 | 0.186 | 0.031 | 1.109 |
| Six-month sNfL-adjusted z-score | 3.983 | 1.560 | 6.517 | 1 | 0.011 | 53.66 | 2.52 | 1141.78 |
| RNFL mean thickness | −0.172 | 0.109 | 2.482 | 1 | 0.115 | 0.842 | 0.679 | 1.043 |
| GCL-IPL mean thickness | −0.187 | 0.103 | 3.274 | 1 | 0.70 | 0.830 | 0.678 | 1.043 |
| Constant | 27.624 | 11.525 | 5.745 | 1 | 0.17 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiu, V.E.; Popescu, B.O.; Enache, I.I.; Tiu, C.; Cherecheanu, A.P.; Panea, C.A. Serum Neurofilaments and OCT Metrics Predict EDSS-Plus Score Progression in Early Relapse-Remitting Multiple Sclerosis. Biomedicines 2023, 11, 606. https://doi.org/10.3390/biomedicines11020606
Tiu VE, Popescu BO, Enache II, Tiu C, Cherecheanu AP, Panea CA. Serum Neurofilaments and OCT Metrics Predict EDSS-Plus Score Progression in Early Relapse-Remitting Multiple Sclerosis. Biomedicines. 2023; 11(2):606. https://doi.org/10.3390/biomedicines11020606
Chicago/Turabian StyleTiu, Vlad Eugen, Bogdan Ovidiu Popescu, Iulian Ion Enache, Cristina Tiu, Alina Popa Cherecheanu, and Cristina Aura Panea. 2023. "Serum Neurofilaments and OCT Metrics Predict EDSS-Plus Score Progression in Early Relapse-Remitting Multiple Sclerosis" Biomedicines 11, no. 2: 606. https://doi.org/10.3390/biomedicines11020606





