1. Introduction
Macrophages (Mφ) are part of the “monocyte-phagocyte system” which consists of tissue macrophages and their progenitors—monocytes present in the peripheral blood [
1]. Recruited circulating monocytes leave the blood stream and differentiate into macrophages as a response to different stimuli in their respective tissue microenvironment [
2]. Once differentiated, macrophages become long-lived cells and develop specialized functions. Cell numbers in the tissues are maintained by resistance to constitutive apoptosis, recruitment of further monocytes from the blood, and/or replication of local intermediates [
3,
4,
5].
Mφ are essential for clearance of infections, induction of adaptive immunity, removal of old and dead cells and debris, promoting tissue repair, and wound healing [
2,
6]. Macrophages exercise all these functions via multiple key processes [
2]:
Phagocytosis (pathogens, debris, and dead and infected cells);
Antigen presentation via MHCII molecules (major histocompatibility complex);
Production of cytokines, chemokines, and growth factors.
For many years, the prevailing notion was that macrophages solely arose from the differentiation of circulating monocytes. However, more recent studies provided evidence that most adult tissue-resident macrophages are seeded before birth, derived from the yolk sac during embryonic development, and have a self-renewal capacity [
7]. Tissue-resident macrophages play fundamental roles in tissue homeostasis and immune-surveillance. After the initial recognition of a microbial challenge, resident macrophages drive the influx of leukocytes, including monocytes, as a source of inflammatory macrophages. Therefore, the main role of monocyte-derived macrophages is host defense and subsequential post-inflammatory wound-healing and tissue remodeling [
8].
Besides the basic distinction of tissue-resident and monocyte-derived macrophages, these cells can be divided into several sub-populations based on the differentiation stimuli and function [
2]. In tissues, tissue-resident Mφ and monocytes recruited from the peripheral blood respond to different micro-environmental stimuli (e.g., damaged cells or pathogens and their products) with the acquirement of a functional phenotype [
9]. Therefore, several classes of macrophages have already been described based mainly on the expression of surface markers, production of cytokines, chemokines, and growth factors, and their biological activities [
10]. Two major and best characterized macrophage sub-populations are classically activated (M1) macrophages with their main role in host defense against pathogens, and alternatively activated (M2) macrophages (resolution of inflammation and tissue repair). This phenomenon of the two different M1/M2 phenotypes is referred to as “macrophage polarization”. However, it should be noted that M1 and M2 activation phenotypes represent two ends of a functional spectrum of macrophage polarization states [
2,
11]. On the other hand, some authors claim that due to the plasticity of Mφ, they constantly adapt to different micro-environmental stimuli. Since any analysis is only a “snapshot” of the current situation in the tissue, the plasticity of Mφ and their constant adaptations create an impression that there are numerous subtypes of macrophages [
6,
12].
Scientific papers use various nomenclature for the two main types of Mφ. Mostly they are referred to as M1/M2 macrophages or classically/alternatively activated macrophages. Another commonly used nomenclature is inflammatory (M1) and anti-inflammatory (M2) macrophages [
11]. Although these terms are often used interchangeably, historically they represented macrophages that were identified under strikingly different conditions (reviewed in [
6]). Some authors have proven that based on the gene expression, M1/M2 terms should not be considered equivalent to classically/alternatively activated Mφ [
13]. In this article, we will be referring to polarized Mφ mainly as M1/M2 for simplicity and due to the common usage of this nomenclature across the scientific community. Still, it is important to acknowledge that according to the historical aspects of the nomenclature only, Mφ in the presented study should be considered rather as classically/alternatively activated.
There are several ways to study the differentiation and function of macrophages in vitro. The most accurate way to study tissue macrophages is to obtain specific Mφ from the tissue of interest. However, the isolation of these cells requires blood donation or invasive procedures, such as tissue biopsy, which often yield low amounts of tissue samples [
14,
15]. Moreover, Mφ isolated from the tissues have only a limited capacity to proliferate therefore only a relatively small number of cells is available for analysis [
16,
17]. To overcome this problem, immortalized monocytic cell lines of varying degrees of differentiation (e.g., KG1, HL-60, U937, and THP-1) are frequently used to model macrophage differentiation and function [
18]. One of the most used monocytic cell lines is THP-1 (Tohoku Hospital Pediatrics-1 cells), which displays commitment towards macrophage differentiation. This cell line was isolated from a boy with acute monocytic leukemia by Tsuchiya et al. [
19]. It resembles many aspects of human monocytes, such as morphology, expression of membrane antigens, and secretory products [
18]. While differentiation of macrophages using monocytic cell lines such as THP-1 has obvious advantages in terms of simplicity, cost effectiveness, and ease of acquisition, results from these experiments may not always accurately predict the behavior of differentiated tissue macrophages [
5,
17]. To address this, differentiation protocols have been developed to obtain macrophages, which best resemble differentiated tissue macrophages regarding their function as well as their morphology, surface markers, gene expression, etc. [
17]. Most of the protocols for polarization of M1 and M2 macrophages use cytokine stimuli [
5]. First, the monocytes differentiate into “M0” macrophages, which represent a transitory state. This so-called activation of macrophages is obtained using phorbol-12-myristate-13-acetate (PMA). Activation is followed by polarization into M1 macrophages using interferon gamma (IFN-γ) and a toll-like receptor (TLR) agonist, such as lipopolysaccharides (LPS). Alternatively, M2 macrophages are polarized by stimulation with interleukin 4 (IL-4) and IL-13 [
20,
21]. However, some protocols use phorbol-12-myristate-13-acetate (PMA) as the sole polarization stimulus for M1 macrophages and 1,25-(OH)
2-Vitamin D
3 (Vit D
3) to generate M2 macrophages [
17,
22].
The initial steps of biological research often depend on using cell cultures and in vitro assays to predict the function of different cells, their physiology, pathological changes, and treatment options in vivo. Therefore, besides the selection of the most appropriate and representative cell line, the most important component of in vitro experiments is the protocol used to stimulate and/or differentiate said cells. A standardized protocol is crucial to ensure the reproducibility of the experiment and most importantly the conditions which resemble the in vivo environment the most. The development of such a protocol is necessary to obtain results that would be relevant and applicable in in vivo settings. However, even though macrophages differentiated from the THP-1 cell line are widely used to study the function of human macrophages, there is currently no consensus on the best protocol for macrophage polarization from THP-1 cells [
21,
23]. Therefore, we decided to compare different polarization protocols from the perspective of the transcriptome of M1/M2 macrophages polarized according to these protocols. Firstly, we chose a protocol that uses PMA and Vit D
3, and a protocol that involves activation with PMA followed by stimulation with cytokines. Lastly, we involved a third protocol in our analysis which also uses cytokine stimuli but applies a markedly different concentration of PMA to obtain M0 macrophages and a rest period between the activation phase and polarization of macrophages. The authors of this protocol claim that high concentrations of PMA block the subsequent polarization of the M2 macrophages [
21].
4. Discussion
Macrophages are important cells of innate immunity with many functions regarding defense against pathogens, inflammatory processes, and anti-tumor activities. Moreover, they play a key part in tissue regeneration, wound healing, and clearance of old cells and debris [
29,
30]. These processes are carried out by different subtypes of Mφ polarized in response to stimuli present in their micro-environment [
5]. Immortalized monocytic cell lines, such as THP-1, are often used to study the differentiation and function of Mφ [
31]. In order to achieve results from these studies that would accurately predict the function and responses of in vivo tissue Mφ, different polarization protocols have been proposed and are regularly optimized. Still to this date, there is not a consensus in the scientific community regarding the most suitable protocol to achieve Mφ which would resemble the macrophages in a living organism the best [
13,
17,
21].
Nowadays, gene and protein expression differences are considered to be the clearest and simplest way to distinguish the polarization state of macrophages [
5]. Many authors analyze the gene expression or the whole transcriptome to characterize the polarization status of Mφ under different experimental conditions or in various diseases [
32,
33,
34,
35]. Some reviews try to summarize these data and present gene expression panels for M1 and M2 macrophages [
5].
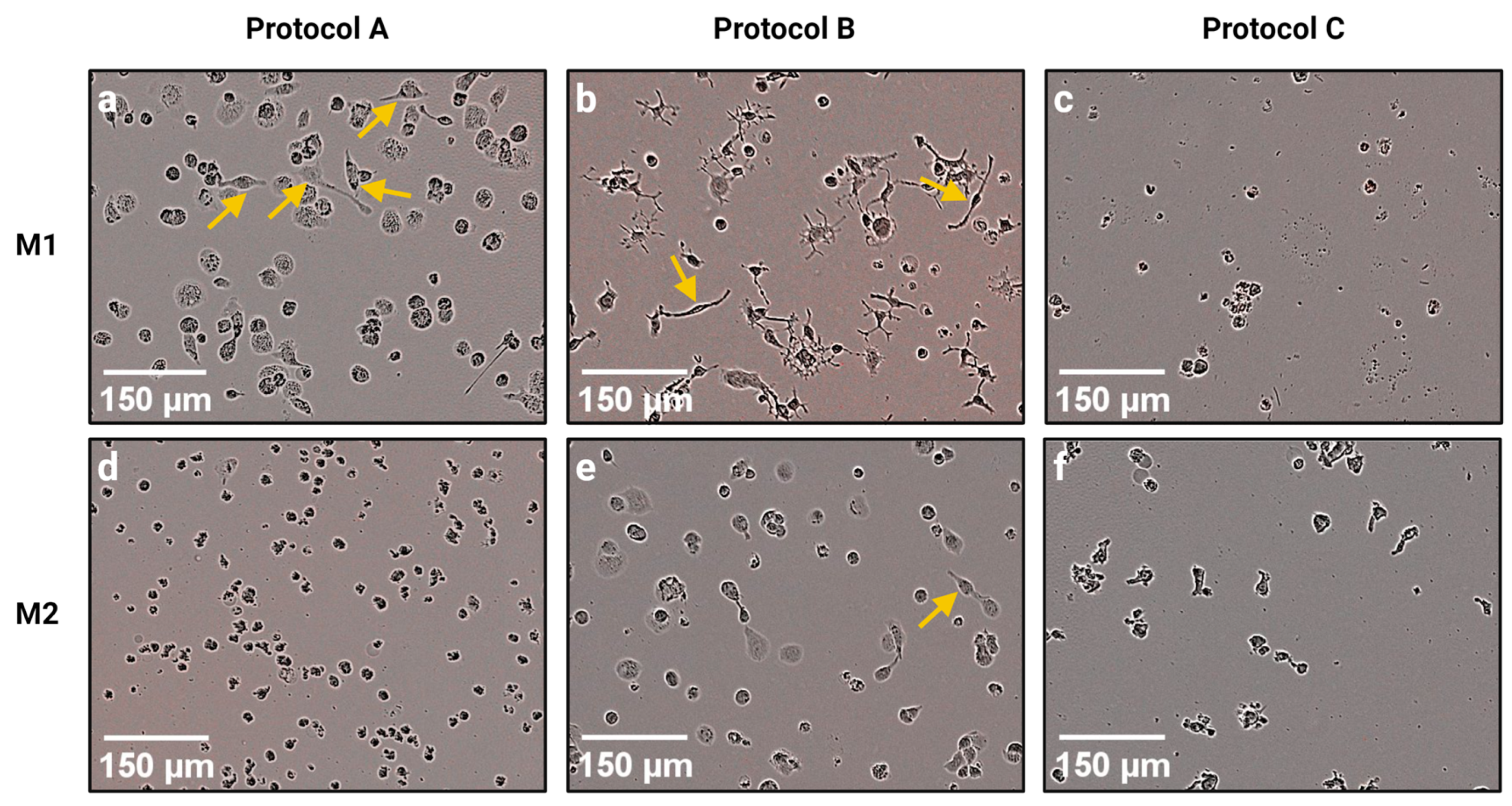
Therefore, in this study we carried out an RNA-seq transcriptome analysis of M1 and M2 macrophages polarized according to three different protocols. Although all three protocols were supposed to generate inflammatory M1 and anti-inflammatory M2 macrophages from THP-1 monocytes, we found great differences between macrophages generated by these protocols at the gene expression level, and also at the morphological level. Several authors have reported that polarized M1 macrophages have an elongated spindle-like morphology, while M2 macrophages are more roundly shaped [
36,
37,
38,
39,
40]. Some of the cells in M1_A and M1_B samples had an elongated morphology, while M1_C macrophages had a round or rugged shape even though LPS was reported to stimulate the formation of spindle-like morphology in M1 macrophages [
39]. M2 macrophages from all protocols were rounded, but occasionally we observed spindle-like cells in the M2_B sample. It should be noted that the data about macrophage morphology is contradictory since there are also studies reporting round M1 cells and spindle-like M2 cells [
41,
42,
43]. Most of the M1_B macrophages had several projections, which were either smooth or ramified. Some papers have already described similar morphology of M1 macrophages polarized from monocytic cell lines [
44,
45] or peripheral blood monocytes [
46,
47]. Projections of the cell surface of macrophages allow them to move and to survey the extracellular milieu. Macrophage projections, e.g., filopodia, lamellipodia, and dorsal ruffles are essential for the migration and chemotaxis of immune cells. Moreover, they function in pathogen detection via toll-like receptors [
48]. Later studies also proved that macrophage filopodia can pull pathogens towards the cell body and are crucial in the initiation of phagocytosis [
49,
50]. We can assume that due to their numerous cell projections, M1_B macrophages would be able to readily migrate towards pathogens and detect them. However, this hypothesis should be confirmed with further testing. It should be also noted that in our morphological analysis Protocol C differed from the other two chosen protocols also in regard to cell numbers. In Protocol C, we observed far less cells despite seeding the same number of THP-1 monocytes in all protocols prior to adding polarization stimuli. Since we did not observe any dead cells, we assume that the reduced number of cells was not due to the effect of the polarization stimuli on cell viability. On the other hand, reduced adherence of Mφ could offer a possible explanation for low cell numbers in Protocol C. A study by Lund et al. (2016) proved that both the concentration of PMA and the length of the post-stimulation PMA-deprivation period have a major effect on the adherence of THP-1 cells. According to this study, lower concentrations of PMA and longer rest periods reduce the adherence of stimulated THP-1 cells with only around 50% of cells remaining attached to the dish after exposure to PMA with a concentration of 8 ng/mL followed by a 48 h PMA-deprivation period [
23]. Therefore, Mφ polarized according to Protocol C (PMA concentration c = 5 ng/mL; rest period 72 h) could have a reduced adherence which would result in unwanted aspiration of cells during the change in the growth medium and/or removal of PMA.
Major differences between the polarization protocols were also confirmed and further studied via the transcriptome analysis. Firstly, when comparing transcriptomic profiles using HCL, we found out that neither the macrophage subtype nor the polarization protocol seemed to be the main clustering factor. Despite that, M1 and M2 macrophages from Protocol B were clustered together (along with M0_C) and separated from the other samples. Moreover, M1_C and M2_C were also clustered closely together. Therefore, the differences between polarization protocols seemed to be greater than differences between M1 and M2 macrophages, especially in Protocol B. Since M1 and M2 macrophages should in many ways have opposite functions, they tend to separate in HCL [
35]. Therefore, we expected that the macrophage subtype would be a better clustering factor than the polarization protocol. However, this was not proven by the HCL. According to both the HCL and Venn diagrams of co-expression, the greatest differences between M1 and M2 macrophages were in Protocol A. Experiments using THP-1 and monocytic cell line HL-60 have revealed that PMA induces a greater degree of differentiation than Vit D
3 [
51]. HL-60 cells stimulated with Vit D
3 were observed to have a monocytic phenotype [
52] while phorbol esters (e.g., PMA) induce differentiation into macrophage-like cells [
53]. The degree of differentiation may induce greater differences in gene expression profiles than polarization into M1 or M2 subtypes; therefore, differences between macrophage polarization subtypes in Protocol A would be greater than between the respective subtypes in Protocols B and C.
Our analysis of DEGs focused mainly on three gene sets: regulators of macrophage polarization, cytokines and chemokines produced by Mφ, and macrophage receptors and enzymes. Regarding the regulators of M1 macrophage polarization, we observed the highest number of genes with significant over-expression in M1_B when compared with M0_B and M1 macrophages polarized according to other polarization protocols. The only gene that was not over-expressed was
AKT2, which is a part of the PI3K/Akt/mTORC1 signaling [
54]. Due to the activity of the PI3K/Akt pathway, differentiated macrophages are resistant to apoptotic stimuli. Moreover, differential expression of
AKT1 and
AKT2 homologues regulates immune response, in which the
AKT2 homologue stimulates inflammation. Many studies have confirmed that
AKT2 (−/−) deletion promotes the anti-inflammatory M2 phenotype while
AKT1 (−/−) deletion skews cells towards the M1 phenotype [
55]. There were no significant differences between the expression of
AKT2 in our samples. However, it is important to note that studies proposing the role of
AKT2 in M1 macrophages used genetically modified animals and cells with deletion of
AKT2 [
55,
56]. Therefore, lack of publications regarding the expression of
AKT2 in M1 and M2 macrophages without genetic modification makes it difficult to establish whether
AKT2 is differentially expressed between M1 and M2 macrophages. Overall, M1_A and M1_C showed lower levels of the regulatory genes’ expression compared with M1_B.
When analyzing the M1 cytokine and chemokine gene set we obtained similar results. The highest number of over-expressed genes was in M1_B which may suggest the highest production of inflammatory cytokines and chemokines in M1_B cells. However, a study by Raza et al. proved that some of the chemokine genes (including
CXCL9,
CXCL10, and
CXCL11) reach their highest expression level between 8–24 h post stimulation by LPS [
57]. Therefore, RNA from M1_B cells (stimulated with cytokines for 18 h) was isolated right in the window with the highest expression of the aforementioned chemokines. The only gene that was not differentially expressed in M1_B when compared with M1 macrophages from other polarization protocols and M0_B was
IL12A. This gene codes inflammatory cytokine which drives macrophages toward the M1 phenotype. Moreover, M1 macrophages are the main source of IL-12 [
58,
59]. Our results show that there was no significant difference between the levels of expression of
IL12A in Mφ subtypes and different protocols (except when comparing M1_C and M0_C) even though expression of this gene is heavily associated with M1 macrophages [
59].
The analysis of the M1 receptor and enzyme set showed that only the
CD80 gene was over-expressed in M1 macrophages polarized by any of the three tested protocols when compared with M2 macrophages, with the highest level of expression in M1_B. CD80 is a membrane receptor on the surface of antigen-presenting cells including macrophages, and it also serves as a ligand to CD28 on T-lymphocytes and promotes T-cell response [
60]. CD80 is considered one of the key markers of M1 macrophages with the power to discriminate between M1 and M2 polarization states [
61,
62]. Another ligand to CD28 is CD86, which was over-expressed only in M1_C. CD86 is also considered an M1 polarization marker, but its role in T-cell activation remains elusive. Although some studies have shown that CD86 is the dominant ligand for regulation of immune response and proliferation of regulatory T-cells (Treg) [
63,
64], other authors report the role of CD86
+ macrophages in inflammatory response [
65], and favorable prognosis in colorectal cancer and hepatocellular carcinoma possibly due to a higher degree of M1 polarization and the subsequent anti-tumor activities [
66,
67]. It was also reported that the presence of CD86
+ macrophages correlated with the severity of tubulointerstitial inflammation in human glomerulonephritis [
68]. However, it should be noted that M1_B macrophages maybe did not express
CD86 due to a short exposure time. A study by Parise et al. showed that most chemical sensitizers induced the transcription of
CD86 in THP-1 cells only after a 48 h of exposure to the chosen sensitizer [
69]. Therefore, Mφ polarized according to Protocol B could not up-regulate the transcription of
CD86, since they were exposed to cytokine stimuli for only 18 h. The gene for the IL-1 receptor was over-expressed in M1_A and M1_C macrophages when compared with M2 macrophages from their respective polarization protocols while
IL1R1 was over-expressed in M1_B when compared with M0_B but not when compared with M2_B. According to a study by Baxter et al., the rest period between the M0 state and the polarization of M2 macrophages is necessary for the down-regulation of M1 gene expression [
21]. A relatively high concentration of PMA combined with the lack of a rest period in Protocol B may have caused the expression of
IL1R1 to not be down-regulated during the polarization of M2_B; therefore, it was not differentially expressed when comparing M1 and M2 macrophages from Protocol B. Moreover,
IL1R1 was not over-expressed in M1_C when compared with M0_C probably because PMA on its own can be considered an M1 polarization stimulus [
70] and can up-regulate the transcription of M1-associated genes [
21]. Surprisingly, the
MARCO gene was not differentially expressed in many of the comparisons. In addition, the expression of
MARCO was higher in M2_C when compared with M1_C and in M0_B when compared with M1_B. MARCO is a member of the class A scavenger receptor (SR-A) family and is mainly expressed on macrophages [
71]. This receptor is considered a marker of M1 macrophages [
72] mainly because it functions as a major phagocytic receptor mediating the binding and uptake of bacteria [
73] and viruses [
74]. Despite these functions, recent studies have found a positive correlation between the expression of
MARCO and production of anti-inflammatory cytokine IL-37. Similarly, MARCO
+ macrophages enhanced Treg cell proliferation and IL-10 production [
75]. Additionally, Chen et al. reported that MARCO
+ macrophages drive tumor progression in glioblastomas and proposed that MARCO could be used as a mesenchymal pro-tumor marker [
76]. The only enzyme-coding gene in this M1 panel is the
NOS2 gene, which codes the enzyme nitric oxide synthase 2. This enzyme, along with the arginase 1 and arginase 2 enzymes (coded by
ARG1 and
ARG2 genes), represent key molecules in macrophage polarization and are closely linked to the functional phenotype of Mφ [
2]. Depending on the stimuli present in their surrounding micro-environment, Mφ, in the process of polarization, alter their arginine metabolism by which they “decide” to execute the “inhibit” or “heal” function [
12]. In the presence of inflammatory stimuli, Mφ can metabolize arginine into nitric oxide (NO) and citrulline by the means of the up-regulated NOS enzyme. NO production is an important effector for the anti-microbial activity of M1 macrophages [
2,
77]. To execute the “heal” function, Mφ actively metabolize arginine by arginase into ornithine and urea. Macrophage production of ornithine is essential for many repair processes because it serves as a precursor of the polyamines required for cell proliferation. Ornithine also serves as a precursor of collagen which is important for the construction of the extracellular matrix [
78]. Despite the crucial role of NOS2 in M1 macrophages and the dichotomy of NOS/ARG expression being considered the hallmark of macrophage polarization, we have found that
NOS2 was over-expressed only in M1_A. The overall expression of
NOS2 gene in all samples was very low based on the gene expression quantification data (FPKM < 2.2). However, several studies have already shown that this heal/inhibit dichotomy based on the changes in arginase metabolism does not apply equally to human and mouse Mφ [
79]. While mouse Mφ express NOS and produce high amounts of NO as a response to inflammatory stimuli, human macrophages make little to no NO due to the high level of methylation around the
NOS2 transcription start site [
80]. Therefore, the use of the expression and activity of NOS/ARG for the determination of the macrophage function and subtype should be reassessed. It should be noted that differences between Mφ generated by different protocols could arise due to different times of exposure to polarization stimuli. Firstly, it has been proven that exposure time can significantly alter the effect of the stimuli, including promoting or inhibiting the transcription of genes [
81]. Moreover, the inflammatory response includes early response genes, which are transcribed almost immediately after the exposure to the stimulus, and late response genes, which are transcribed later on [
82]. An extensive study of murine macrophages by Raza et al. divided the transcribed genes into three clusters: early, mid, and late responses [
57]. Since each protocol implemented different exposure times and we performed the RNA isolation immediately after removing the polarization stimuli, our data represent the transcriptome profiles of macrophages in different time points. Therefore, differences between gene expression levels of macrophages from the three chosen protocols may not mean that these cells express the analyzed genes in a different way. It may simply mean that the gene expression was analyzed at a different time for each protocol and therefore cannot be compared.
The differential gene expression of M2 macrophages was also analyzed. Most of the genes in both the regulatory gene set and the panel of genes for receptors and enzymes were not differentially expressed in M2 macrophages when comparing with other cells and protocols, which suggests that the expression of these genes was not up-regulated in the M2 macrophages. The causes differ between the chosen polarization protocols. Protocol A uses Vit D
3 for the polarization of M2 macrophages [
22]. Even though this compound is usually considered an M2 macrophage polarization stimulus [
83,
84,
85,
86], there is evidence regarding its effect on the up-regulation of the expression of inflammatory cytokines and antimicrobial peptides [
36], increase in the M1/M2 macrophage ratio [
87], or enhancement of bactericidal activity and superoxide production in macrophages [
88], which are all associated with the M1 phenotype. Moreover, Neme et al. reported that gene ontology assessment of the Vit D
3-stimulated THP-1 monocytes revealed anti-microbial response as their top-ranking early physiological function [
89]. Therefore, the aforementioned studies along with our results suggest that the role of Vit D
3 as an M2 macrophage stimulator should be reassessed. The effect of the stimulation time should also be taken into consideration. A recent study by Unuvar Purcu et al. revealed the effect of the stimulation time on the expression of macrophage markers [
90]. According to their study, M2 macrophages had the highest level of
MRC1 and
CCL22 expression after 48–72 h of stimulation. Since macrophages in Protocol B were stimulated with IL-4 and IL-13 for 18 h, this time was probably not sufficient to up-regulate the expression of
MRC1 and
CCL22, unlike in Protocol C where the M0 cells were stimulated with IL-4 for 48 h. The relatively high concentration of PMA used in Protocol B (61.3 ng/mL) could be another reason for the failed expression of M2-associated genes. A study by Chanput et al. suggests that initial priming with a high concentration of PMA (100 ng/mL) compromises polarization of M2 macrophages [
91]. Baxter et al. also confirmed that priming of THP-1 cells with concentrations of PMA above 50 ng/mL inhibited transcription of M2-associated genes [
21]. Based on these studies, we also incorporated Protocol C in our experiment, which is suggested for the polarization of M2 macrophages by Baxter et al. [
21]. It should be noted that their study analyzed the expression of only a few chosen M2-related genes (
MRC1,
CD200R,
CCL17,
ALOX15, and
TGM2). These genes were also over-expressed in M2_C macrophages in our study (see
Figure 6b and
Figure 7b; data for
CD200R,
ALOX15, and
TGM2 are not shown). However, our transcriptome analysis showed that M2_C cells failed to up-regulate other genes which are associated with the M2 phenotype. Only the genes for chemokines along with
MRC1 and
CLEC7A were significantly over-expressed when comparing with other macrophage subtypes and polarization protocols. Therefore, further optimization of the M2-polarization protocol is needed.
Results from the differential gene expression analysis were supported by the GO enrichment analysis. DEGs of M1_B (when compared with M0_B) were functionally enriched in biological processes which are generally associated with the function of M1 macrophages [
2], e.g., defense response to another organism (GO:0098542), response to bacterium (GO:0009217), adaptive immune response (GO:0002250), response to a virus (GO:0009615), or defense response to a virus (GO:0051607). We also identified functional enrichment for M1-associated processes in genes that were differentially expressed in M1_C when comparing with M0_C, e.g., defense response to another organism (GO:0098542), response to bacterium (GO:0009217), regulation of immune effector process (GO:0002697), and activation of immune response (GO:0002253). However, comparisons between M1_B, M1_A, and M1_C revealed that the M1-associated processes mentioned earlier (see
Table 5 and
Table 6) were over-represented in M1 macrophages polarized according to Protocol B. These results suggest that M1_B could have the strongest antimicrobial, antiviral, and overall defense response out of all tested M1 macrophages.
According to the differential gene expression analysis, neither of the protocols could be considered a reliable model for the polarization of M2 macrophages. Even though M2_C cells expressed some of the genes that are associated with the M2 phenotype (e.g., genes for chemokines:
MRC1 and
CLEC7A) [
2,
5], functionally (see
Table 8) they could not be considered as M2 macrophages. On the other hand, M2_B did not differentially express the genes for M2 regulatory factors, chemokines, or receptors and enzymes, but according to the GO enrichment analysis they could perform some of the biological processes which are traditionally associated with the M2 phenotype. It has been proven that M2 macrophages promote angiogenesis [
92,
93], remodeling of the extracellular matrix, phagocytosis of old and dead cells, and coordination of tissue regeneration (reviewed in [
12,
94,
95]). We identified some of these processes among the GO terms significantly enriched in DEGs of M2_B macrophages: e.g., phagocytosis (GO:0006909), collagen metabolic process (GO:0032963), angiogenesis (GO:0001525), or extracellular matrix organization (GO:0030198). As previously mentioned, Baxter et al. proved that priming THP-1 monocytes with relatively high concentrations of PMA could block the expression of M2-associated genes [
21]. However, the reasons why the M2 macrophages from Protocol B lack the established markers for M2 polarization but show enrichment for biological processes of the M2 macrophages should be further investigated and supported by experiments.
Our study provided new remarkable information regarding the reliability of commonly used macrophage polarization protocols. Despite that, we acknowledge the limitations of our study. First, transcriptomic data are not supported by RT-PCR or proteomic analysis; therefore, conclusions from this study need to be supported by further experiments. Moreover, this research is primarily a hypothesis-generating study, rather than a hypothesis-testing study.