Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery
Abstract
:1. Introduction
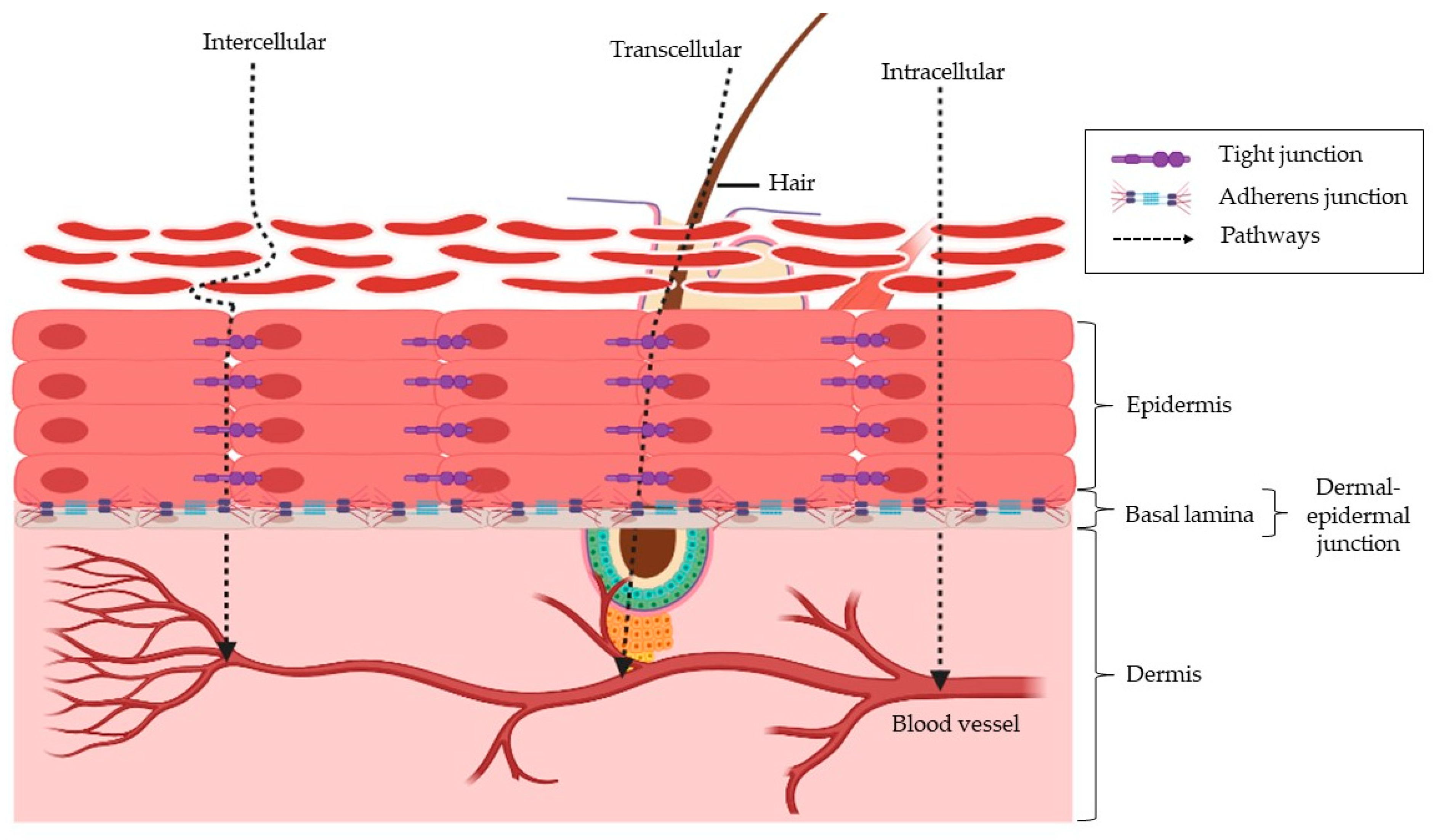
2. The Skin Structure and How This Affects Insulin Penetration
3. Chemical Enhancers
3.1. Transdermal Insulin Delivery
3.1.1. Ionic Liquids
3.1.2. Skin Pre-Treatment
3.1.3. Nanoparticles
3.1.4. Carrier
3.1.5. Peptides
3.1.6. Virtual Design Algorithm for Screening of Chemical Enhancers
3.2. Transdermal Delivery of Other Pharmaceutical Drugs
3.2.1. Dimethyl Sulfoxide
3.2.2. Ionic Liquids
3.2.3. Deep Eutectic Solvents
3.2.4. Essential Oils
3.3. Section Summary
4. Concluding Remark and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemmochi, Y.; Fukui, K.; Maki, M.; Kimura, S.; Ishii, Y.; Sasase, T.; Miyajima, K.; Ohta, T. Metabolic Disorders and Diabetic Complications in Spontaneously Diabetic Torii Lepr (fa) Rat: A New Obese Type 2 Diabetic Model. J. Diabetes Res. 2013, 2013, 948257. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Colagiuri, S.; Chan, J.; Gregg, E.; Ke, C.; Lim, L.-L.; Yang, X. IDF Atlas, 9th ed.; 2019; Available online: https://diabetesatlas.org/atlas/ninth-edition/ (accessed on 26 January 2023).
- Animaw, W.; Syoum, Y. Increasing prevalence of diabetes mellitus in a developing country and its related factors. PLoS ONE 2017, 12, e0187670. [Google Scholar] [CrossRef]
- Grintsova, O.; Maier, W.; Mielck, A. Inequalities in health care among patients with type 2 diabetes by individual socio-economic status (SES) and regional deprivation: A systematic literature review. Int. J. Equity Health 2014, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Cantley, J.; Ashcroft, F. Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Biol. 2015, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Kahanovitz, L.; Sluss, P.M.; Russell, S.J. Type 1 Diabetes—A Clinical Perspective. Point Care 2017, 16, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [Green Version]
- Chiasson, J.-L.; Aris-Jilwan, N.; Bélanger, R.; Bertrand, S.; Beauregard, H.; Ekoé, J.-M.; Fournier, H.; Havrankova, J. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ Can. Med. Assoc. J. 2003, 168, 859–866. [Google Scholar]
- Konstantinov, N.K.; Rohrscheib, M.; Agaba, E.I.; Dorin, R.I.; Murata, G.H.; Tzamaloukas, A.H. Respiratory failure in diabetic ketoacidosis. World J. Diabetes 2015, 6, 1009–1023. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Hirsch, I.B. Type 1 diabetes mellitus and the use of flexible insulin regimens. Am. Fam. Physician 1999, 60, 2343–2352. [Google Scholar]
- Misso, M.L.; Egberts, K.J.; Page, M.; O’Connor, D.; Shaw, J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 20, CD005103. [Google Scholar] [CrossRef]
- Jeitler, K.; Horvath, K.; Berghold, A.; Gratzer, T.W.; Neeser, K.; Pieber, T.R.; Siebenhofer, A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: Systematic review and meta-analysis. Diabetologia 2008, 51, 941–951. [Google Scholar] [CrossRef] [Green Version]
- St Charles, M.; Lynch, P.; Graham, C.; Graham, C.; Minshall, M.E. A cost-effectiveness analysis of continuous subcutaneous insulin injection versus multiple daily injections in type 1 diabetes patients: A third-party US payer perspective. Value Health 2009, 12, 674–686. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, E.; Jahnke, J.; Fischer, A.; Kapitza, C.; Forst, T. Impact of Injection Speed, Volume, and Site on Pain Sensation. J. Diabetes Sci. Technol. 2018, 12, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Cemeroglu, A.P.; Can, A.; Davis, A.T.; Cemeroglu, O.; Kleis, L.; Daniel, M.S.; Bustraan, J.; Koehler, T.J. Fear of needles in children with type 1 diabetes mellitus on multiple daily injections and continuous subcutaneous insulin infusion. Endocr. Pract. 2015, 21, 46–53. [Google Scholar] [CrossRef]
- Aronson, R. The role of comfort and discomfort in insulin therapy. Diabetes Technol. Ther. 2012, 14, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Johansen, N.J.; Christensen, M.B. A Systematic Review on Insulin Overdose Cases: Clinical Course, Complications and Treatment Options. Basic Clin. Pharm. Toxicol. 2018, 122, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Richardson, T.; Kerr, D. Skin-related complications of insulin therapy: Epidemiology and emerging management strategies. Am. J. Clin. Dermatol. 2003, 4, 661–667. [Google Scholar] [CrossRef]
- Mokta, J.K.; Mokta, K.K.; Panda, P. Insulin lipodystrophy and lipohypertrophy. Indian J. Endocrinol. Metab. 2013, 17, 773–774. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Q.; Zhang, L. Transdermal delivery of insulin in mice by using lecithin vesicles as a carrier. Drug Deliv. 2000, 7, 113–116. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef]
- Sintov, A.C.; Wormser, U. Topical iodine facilitates transdermal delivery of insulin. J. Control. Release 2007, 118, 185–188. [Google Scholar] [CrossRef]
- Zou, J.J.; Le, J.Q.; Zhang, B.C.; Yang, M.Y.; Jiang, J.L.; Lin, J.F.; Wu, P.Y.; Li, C.; Chen, L.; Shao, J.W. Accelerating transdermal delivery of insulin by ginsenoside nanoparticles with unique permeability. Int. J. Pharm. 2021, 605, 120784. [Google Scholar] [CrossRef]
- Tahara, Y.; Honda, S.; Kamiya, N.; Piao, H.; Hirata, A.; Hayakawa, E.; Fujii, T.; Goto, M. A solid-in-oil nanodispersion for transcutaneous protein delivery. J. Control. Release 2008, 131, 14–18. [Google Scholar] [CrossRef]
- Sugumar, V.; Ang, K.P.; Alshanon, A.F.; Sethi, G.; Yong, P.V.C.; Looi, C.Y.; Wong, W.A.-O. A Comprehensive Review of the Evolution of Insulin Development and Its Delivery Method. Pharmaceutics 2022, 14, 1406. [Google Scholar] [CrossRef]
- Jhawat, V.; Saini, V.; Kamboj, S.; Maggon, N. Transdermal Drug Delivery Systems: Approaches and Advancements in Drug Absorption through Skin. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 47–56. [Google Scholar]
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira, J.M.; Tubino, M.; Vila, M.M.D.C.; et al. Non-invasive Transdermal Delivery of Human Insulin Using Ionic Liquids: In vitro Studies. Front. Pharmacol. 2020, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2005, 58, 68–89. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product Against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Li, S.K. Effects of chemical enhancers on human epidermal membrane: Structure-enhancement relationship based on maximum enhancement (E(max)). J. Pharm. Sci. 2009, 98, 926–944. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.; Roberts, M. Dermatological and Transdermal Formulations; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–39. [Google Scholar]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Kydonieus, A.F.; Berner, B. Transdermal Delivery of Drugs; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Kirschner, N.; Houdek, P.; Fromm, M.; Moll, I.; Brandner, J.M. Tight junctions form a barrier in human epidermis. Eur. J. Cell Biol. 2010, 89, 839–842. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of Chemical Permeation Enhancers on Skin Permeability: In silico screening using Molecular Dynamics simulations. Sci. Rep. 2019, 9, 1456. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, N.; Atkinson, J.P.; Maibach, H.I. Chemical Penetration Enhancers: Classification and Mode of Action. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–27. [Google Scholar]
- Lopes, L.B.; Garcia, M.T.; Bentley, M.V. Chemical penetration enhancers. Ther. Deliv. 2015, 6, 1053–1061. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.; Silva, S.G.; Pinheiro, M.; Reis, S.; Vale, M.L. Current Status of Amino Acid-Based Permeation Enhancers in Transdermal Drug Delivery. Membranes 2021, 11, 343. [Google Scholar] [CrossRef]
- Moghadam, S.H.; Saliaj, E.; Wettig, S.D.; Dong, C.; Ivanova, M.V.; Huzil, J.T.; Foldvari, M. Effect of Chemical Permeation Enhancers on Stratum Corneum Barrier Lipid Organizational Structure and Interferon Alpha Permeability. Mol. Pharm. 2013, 10, 2248–2260. [Google Scholar] [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Agostinho, D.A.S.; Santos, F.; Esperança, J.M.S.S.; Duarte, A.R.C.; Reis, P.M. New non-toxic biocompatible dianionic ionic liquids that enhance the solubility of oral drugs from BCS class II. J. Ion. Liq. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [Green Version]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity-Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Jesus, A.R.; Raposo, L.R.; Soromenho, M.R.C.; Agostinho, D.A.S.; Esperança, J.M.S.S.; Baptista, P.V.; Fernandes, A.R.; Reis, P.M. New Non-Toxic N-alkyl Cholinium-Based Ionic Liquids as Excipients to Improve the Solubility of Poorly Water-Soluble Drugs. Symmetry 2021, 13, 2053. [Google Scholar] [CrossRef]
- Jesus, A.R.; Soromenho, M.R.C.; Raposo, L.R.; Esperança, J.; Baptista, P.V.; Fernandes, A.R.; Reis, P.M. Enhancement of water solubility of poorly water-soluble drugs by new biocompatible N-acetyl amino acid N-alkyl cholinium-based ionic liquids. Eur. J. Pharm. Biopharm. 2019, 137, 227–232. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal Protein Delivery Using Choline and Geranate (CAGE) Deep Eutectic Solvent. Adv. Healthc. Mater. 2017, 6, 1601411. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Uddin, S.; Chowdhury, M.R.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Insulin Transdermal Delivery System for Diabetes Treatment Using a Biocompatible Ionic Liquid-Based Microemulsion. ACS Appl. Mater. Interfaces 2021, 13, 42461–42472. [Google Scholar] [CrossRef]
- Guncheva, M.; Ossowicz, P.; Janus, E.; Todinova, S.; Yancheva, D. Elucidation of the effect of some cholinium amino acid ionic liquids on the thermal and the conformational stability of insulin. J. Mol. Liq. 2019, 283, 257–262. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Quan, Y.S.; Zang, L.; Jin, M.N.; Kamiyama, F.; Katsumi, H.; Yamamoto, A.; Tsutsumi, S. Transdermal Delivery of Insulin Using Trypsin as a Biochemical Enhancer. Biol. Pharm. Bull. 2008, 31, 1574–1579. [Google Scholar] [CrossRef] [Green Version]
- Choe, C.; Schleusener, J.; Lademann, J.; Darvin, M.E. Keratin-water-NMF interaction as a three layer model in the human stratum corneum using in vivo confocal Raman microscopy. Sci. Rep. 2017, 7, 15900. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Quan, Y.S.; Zang, L.; Jin, M.N.; Kamiyama, F.; Katsumi, H.; Tsutsumi, S.; Yamamoto, A. Trypsin as a novel potential absorption enhancer for improving the transdermal delivery of macromolecules. J. Pharm. Pharmacol. 2009, 8, 1005–1012. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Sankaranarayanan, R.; Kumar, J.P.S. 2—Nanoparticles and medicine. In Nanomedicine Manufacturing and Applications; Verpoort, F., Ahmad, I., Ahmad, A., Khan, A., Chee, C.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–37. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Zhong, Z. 1.3.8B—Nanoparticles. In Biomaterials Science, 4th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 453–483. [Google Scholar]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Development and evaluation of microemulsions for transdermal delivery of insulin. Pharmaceutics 2011, 2011, 780150. [Google Scholar] [CrossRef] [Green Version]
- Higaki, M.; Kameyama, M.; Udagawa, M.; Ueno, Y.; Yamaguchi, Y.; Igarashi, R.; Ishihara, T.; Mizushima, Y. Transdermal Delivery of CaCO3-Nanoparticles Containing Insulin. Diabetes Technol. Ther. 2006, 8, 369–374. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Zhao, X.; Shan, C.; Zu, S.; Wang, K.; Li, Y.; Ge, Y. Preparation and characterization of chitosan-polyvinyl alcohol blend hydrogels for the controlled release of nano-insulin. Macromolecules 2012, 50, 82–87. [Google Scholar] [CrossRef]
- King, M.; Badea, I.; Solomon, J.; Kumar, P.; Gaspar, K.; Foldvari, M. Transdermal Delivery of Insulin from a Novel Biphasic Lipid System in Diabetic Rats. Diabetes Technol. Ther. 2002, 4, 479–488. [Google Scholar] [CrossRef]
- King, M.J.; Michel, D.; Foldvari, M. Evidence for lymphatic transport of insulin by topically applied biphasic vesicles. J. Pharm. Pharmacol. 2003, 55, 1339–1344. [Google Scholar] [CrossRef]
- Nose, K.; Pissuwan, D.; Goto, M.; Katayama, Y.; Niidome, T. Gold nanorods in an oil-base formulation for transdermal treatment of type 1 diabetes in mice. Nanoscale 2012, 4, 3776–3780. [Google Scholar] [CrossRef]
- Sadhasivam, L.; Dey, N.; Francis, A.P.; Devasena, T. Trandermal patches of chitosan nanoparticles for insulin delivery. Int. J. Pharm. Pharm. Sci. 2015, 7, 84–88. [Google Scholar]
- Zhao, X.; Zu, Y.; Zu, S.; Wang, D.; Zhang, Y.; Zu, B. Insulin nanoparticles for transdermal delivery: Preparation and physicochemical characterization and in vitro evaluation. Drug Dev. Ind. Pharm. 2010, 36, 1177–1185. [Google Scholar] [CrossRef]
- Chroni, A.; Forys, A.; Sentoukas, T.; Trzebicka, B.; Pispas, S. Poly[(vinyl benzyl trimethylammonium chloride)]-based nanoparticulate copolymer structures encapsulating insulin. Eur. Polym. J. 2022, 169, 111158. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012, 20, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Marwah, H.; Garg, T.; Rath, G.; Goyal, A.K. Development of transferosomal gel for trans-dermal delivery of insulin using iodine complex. Drug Deliv. 2016, 23, 1636–1644. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xin, P.; Ou, Q.; Hollett, G.; Gu, Z.; Wu, J. Poly(ester amide)-based hybrid hydrogels for efficient transdermal insulin delivery. Mater. Chem. 2018, 6, 6723–6730. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.; Guo, X.; Zhang, C.; Yang, W.; Ma, M.; Liu, S.; Zhang, M.; Wen, L.P. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat. Biotechnol. 2006, 24, 455–460. [Google Scholar] [CrossRef]
- Chang, M.; Li, X.; Sun, Y.; Cheng, F.; Wang, Q.; Xie, X.; Zhao, W.; Tian, X. Effect of cationic cyclopeptides on transdermal and transmembrane delivery of insulin. Mol. Pharm. 2013, 10, 951–957. [Google Scholar] [CrossRef]
- Golla, S.; Neely, B.J.; Whitebay, E.; Madihally, S.; Robinson, R.L., Jr.; Gasem, K.A. Virtual design of chemical penetration enhancers for transdermal drug delivery. Chem. Biol. Drug Des. 2012, 4, 478. [Google Scholar] [CrossRef] [Green Version]
- Yerramsetty, K.M.; Rachakonda, V.K.; Neely, B.J.; Madihally, S.V.; Gasem, K.A. Effect of different enhancers on the transdermal permeation of insulin analog. Int. J. Pharm. 2010, 398, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Kahler, C.P. Evaluation of the use of the solvent dimethyl sulfoxide in chemiluminescent studies. Blood Cells Mol. Dis. 2000, 26, 626–633. [Google Scholar] [CrossRef]
- Capriotti, K.; Capriotti, J.A. Dimethyl sulfoxide: History, chemistry, and clinical utility in dermatology. J. Clin. Aesthet. Dermatol. 2012, 5, 24–26. [Google Scholar]
- Notman, R.; den Otter, W.K.; Noro, M.G.; Briels, W.J.; Anwar, J. The permeability enhancing mechanism of DMSO in ceramide bilayers simulated by molecular dynamics. Biophys. J. 2007, 93, 2056–2068. [Google Scholar] [CrossRef] [Green Version]
- Masrijal, C.; Harmita, H.; Iskandarsyah, I. Improving transdermal drug delivery system for medroxyprogesterone acetate by olive oil and dimethylsulfoxide (dmso) as penetration enhancers: In vitro penetration study. Int. J. Pharm. Pharm. Sci. 2020, 12, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Al-Saidan, S.M.; Selkirk, A.B.; Winfield, A.J. Effect of dimethylsulfoxide concentration on the permeability of neonatal rat stratum corneum to alkanols. J. Investig. Dermatol. 1987, 89, 426–429. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Shabbir, M.; Farooq, M.; Adnan, S.; Nabeel Shahid, M. The enhancement effect of permeation enhancers on Bisoprolol fumarate across animal membrane using Franz diffusion cell. Res. J. Pharm. Technol. 2014, 7, 1391–1395. [Google Scholar] [CrossRef]
- Fuller, P.; Roth, S. Diclofenac sodium topical solution with dimethyl sulfoxide, a viable alternative to oral nonsteroidal anti-inflammatories in osteoarthritis: Review of current evidence. J. Multidiscip. Healthc. 2011, 4, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Nayakal, O.; Patil, P. Evaluation of dimethylsulfoxide and Aloe vera as penetration enhancers for cutaneous application of lidocaine. Ars Pharm. 2019, 60, 85–92. [Google Scholar] [CrossRef]
- Otterbach, A.; Lamprecht, A. Enhanced skin permeation of estradiol by dimethyl sulfoxide containing transdermal patches. Pharmaceutics 2021, 13, 320. [Google Scholar] [CrossRef]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Dobler, D.; Schmidts, T.; Klingenhöfer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef]
- Sadaf, A.; Sinha, R.; Ekka, M.K. Ionic liquid-mediated skin technologies: Recent advances and prospects. Curr. Res. Biotechnol. 2022, 4, 514–529. [Google Scholar] [CrossRef]
- Qi, Q.M.; Mitragotri, S. Mechanistic study of transdermal delivery of macromolecules assisted by ionic liquids. J. Control. Release 2019, 311–312, 162–169. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Faulds, D.; Goa, K.L. Aciclovir. Drugs 1994, 47, 153–205. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Z.; Yang, Y.; Li, Y.; Wang, C. Novel skin permeation enhancers based on amino acid ester ionic liquid: Design and permeation mechanism. Int. J. Pharm. 2020, 576, 119031. [Google Scholar] [CrossRef]
- Janůšová, B.; Skolová, B.; Tükörová, K.; Wojnarová, L.; Simůnek, T.; Mladěnka, P.; Filipský, T.; Ríha, M.; Roh, J.; Palát, K.; et al. Amino acid derivatives as transdermal permeation enhancers. J. Control. Release 2013, 165, 91–100. [Google Scholar] [CrossRef]
- Vávrová, K.; Hrabálek, A. Amino Acid-Based Transdermal Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 325–336. [Google Scholar]
- Md Moshikur, R.; Chowdhury, M.R.; Fujisawa, H.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Design and Characterization of Fatty Acid-Based Amino Acid Ester as a New “Green” Hydrophobic Ionic Liquid for Drug Delivery. ACS Sustain. Chem. Eng. 2020, 8, 13660–13671. [Google Scholar] [CrossRef]
- Warner, K.S.; Li, S.K.; Higuchi, W.I. Influences of alkyl group chain length and polar head group on chemical skin permeation enhancement. J. Pharm. Sci. 2001, 90, 1143–1153. [Google Scholar] [CrossRef]
- Warner, K.S.; Li, S.K.; He, N.; Suhonen, T.M.; Chantasart, D.; Bolikal, D.; Higuchi, W.I. Structure–Activity Relationship for Chemical Skin Permeation Enhancers: Probing the Chemical Microenvironment of the Site of Action. J. Pharm. Sci. 2003, 92, 1305–1322. [Google Scholar] [CrossRef]
- Vávrová, K.; Hrabálek, A.; Doležal, P.; Šámalová, L.; Palát, K.; Zbytovská, J.; Holas, T.; Klimentová, J. Synthetic ceramide analogues as skin permeation enhancers: Structure–Activity relationships. Bioorg. Med. Chem. 2003, 11, 5381–5390. [Google Scholar] [CrossRef]
- Vávrová, K.; Hrabálek, A.; Dolezal, P.; Holas, T.; Zbytovská, J. L-Serine and glycine based ceramide analogues as transdermal permeation enhancers: Polar head size and hydrogen bonding. Bioorg. Med. Chem. Lett. 2003, 13, 2351–2353. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.; Santos, F.; Duarte, A.R.C. Therapeutic Deep Eutectic Systems towards the Treatment of Tuberculosis and Colorectal Cancer: Opportunities and Challenges. Molecules 2021, 26, 7022. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Mohammed Ali, H.H.; Ghareeb, M.M.; Al-Remawi, M. Therapeutic deep eutectic system of capric acid and menthol: Characterization and pharmaceutical application. J. Drug Deliv. Sci. Technol. 2019, 53, 101159. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Kasting, G.B. Lipid solubility and molecular weight: Whose idea was that. Skin Pharmacol. Physiol. 2013, 26, 295–301. [Google Scholar] [CrossRef]
- Kang, L.; Jun, H.W.; McCall, J.W. Physicochemical studies of lidocaine–menthol binary systems for enhanced membrane transport. Int. J. Pharm. 2000, 206, 35–42. [Google Scholar] [CrossRef]
- Kaplun-Frischoff, Y.; Touitou, E. Testosterone Skin Permeation Enhancement by Menthol through Formation of Eutectic with Drug and Interaction with Skin Lipids. J. Pharm. Sci. 1997, 86, 1394–1399. [Google Scholar] [CrossRef]
- Liu, C.; Qu, X.; Song, L.; Shang, R.; Wan, X.; Fang, L. Investigation on the effect of deep eutectic formation on drug-polymer miscibility and skin permeability of rotigotine drug-in-adhesive patch. Int. J. Pharm. 2020, 574, 118852. [Google Scholar] [CrossRef]
- Mokhtarpour, M.; Shekaari, H.; Shayanfar, A. Design and characterization of ascorbic acid based therapeutic deep eutectic solvent as a new ion-gel for delivery of sunitinib malate. J. Drug Deliv. Sci. Technol. 2020, 56, 101512. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2003, 93, 377–387. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zheng, L.; Yang, Y.; Cui, X.; Xu, T.; Zhang, W.; Wang, C. Noninvasive transdermal delivery of mesoporous silica nanoparticles using deep eutectic solvent. J. Control. Release 2022, 343, 43–56. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Jiang, W.; Zhu, C.; Yao, S. Transdermal release behaviors of bioactive deep eutectic solvents as natural skin care and mechanism. J. Mol. Liq. 2022, 367, 120412. [Google Scholar] [CrossRef]
- Qu, W.; Qader, I.B.; Abbott, A.P. Controlled release of pharmaceutical agents using eutectic modified gelatin. Drug Deliv. Transl. Res. 2022, 12, 1187–1194. [Google Scholar] [CrossRef]
- Araki, Y.; Hamada, Y.; Imamura, N.; Yamasaka, K.; Sakuragi, M. Evaluation of terpene-based hydrophobic deep eutectic solvents as skin permeation enhancers. Jpn. J. Appl. Phys. 2023. [Google Scholar] [CrossRef]
- Hayyan, M. Versatile applications of deep eutectic solvents in drug discovery and drug delivery systems: Perspectives and opportunities. Asian J. Pharm. Sci. 2023, 100780. [Google Scholar] [CrossRef]
- Caliskan, U.K.; Karakus, M.M. Essential oils as skin permeation boosters and their predicted effect mechanisms. J. Dermatol. Skin Sci. 2020, 2, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Farooqui, H. A Review on Role of Essential Oil as Penetration Enhancer in Transdermal Drug Delivery System. Syst. Rev. Pharm. 2021, 12, 439–444. [Google Scholar]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Khan, N.R.; Khan, G.M.; Wahab, A.; Khan, A.R.; Hussain, A.; Nawaz, A.; Akhlaq, M. Formulation, and physical, in vitro and ex vivo evaluation of transdermal ibuprofen hydrogels containing turpentine oil as penetration enhancer. Die Pharm.-An Int. J. Pharm. Sci. 2011, 66, 849–852. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112–116. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Essential oils as novel human skin penetration enhancers. Int. J. Pharm. 1989, 57, R7–R9. [Google Scholar] [CrossRef]
- Wang, L.-H.; Chen, J.-X. Study of p-aminobenzoic acid and its metabolites in human volunteers treated with essential oil formulations using attenuated total reflection-Fourier transform infrared spectroscopy and HPLC with fluorometric detection. Microchim. Acta 2010, 168, 93–98. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.; Kohli, K.; Pillai, K.; Rahman, Z. Improvement in bioavailability of transdermally applied flurbiprofen using tulsi (Ocimum sanctum) and turpentine oil. Colloids Surf. B Biointerfaces 2008, 65, 300–307. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Hwang, T.-L.; Cheng, H.-C. Essential oils from sweet basil (Ocimum basilicum) as novel enhancers to accelerate transdermal drug delivery. Biol. Pharm. Bull. 2004, 27, 1819–1825. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Aqil, M.; Ahad, A.; Ali, A.; Khar, R.K. Basil oil is a promising skin penetration enhancer for transdermal delivery of labetolol hydrochloride. Drug Dev. Ind. Pharm. 2008, 34, 384–389. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Hwang, T.-L.; Cheng, H.-C.; Hung, C.-F. Development of sesquiterpenes from Alpinia oxyphylla as novel skin permeation enhancers. Eur. J. Pharm. Sci. 2003, 19, 253–262. [Google Scholar] [CrossRef]
- Jain, A.K.; Thomas, N.S.; Panchagnula, R. Transdermal drug delivery of imipramine hydrochloride. I. Effect of terpenes. J. Control. Release 2002, 79, 93–101. [Google Scholar] [CrossRef]
- Fang, J.Y.; Tsai, T.H.; Hung, C.F.; Wong, W.W. Development and evaluation of the essential oil from Magnolia fargesii for enhancing the transdermal absorption of theophylline and cianidanol. J. Pharm. Pharmacol. 2004, 56, 1493–1500. [Google Scholar] [CrossRef]
- Vashisth, I.; Ahad, A.; Aqil, M.; Agarwal, S.P. Investigating the potential of essential oils as penetration enhancer for transdermal losartan delivery: Effectiveness and mechanism of action. Asian J. Pharm. Sci. 2014, 9, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.F.C.; Liu, X.Y.; Kang, L.; Ho, P.C.L.; Chan, Y.W.; Chan, S.Y. Limonene GP1/PG organogel as a vehicle in transdermal delivery of haloperidol. Int. J. Pharm. 2006, 311, 157–164. [Google Scholar] [CrossRef]
- Rhee, Y.S.; Choi, J.G.; Park, E.S.; Chi, S.C. Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.-P.; Ruan, J.W. Transdermal drug delivery system of aceclofenac for rheumatoid arthritis and the effect of permeation enhancers: In vitro and in vivo characterization. Int. J. Pharmacol. 2015, 11, 456. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-H.; Chang, F.-Y.; Hung, D.-K. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf. B Biointerfaces 2011, 82, 63–70. [Google Scholar] [CrossRef]
- Amnuaikit, C.; Ikeuchi, I.; Ogawara, K.-i.; Higaki, K.; Kimura, T. Skin permeation of propranolol from polymeric film containing terpene enhancers for transdermal use. Int. J. Pharm. 2005, 289, 167–178. [Google Scholar] [CrossRef]
- Ali, F.R.; Shoaib, M.H.; Ali, S.A.; Yousuf, R.I.; Siddiqui, F.; Raja, R.; Jamal, H.S.; Saleem, M.T.; Ahmed, K.; Imtiaz, M.S.; et al. A nanoemulsion based transdermal delivery of insulin: Formulation development, optimization, in-vitro permeation across Strat-M® membrane and its pharmacokinetic/pharmacodynamic evaluation. J. Drug Deliv. Sci. Technol. 2022, 71, 103338. [Google Scholar] [CrossRef]
- Alyoussef Alkrad, J.; Neubert, R.H.H. Dermal and transdermal peptide delivery using enhancer molecules and colloidal carrier systems. Part V: Transdermal administration of insulin. Int. J. Pharm. 2022, 616, 121511. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, F.; Dun, J.; Qi, X.; Cao, D. Lecithin/isopropyl myristate reverse micelles as transdermal insulin carriers: Experimental evaluation and molecular dynamics simulation. J. Drug Deliv. Sci. Technol. 2020, 59, 101891. [Google Scholar] [CrossRef]
- Boscariol, R.; Oliveira Junior, J.M.; Baldo, D.A.; Balcão, V.M.; Vila, M.M.D.C. Transdermal permeation of curcumin promoted by choline geranate ionic liquid: Potential for the treatment of skin diseases. Saudi Pharm. J. 2022, 30, 382–397. [Google Scholar] [CrossRef]





| Routes of Administration | Oral | * IV | Nasal | Transdermal |
|---|---|---|---|---|
| Avoid first-pass effect | No | Yes | Yes | Yes |
| Constant drug level | No | Yes | No | Yes |
| Self-administration | Yes | No | Yes | Yes |
| Unrestricted activity | Yes | No | Yes | Yes |
| Better patient compliance | Yes | No | Yes | Yes |
| Absorption issue | Yes | No | Yes | No |
| Chemical Penetration Enhancers | Pharmaceutical Drugs | Transdermal Permeation Model | Mechanism of Action | References | |
|---|---|---|---|---|---|
| ILs | Choline bicarbonate and geranic acid (CAGE)- ratio 1:2 | Insulin | Ex vivo (Porcine skin) In vivo (Diabetic male Wistar rats) | Extracting lipids from the stratum corneum | [56] |
| (CAGE)- ratio 1:2, 1:4 | Insulin | Ex vivo (Porcine skin) | Extracting lipids from the stratum corneum | [22] | |
| Microemulsion of choline-fatty acids [Chl][FAs] | Insulin | Ex vivo (Yucatan micro-pig skin) In vivo (Diabetic mice) | Activating the fluidizing effect on the lipid bilayer, altering the lamellar structure of the SC lipid | [57] | |
| Skin pre-treatment chemicals | Iodine (povidone-iodine ointment) | Insulin | In vivo (Diabetic Sprague–Dawley rats) | Inactivate thiols on the skin which would significantly increase the bioavailability of active insulin | [23] |
| Trypsin | Insulin | Ex vivo (Male Wistar rat skin) In vivo (Diabetic male Wistar rats) | Alter the stratum corneum structure from alpha- to beta-form and decreases the electrical resistance of the skin | [60] | |
| NPs | Biphasic insulin vesicles patches | Insulin | In vivo (Diabetic Sprague–Dawley rats) | - | [70] |
| Solid-in-oil Gold nanorods | Insulin | Ex vivo (Sea:ddY male mice skin) In vivo (diabetic Sea:ddY male mice) | Gold nanorods absorb irradiation from near-infrared light converting light energy to heat energy which eventually breaks the stratum corneum layer | [72] | |
| Solid-in-oil nanodispersion containing oligo-arginine peptides | Insulin | Ex vivo (Yucatan micro-pig skin) | Disruption to the stratum corneum layer | [25] | |
| Calcium carbonate (CaCO3) | Insulin | In vivo (Normal ddY mice, diabetic dB/dB, and kkAY mice) | - | [68] | |
| Water-in-oil nanoemulsion containing oleic acid | Insulin | Strat-M®-surrogate to human skin | - | [143] | |
| Carriers | Flexible lecithin vesicles with sodium cholate | Insulin | In vivo (Normal Kunming mice) | Sodium cholate could alter properties of lipid bilayer (lecithin alky chain) to increase fluidization of and flexibility of vesicle | [21] |
| Transferosome gel | Insulin | Ex vivo (Goat skin) In vivo (Diabetic Wistar rat) | Membrane fluidization and stratum corneum alteration | [77,78] | |
| Arginine-based unsaturated poly (ester amides) (Arg-PEA) based hydrogel | Insulin | In vivo (Diabetic ICR mice) | - | [79] | |
| Ginsenosides based nano-formulation | Insulin | Ex vivo (Sprague Dawley rat skin) | Ginsenosides are natural triterpenoid saponin compounds that could essentially create transient pores in the membrane via interactions with components like phospholipids and steroids, which could promote skin penetration, disruption of intracellular lipid barriers of the stratum corneum | [24] | |
| DMSO | colloidal carrier systems consisting of DMSO | Ex vivo (Male Wistar rat skin) In vivo (Male Wistar rat skin | - | [144] | |
| Lecithin/isopropyl myristate reverse micelles | Ex vivo (Hairless mice) In vivo (Diabetic New Zealand white rabbits) | - | [145] | ||
| 14C labeled propan-l-ol and hexan-l-ol | Ex vivo (Rat skin) | Hydrogen bond mediated transdermal permeation | [88] | ||
| bisoprolol fumarate | Ex vivo (Rabbit skin) | - | [89] | ||
| Diclofenac sodium solution | Human skin | - | [90] | ||
| Lidocaine | Ex vivo (dialysis membrane) | - | [91] | ||
| Esterdiol | Ex vivo (Porcine ear skin) | - | [92] | ||
| Idoxuridin | Clinical trial (human skin) | [93] | |||
| ILs | Hydrophobic and hydrophilic ILs | [HMIM] [Cl], [BMIM] [PF6] | Ex vivo (Porcine ear skin) | - | [94] |
| CAGE—ratio 1:2 | Dextran | Ex vivo (female Yorkshire pigs) | Lipid extraction | [96] | |
| IL- dimethylimidazolium dimethylphosphate [C1mim][(MeO)2PO2] | Acyclovir | Ex vivo (Yucatan micropig skin) | - | [98] | |
| Amino-acid ester-based IL (AAE)Cl | Hydrocortisone, 5 -Fluorouracil | Ex vivo (Kunming mice) In vivo (Wistar rats) | Interacting with the intercellular lipid domain by lipid fluidization and lipid extraction | [99,100,101] | |
| fatty acid-based amino acid ILs (FAAAE-IL) | Ibuprofen, peptide | Ex vivo (Yucatan micropig skin) | Enhancing penetration of drugs across the skin via the fluidizing lipid of the stratum corneum | [102] | |
| CAGE- ratio 1:2 | Curcumin | Ex vivo (Porcine ear skin) | Increase in solubilization of curcumin by the ILs and by momentary changes in the corneal extract. | [146] | |
| DESs | Terpenes (l-Menthol, LD-Menthol, Thymolm 1,8-Cineole) | Ibuprofen | Ex vivo (Human epidermal membranes) | - | [112] |
| Lidocaine-L-Menthol | Lidocaine | Ex vivo (Snake skin) | - | [114] | |
| Menthol-testosterone | Testosterone | Ex vivo (Silastic membrane and nude mouse Skin) | Alteration of skin lipids. Interaction between menthol and testosterone where it increases the solubility of drug, allowing the increase in skin permeation. | [115] | |
| Rotigotine-Lauric acid | Rotigotine | Ex vivo (Male Wistar rat skin) | Modification of physiochemical properties of drugs. | [116] | |
| Cannabidiol-phosphatidylcholine ethosomes | Cannabidiol | Ex vivo (CDI nude mice) | Enhanced fluidity of ethosomes through enhancer-membrane and drug-enhancer interactions. | [118] | |
| Citric acid: Lysine 1:1 | Mesoporous silica NPs | Ex vivo (Porcine ear skin, hairless mice skin, biomimetic membrane, rat skin) In vivo (Female hairless mice) | ‘Erosion’ effect of stratum corneum whereby the tight structure of the stratum corneum is altered, allowing fluidization of membrane, hence enabling the permeation of MSNs deep into the skin | [119] | |
| Phytic acid-betaine (3:1) | Betaine | Ex vivo (polymethylsiloxane, mice skin) In vivo (Healthy human skin) | - | [120] | |
| catechol: ChCl (1:1), imipramine HCl: glycerol (1:2), and ascorbic acid: ChCl (1:2)) | - | Ex vivo (Porcine loin cut, saline-soaked bovine hides) | - | [121] | |
| Essential oil | Turpentine oil, tulsi oil (Ocimum sanctum) | Flurbiprofen | In vivo (Albino rats) | Turpentine oil exhibited the disruption of normal stratification of the stratum corneum. Tulsi oil, there was an extensive disruption of the stratum corneum with condensation of the normal stratified corneal layers with an increase in epidermal thickness | [131] |
| Sweet basil (Ocimum basilicum) | Indomethacin | Ex vivo (Wistar rat skin) | Effective partitioning of OB essential oil between the stratum corneum, which in turn reduces the polarity of the stratum corneum, facilitating the permeation of lipophilic indomethacin into the skin | [132] | |
| Basil oil | Labetalol hydrochloride | Ex vivo (Wistar rat skin) | Interacting with the intracellular lipid of the stratum corneum | [133] | |
| Alpinia oxyphylla oil | Indomethacin | Ex vivo (Wistar rat skin) In vivo (Wistar rat skin) | Reduces the polarity of the stratum corneum allowing the flux of lipophilic indomethacin across the skin. | [134] | |
| Magnolia fargesii | Theophylline and cianidanol | Ex vivo (Wistar rat skin), In vivo (Wistar rat skin) | skin-vehicle partitioning by Magnolia fargesii | [136] | |
| Aloe vera oil | losartan potassium | Ex vivo (Wistar rat skin), | Disruption of hydrogen bonding of drug with intracellular lipid. Low activation energy of losartan potassium with the use of aloe vera oil suggests a new polar pathway where aloe vera oil interacts with the polar head group region of stratum corneum lipid bilayer | [137] | |
| Limonene oil microemulsion | Curcumin | Ex vivo (Pig ear skin) | - | [141] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugumar, V.; Hayyan, M.; Madhavan, P.; Wong, W.F.; Looi, C.Y. Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery. Biomedicines 2023, 11, 664. https://doi.org/10.3390/biomedicines11030664
Sugumar V, Hayyan M, Madhavan P, Wong WF, Looi CY. Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery. Biomedicines. 2023; 11(3):664. https://doi.org/10.3390/biomedicines11030664
Chicago/Turabian StyleSugumar, Vaisnevee, Maan Hayyan, Priya Madhavan, Won Fen Wong, and Chung Yeng Looi. 2023. "Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery" Biomedicines 11, no. 3: 664. https://doi.org/10.3390/biomedicines11030664
APA StyleSugumar, V., Hayyan, M., Madhavan, P., Wong, W. F., & Looi, C. Y. (2023). Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery. Biomedicines, 11(3), 664. https://doi.org/10.3390/biomedicines11030664








