Glucose Homeostasis, Diabetes Mellitus, and Gender-Affirming Treatment
Abstract
:1. Background
1.1. Physiology of Insulin
1.2. Blood Glucose Regulation and Insulin Resistance
1.3. Diabetes Mellitus
2. Caring for Transgender People
2.1. Sex, Gender Identity, and Transgender People
2.2. Medical Treatment for Gender Transition
3. Sex-Specific Differences in Glucose Metabolism
4. The Impact of Gender-Affirming Treatment on Glycemia
4.1. Glycemia in Transgender Males
| Study | Method | N | Duration | Insulin Resistance | |||
|---|---|---|---|---|---|---|---|
| Fasting Glucose | Fasting Insulin | HOMA-IR | Hyperinsulinemic Euglycemic Clamp | ||||
| Shadid et al., 2020 | Prospective (crossover control) | 35 | 1 year | ↔ | ↓ | ↓ | |
| Auer et al., 2018 | Prospective (crossover control) | 24 | 1 year | ↔ | ↓ | ↓ | |
| Bretherton et al., 2021 | Prospective (compared with cisgender women) | 43 | 1 year | ↔ | |||
| Elbers et al., 2003 | Prospective (crossover control) | 17 | 1 year | ↓ | ↔ | ↔ | |
| Polderman et al., 1994 | Prospective (crossover control) | 13 | 4 months | ↑ | |||
4.2. Glycemia in Transgender Females
| Study | Method | N | Duration | Insulin Resistance | |||
|---|---|---|---|---|---|---|---|
| Fasting Glucose | Fasting Insulin | HOMA-IR | Hyperinsulinemic euglycemic clamp | ||||
| Shadid et al., 2020 | Prospective (crossover control) | 55 | 1 year | ↔ | ↑ | ↑ | |
| Auer et al., 2018 | Prospective (crossover control) | 45 | 1 year | ↔ | ↑ | ↑ | |
| Bretherton et al., 2021 | Prospective (compared with cisgender men) | 41 | 1 year | ↑ | |||
| Elbers et al., 2003 | Prospective (crossover control) | 20 | 1 year | ↔ | ↑ | ↑ | |
| Polderman et al., 1994 | Prospective (crossover control) | 18 | 4 months | ↑ | |||
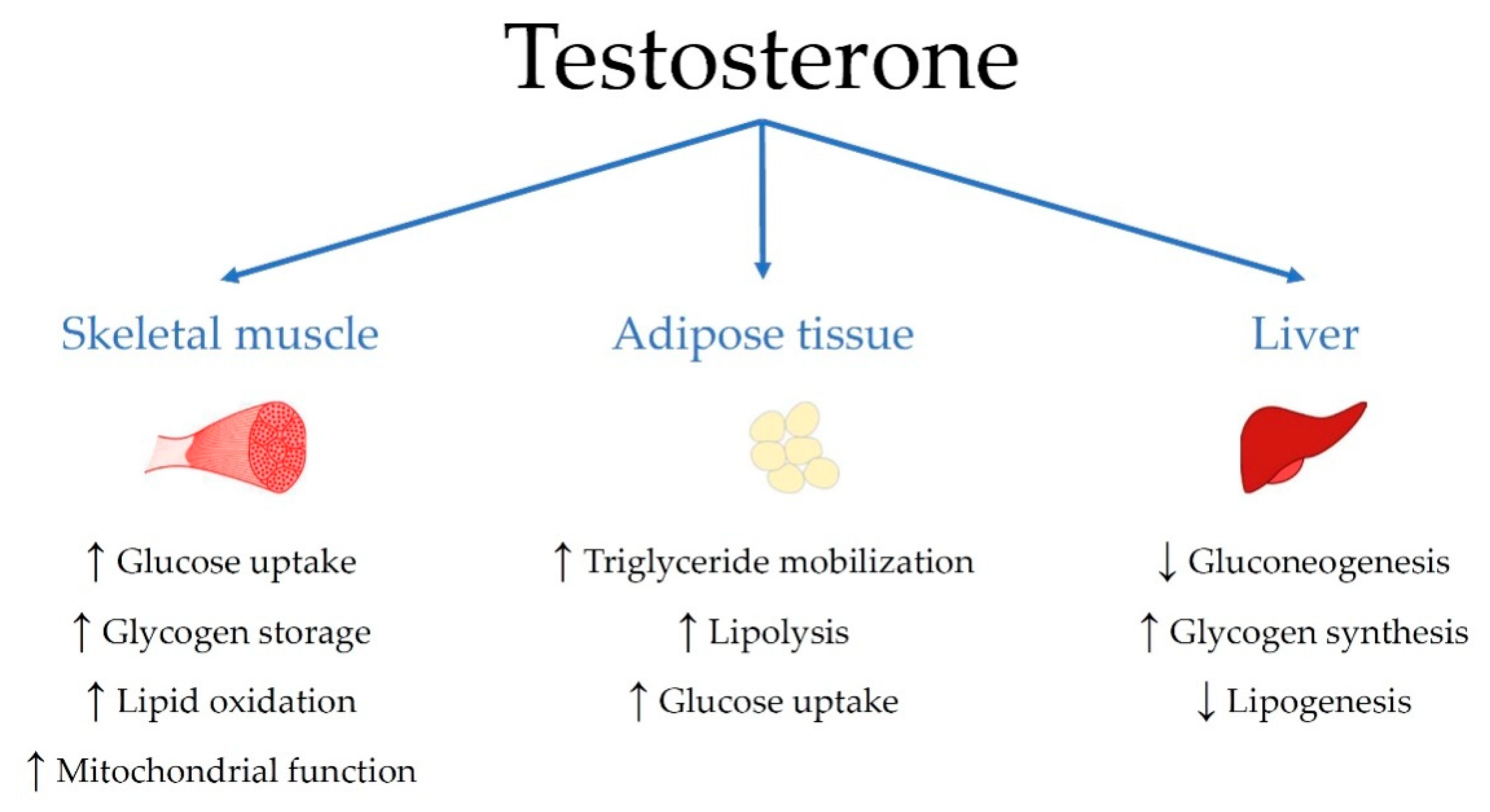
4.3. Sex Steroids and Insulin Resistance
4.4. Treatment of Trans Individuals with Diabetes Mellitus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of insulin in health and disease: An update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes 2014, 5, 89–96. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.M.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; Cockram, C.S.; Critchley, J.A.J.H. Drug-induced disorders of glucose metabolism. Drug Saf. 1996, 15, 135–157. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Chawla, R.; Chawla, A.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, L.; Pan, Q.; Mather, K.J.; Watson, K.E.; Hamman, R.F.; Kahn, S.E.; Diabetes Prevention Program Research Group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: Results from the Diabetes Prevention Program Outcomes Study. Lancet 2012, 379, 2243–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stévant, I.; Nef, S. Genetic control of gonadal sex determination and development. Trends Genet. 2019, 35, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Russell, A.; Mandy, W.; Butler, C. The phenomenology of gender dysphoria in adults: A systematic review and meta-synthesis. Clin. Psychol. Rev. 2020, 80, 101875. [Google Scholar] [CrossRef]
- T’Sjoen, G.; Arcelus, J.; Gooren, L.; Klink, D.T.; Tangpricha, V. Endocrinology of transgender medicine. Endocr. Rev. 2018, 40, 97–117. [Google Scholar] [CrossRef] [Green Version]
- Zucker, K.J. Epidemiology of gender dysphoria and transgender identity. Sex. Health 2017, 14, 404–411. [Google Scholar] [CrossRef]
- Collin, L.; Reisner, S.L.; Tangpricha, V.; Goodman, M. Prevalence of transgender depends on the “case” definition: A systematic review. J. Sex. Med. 2016, 13, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Leinung, M.C.; Joseph, J. Changing demographics in transgender individuals seeking hormonal therapy: Are trans women more common than trans men? Transgend. Health 2020, 5, 241–245. [Google Scholar] [CrossRef]
- Safer, J.D.; Tangpricha, V. Care of the Transgender Patient. Ann. Intern. Med. 2019, 171, ITC1–ITC16. [Google Scholar] [CrossRef]
- Hembree, W.C.; Cohen-Kettenis, P.T.; Gooren, L.; E Hannema, S.; Meyer, W.J.; Murad, M.H.; Rosenthal, S.M.; Safer, J.D.; Tangpricha, V.; T’Sjoen, G.G. Endocrine treatment of gender-dysphoric/gender-incongruent persons: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 3869–3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, G.; Mayer, M.; Mondorf, A.; Fluegel, A.K.; Herrmann, E.; Bojunga, J. Safety and rapid efficacy of guideline-based gender-affirming hormone therapy: An analysis of 388 individuals diagnosed with gender dysphoria. Eur. J. Endocrinol. 2020, 182, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.E.; Wilson, L.M.; Sharma, R.; Dukhanin, V.; McArthur, K.; Robinson, K.A. Therapy, mental health, and quality of life among transgender people: A systematic review. J. Endocr. Soc. 2021, 5, bvab011. [Google Scholar] [CrossRef]
- Cocchetti, C.; Romani, A.; Collet, S.; Greenman, Y.; Schreiner, T.; Wiepjes, C.; den Heijer, M.; T’Sjoen, G.; Fisher, A.D. The ENIGI (European Network for the Investigation of Gender Incongruence) Study: Overview of acquired endocrine knowledge and future perspectives. J. Clin. Med. 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, T.; Zajac, J.D.; Cheung, A.S. Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals-a systematic review. Clin. Endocrinol. 2018, 89, 700–711. [Google Scholar] [CrossRef] [Green Version]
- Bruns, C.M.; Kemnitz, J.W. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J. 2004, 45, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Gao, A.; Su, J.; Liu, R.; Zhao, S.; Li, W.; Xu, X.; Li, D.; Shi, J.; Gu, B.; Zhang, J.; et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 2021, 12, 7080. [Google Scholar] [CrossRef]
- Gooren, L.J.; T’Sjoen, G. Endocrine treatment of aging transgender people. Rev. Endocr. Metab. Disord. 2018, 19, 253–262. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex. Differ. 2015, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Wierckx, K.; Elaut, E.; Declercq, E.; Heylens, G.; De Cuypere, G.; Taes, Y.; Kaufman, J.M.; T’Sjoen, G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: A case-control study. Eur. J. Endocrinol. 2013, 169, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.; Nash, R.; Zhang, Q.; Panagiotakopoulos, L.; Daley, T.; Bhasin, S.; Getahun, D.; Sonya Haw, J.; McCracken, C.; Silverberg, M.J.; et al. Is there a link between hormone use and diabetes incidence in transgender people? Data from the STRONG Cohort. J. Clin. Endocrinol. Metab. 2022, 107, e1549–e1557. [Google Scholar] [CrossRef]
- Van Velzen, D.; Wiepjes, C.; Nota, N.; van Raalte, D.; de Mutsert, R.; Simsek, S.; den Heijer, M. Incident diabetes risk is not increased in transgender individuals using hormone therapy. J. Clin. Endocrinol. Metab. 2022, 107, e2000–e2007. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. 2013, 217, R25–R45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Spanos, C.; Bretherton, I.; Zajac, J.D.; Cheung, A.S. Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: A systematic review. World J. Diabetes 2020, 11, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Shadid, S.; Abosi-Appeadu, K.; De Maertelaere, A.S.; Defreyne, J.; Veldeman, L.; Holst, J.J.; Lapauw, B.; Vilsbøll, T.; T’Sjoen, G. Effects of gender-affirming hormone therapy on insulin sensitivity and incretin responses in transgender people. Diabetes Care 2020, 43, 411–417. [Google Scholar] [CrossRef]
- Auer, M.K.; Ebert, T.; Pietzner, M.; Defreyne, J.; Fuss, J.; Stalla, G.K.; T’Sjoen, G. Effects of sex hormone treatment on the metabolic syndrome in transgender individuals: Focus on metabolic cytokines. J. Clin. Endocrinol. Metab. 2018, 103, 790–802. [Google Scholar] [CrossRef]
- Bretherton, I.; Spanos, C.; Leemaqz, S.Y.; Premaratne, G.; Grossmann, M.; Zajac, J.D.; Cheung, A.S. Insulin resistance in transgender individuals correlates with android fat mass. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820985681. [Google Scholar] [CrossRef] [PubMed]
- Elbers, J.M.; Giltay, E.J.; Teerlink, T.; Scheffer, P.G.; Asscheman, H.; Seidell, J.C.; Gooren, L.J. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin. Endocrinol. 2003, 58, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polderman, K.H.; Gooren, L.J.; Asscheman, H.; Bakker, A.; Heine, R.J. Induction of insulin resistance by androgens and estrogens. J. Clin. Endocrinol. Metab. 1994, 79, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Klaver, M.; Dekker, M.J.H.J.; de Mutsert, R.; Twisk, J.W.R.; den Heijer, M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: A meta-analysis. Andrologia 2017, 49, e12660. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Lalik, A.; Soveral, G. Recent update on the molecular mechanisms of gonadal steroids action in adipose tissue. Int. J. Mol. Sci. 2021, 22, 5226. [Google Scholar] [CrossRef]
- Kadi, F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br. J. Pharmacol. 2008, 154, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Balistreri, C.R.; Caruso, C.; Candore, G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediat. Inflamm. 2010, 802078. [Google Scholar] [CrossRef] [Green Version]
- Paquin, J.; Lagacé, J.C.; Brochu, M.; Dionne, I.J. Exercising for insulin sensitivity—Is there a mechanistic relationship with quantitative changes in skeletal muscle mass? Front. Physiol. 2021, 12, 656909. [Google Scholar] [CrossRef]
- Yassin, A.; Haider, A.; Haider, K.S.; Caliber, M.; Doros, G.; Saad, F.; Garvey, W.T. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: Eight-year data from a registry study. Diabetes Care 2019, 42, 1104–1111. [Google Scholar] [CrossRef]
- Traish, A.M.; Haider, A.; Doros, G.; Saad, F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: An observational, long-term registry study. Int. J. Clin. Pract. 2014, 68, 314–329. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, M. Testosterone and glucose metabolism in men: Current concepts and controversies. J. Endocrinol. 2014, 220, R37–R55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic—hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, M.E.; Alfaro, A.A. The effects of hormonal contraceptives on glycemic regulation. Linacre Q. 2014, 81, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, J.; Chávez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejías, J.; Calvo, M.; Bermúdez, V. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int. J. Reprod. Med. 2014, 2014, 719050. [Google Scholar] [CrossRef] [Green Version]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Ropero, A.B.; Quesada, I. The role of oestrogens in the adaptation of islets to insulin resistance. J. Physiol. 2009, 587, 5031–5037. [Google Scholar] [CrossRef]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [Green Version]
- Gupte, A.A.; Pownall, H.J.; Hamilton, D.J. Estrogen: An emerging regulator of insulin action and mitochondrial function. J. Diabetes Res. 2015, 2015, 916585. [Google Scholar] [CrossRef] [Green Version]
- Godsland, I.F. Oestrogens and insulin secretion. Diabetologia 2005, 48, 2213–2220. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Podufaly, A.; Dillon, P.F. Estradiol binds to insulin and insulin receptor decreasing insulin binding in vitro. Front. Endocrinol. 2014, 5, 118. [Google Scholar] [CrossRef] [Green Version]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Quesada, I.; Ropero, A.B. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol. Cell Endocrinol. 2009, 304, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Barros, R.P.; Morani, A.; Moriscot, A.; Machado, U.F. Insulin resistance of pregnancy involves estrogen-induced repression of muscle GLUT4. Mol. Cell Endocrinol. 2008, 295, 24–31. [Google Scholar] [CrossRef] [Green Version]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Pharmacologic approaches to glycemic treatment: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S140–S157. [Google Scholar] [CrossRef] [PubMed]
- Kirpichnikov, D.; McFarlane, S.I.; Sowers, J.R. Metformin: An update. Ann. Intern. Med. 2002, 137, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome—Review of classical and new compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinnen, D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, B.; Ahrén, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 2014, 63, 2196–2202. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J. Insulin resistance: Impact on therapeutic developments in diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 128–132. [Google Scholar] [CrossRef]
- Riddle, M.C.; Herman, W.H. The cost of diabetes care—An elephant in the room. Diabetes Care 2018, 41, 929–932. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, G.; Henning-Smith, C. Barriers to care among transgender and gender nonconforming adults. Milbank Q. 2017, 95, 726–748. [Google Scholar] [CrossRef]
- Aylagas-Crespillo, M.; García-Barbero, Ó.; Rodríguez-Martín, B. Barriers in the social and healthcare assistance for transgender persons: A systematic review of qualitative studies. Enferm. Clin. 2017, 28, 247–259. [Google Scholar] [CrossRef]
- Kcomt, L.; Gorey, K.M.; Barrett, B.J.; McCabe, S.E. Healthcare avoidance due to anticipated discrimination among transgender people: A call to create trans-affirmative environments. SSM Popul. Health 2020, 11, 100608. [Google Scholar] [CrossRef] [PubMed]
- White Hughto, J.M.; Reisner, S.L. A systematic review of the effects of hormone therapy on psychological functioning and quality of life in transgender individuals. Transgend Health 2016, 1, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, P.J.; Marie Freel, E.; Perry, C.; Ewan, J.; Touyz, R.M.; Currie, G.; Delles, C. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension 2019, 74, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Tangpricha, V. Gender-affirming hormone therapy and risk of diabetes in transgender persons. J. Clin. Endocrinol. Metab. 2022, 107, e2632–e2633. [Google Scholar] [CrossRef]
- Moverley, J.; Loebner, S.; Carmona, B.; Vuu, D. Considerations for transgender people with diabetes. Clin. Diabetes 2021, 39, 389–396. [Google Scholar] [CrossRef]
- Weinand, J.D.; Safer, J.D. Hormone therapy in transgender adults is safe with provider supervision; a review of hormone therapy sequelae for transgender individuals. J. Clin. Transl. Endocrinol. 2015, 2, 55–60. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milionis, C.; Ilias, I.; Venaki, E.; Koukkou, E. Glucose Homeostasis, Diabetes Mellitus, and Gender-Affirming Treatment. Biomedicines 2023, 11, 670. https://doi.org/10.3390/biomedicines11030670
Milionis C, Ilias I, Venaki E, Koukkou E. Glucose Homeostasis, Diabetes Mellitus, and Gender-Affirming Treatment. Biomedicines. 2023; 11(3):670. https://doi.org/10.3390/biomedicines11030670
Chicago/Turabian StyleMilionis, Charalampos, Ioannis Ilias, Evangelia Venaki, and Eftychia Koukkou. 2023. "Glucose Homeostasis, Diabetes Mellitus, and Gender-Affirming Treatment" Biomedicines 11, no. 3: 670. https://doi.org/10.3390/biomedicines11030670
APA StyleMilionis, C., Ilias, I., Venaki, E., & Koukkou, E. (2023). Glucose Homeostasis, Diabetes Mellitus, and Gender-Affirming Treatment. Biomedicines, 11(3), 670. https://doi.org/10.3390/biomedicines11030670







