The Effects of Topiroxostat, a Selective Xanthine Oxidoreductase Inhibitor, on Arterial Stiffness in Hyperuricemic Patients with Liver Dysfunction: A Sub-Analysis of the BEYOND-UA Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization and Intervention
2.3. Outcomes
2.4. Assessments
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Subjects Treated with Topiroxostat
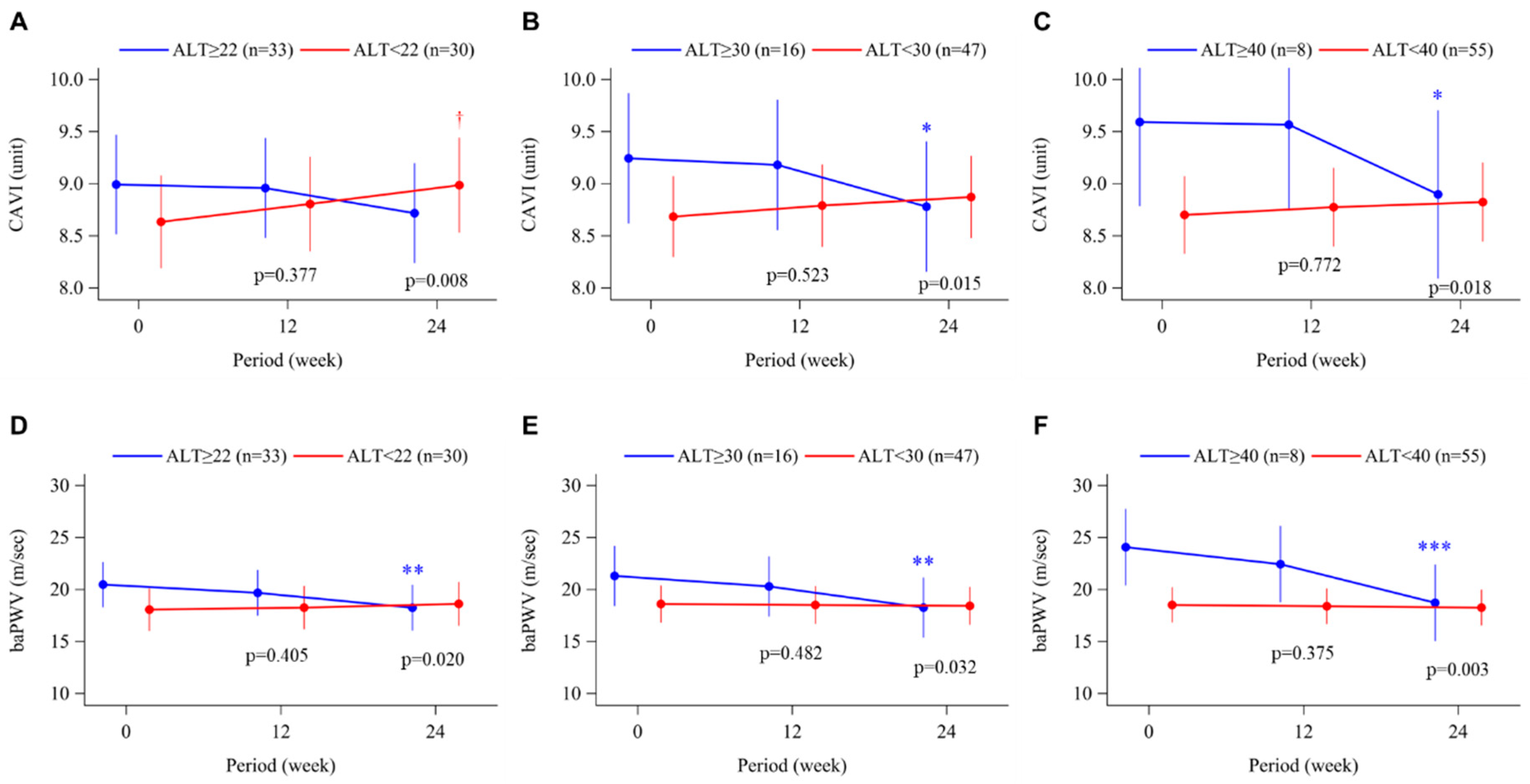
3.2. Arterial Stiffness
3.3. Morning Home Blood Pressure
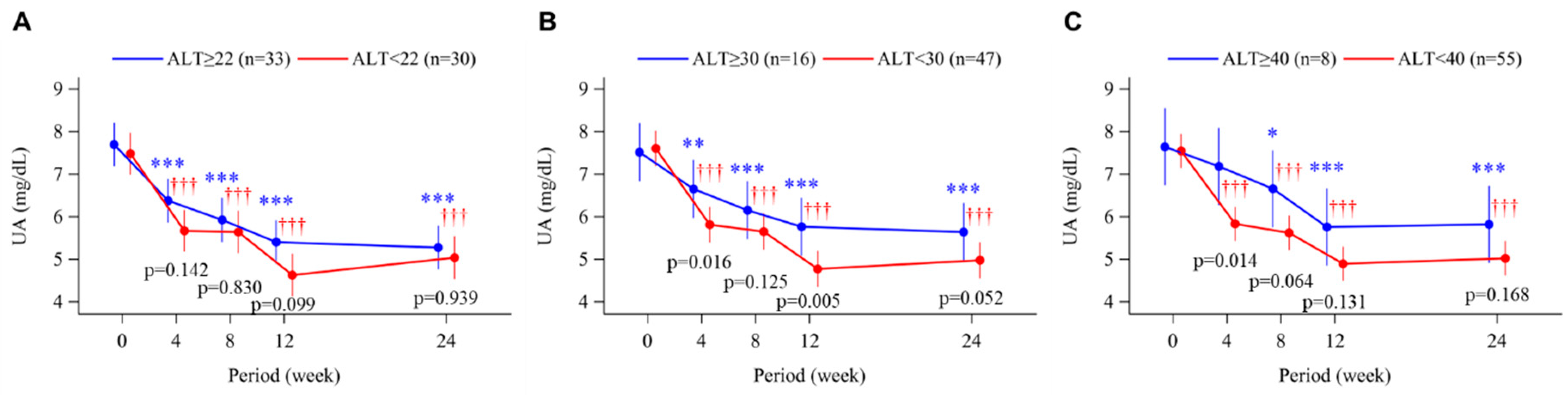
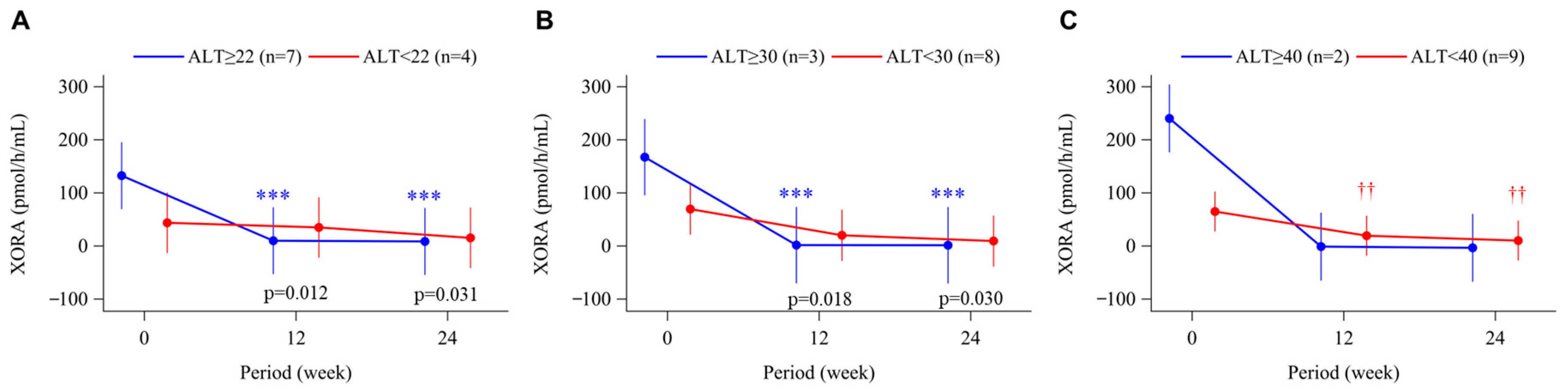
3.4. Uric Acid Levels and Plasma XOR Activity
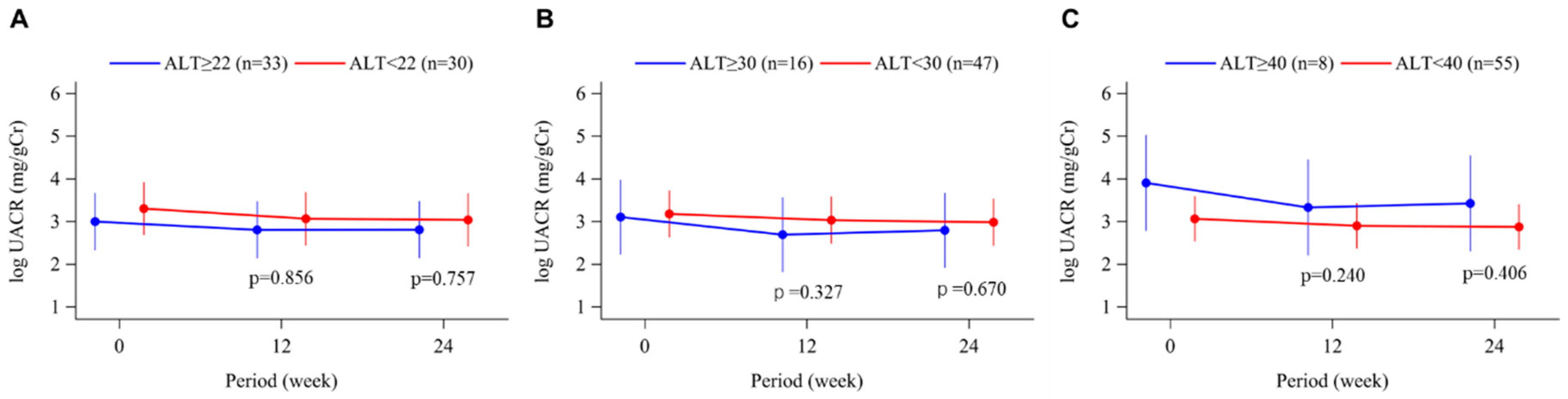
3.5. Urinary Albumin-Creatinine Ratio
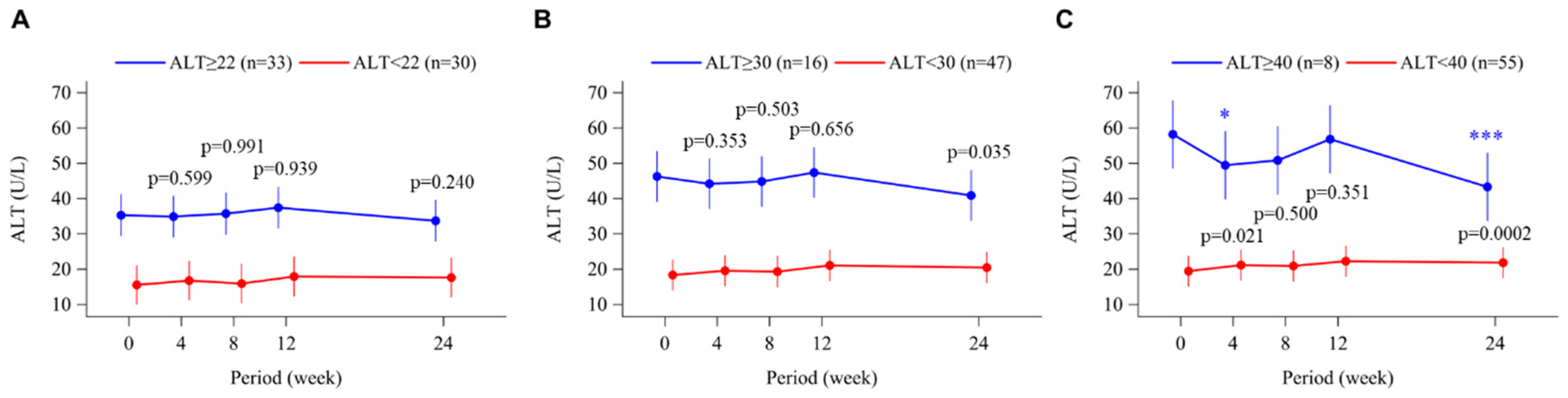
3.6. Alanine Aminotransferase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamei, K.; Konta, T.; Hirayama, A.; Suzuki, K.; Ichikawa, K.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; et al. A slight increase within the normal range of serum uric acid and the decline in renal function: Associations in a community-based population. Nephrol. Dial. Transplant. 2014, 29, 2286–2292. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Han, L.; Xu, G.; Ran, J.M. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: A meta-analysis of 15 cohort studies. PLoS ONE 2014, 24, e100801. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Murakami, Y.; Miura, K.; Nagai, M.; Sugiyama, D.; Ueshima, H.; Okamura, T.; EPOCH-JAPAN GROUP. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN Study. J. Atheroscler. Thromb. 2016, 23, 692–703. [Google Scholar] [CrossRef]
- Nishizawa, H.; Maeda, N.; Shimomura, I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens. Res. 2022, 45, 635–640. [Google Scholar] [CrossRef]
- Hisatome, I.; Ichida, K.; Mineo, I.; Ohtahara, A.; Ogino, K.; Kuwahara, M.; Ishizaka, N.; Uchida, S.; Kurajoh, M.; Kohagura, K.; et al. Japanese Society of Gout and Uric & Nucleic Acids 2019 guidelines for management of hyperuricemia and gout 3rd edition. Gout Uric. Nucleic Acids. 2020, 44, sp-1–sp-40. [Google Scholar] [CrossRef]
- Matsuura, F.; Yamashita, S.; Nakamura, T.; Nishida, M.; Nozaki, S.; Funahashi, T.; Matsuzawa, Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998, 47, 929–933. [Google Scholar] [CrossRef]
- Adachi, T.; Fukushima, T.; Usami, Y.; Hirano, K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem. J. 1993, 289, 523–527. [Google Scholar] [CrossRef]
- Houston, M.; Estevez, A.; Chumley, P.; Aslan, M.; Marklund, S.; Parks, D.A.; Freeman, B.A. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999, 274, 4985–4994. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bolognesi, A.; Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta 2014, 1842, 1502–1517. [Google Scholar] [CrossRef]
- Kelley, E.E. A new paradigm for XOR-catalyzed reactive species generation in the endothelium. Pharmacol. Rep. 2015, 67, 669–674. [Google Scholar] [CrossRef]
- Nagao, H.; Nishizawa, H.; Tanaka, Y.; Fukata, T.; Mizushima, T.; Furuno, M.; Bamba, T.; Tsushima, Y.; Fujishima, Y.; Kita, S.; et al. Hypoxanthine secretion from human adipose tissue and its increase in hypoxia. Obesity 2018, 26, 1168–1178. [Google Scholar] [CrossRef]
- Kawachi, Y.; Fujishima, Y.; Nishizawa, H.; Nagao, H.; Nakamura, T.; Akari, S.; Murase, T.; Taya, N.; Omori, K.; Miyake, A.; et al. Plasma xanthine oxidoreductase activity in Japanese patients with type 2 diabetes across hospitalized treatment. J. Diabetes. Investig. 2021, 12, 1512–1520. [Google Scholar] [CrossRef]
- Kawachi, Y.; Fujishima, Y.; Nishizawa, H.; Nakamura, T.; Akari, S.; Murase, T.; Saito, T.; Miyazaki, Y.; Nagao, H.; Fukuda, S.; et al. Increased plasma XOR activity induced by NAFLD/NASH and its possible involvement in vascular neointimal proliferation. JCI Insight 2021, 6, e144762. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. large-artery stiffness in health and disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Tomiyama, H.; Shiina, K. State of the Art Review: Brachial-Ankle PWV. J. Atheroscler. Thromb. 2020, 27, 621–636. [Google Scholar] [CrossRef]
- Kario, K.; Nishizawa, M.; Kiuchi, M.; Kiyosue, A.; Tomita, F.; Ohtani, H.; Abe, Y.; Kuga, H.; Miyazaki, S.; Kasai, T.; et al. Comparative effects of topiroxostat and febuxostat on arterial properties in hypertensive patients with hyperuricemia. J. Clin. Hypertens 2021, 23, 334–344. [Google Scholar] [CrossRef]
- Nakamura, T.; Murase, T.; Satoh, E.; Miyachi, A.; Ogawa, N.; Abe, K.; Katoh, N.; Nakayama, Y. The influence of albumin on the plasma xanthine oxidoreductase inhibitory activity of allopurinol, febuxostat and topiroxostat: Insight into extra-urate lowering effect. Integr. Mol. Med. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Shimamoto, K.; Ando, K.; Fujita, T.; Hasebe, N.; Higaki, J.; Horiuchi, M.; Imai, Y.; Imaizumi, T.; Ishimitsu, T.; Ito, M.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens. Res. 2014, 37, 253–390. [Google Scholar] [CrossRef]
- Murase, T.; Nampei, M.; Oka, M.; Miyachi, A.; Nakamura, T. A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope-labeled xanthine and LC/TQMS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1039, 51–58. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Zhang, F.; Chen, Y.; Wang, D.; Wang, Y. The Effects of Allopurinol on the Carotid Intima-media Thickness in Patients with Type 2 Diabetes and Asymptomatic Hyperuricemia: A Three-year Randomized Parallel-controlled Study. Intern. Med. 2015, 54, 2129–2137. [Google Scholar] [CrossRef]
- Higgins, P.; Walters, M.R.; Murray, H.M.; McArthur, K.; McConnachie, A.; Lees, K.R.; Dawson, J. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: A randomised controlled trial. Heart 2014, 100, 1085–1092. [Google Scholar] [CrossRef]
- Kojima, S.; Matsui, K.; Hiramitsu, S.; Hisatome, I.; Waki, M.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Jinnouchi, H.; et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur. Heart J. 2019, 40, 1778–1786. [Google Scholar] [CrossRef]
- Mackenzie, I.S.; Hawkey, C.J.; Ford, I.; Greenlaw, N.; Pigazzani, F.; Rogers, A.; Struthers, A.D.; Begg, A.G.; Wei, L.; Avery, A.J.; et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): A multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 1195–1205. [Google Scholar] [CrossRef]
- Ampuero, J.; Gallego-Durán, R.; Romero-Gómez, M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: Meta-analysis. Rev. Esp. Enferm. Dig. 2015, 107, 10–16. [Google Scholar]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci. Rep. 2016, 16, 33386. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef]
- Franzini, M.; Corti, A.; Martinelli, B.; Del-Corso, A.; Emdin, M.; Parenti, G.F.; Glauber, M.; Pompella, A.; Paolicchi, A. Gamma-glutamyltransferase activity in human atherosclerotic plaques--biochemical similarities with the circulating enzyme. Atherosclerosis 2009, 202, 119–127. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Apekey, T.A.; Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 2014, 236, 7–17. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, H.; Que, S.; Wang, L.; Qin, X.; Peng, T. Complex association between alanine aminotransferase activity and mortality in general population: A systematic review and meta-analysis of prospective studies. PLoS ONE 2014, 9, e91410. [Google Scholar] [CrossRef]
- Ohira, M.; Tanaka, S.; Watanabe, Y.; Nakamura, S.; Oka, R.; Yamaguchi, T.; Ban, N.; Saiki, A.; Ishihara, N.; Murano, T.; et al. Association of Plasma Xanthine mean value 8Oxidoreductase with Arterial Stiffness in Type 2 Diabetes with Liver Dysfunction. Am. J. Med. Sci. 2022, 363, 242–250. [Google Scholar] [CrossRef]
- Tanaka, K.; Hyogo, H.; Ono, M.; Takahashi, H.; Kitajima, Y.; Ono, N.; Eguchi, T.; Fujimoto, K.; Cheema, K.; Saibara, T.; et al. Upper limit of normal serum alanine aminotransferase levels in Japanese subjects. Hepatol. Res. 2014, 44, 1196–1207. [Google Scholar] [CrossRef]
- Shiina, K.; Tomiyama, H.; Tanaka, A.; Yoshida, H.; Eguchi, K.; Kario, K.; Kato, T.; Teragawa, H.; Toyoda, S.; Ohishi, M.; et al. Differential effect of a xanthine oxidase inhibitor on arterial stiffness and carotid atherosclerosis: A subanalysis of the PRIZE study. Hypertens. Res. 2022, 45, 602–611. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Andres-Hernando, A.; Kuwabara, M. Uric acid and hypertension. Hypertens. Res. 2020, 43, 832–834. [Google Scholar] [CrossRef]
- Nakatsu, Y.; Seno, Y.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Katasako, A.; Mori, K.; Matsunaga, Y.; Fukushima, T.; Kanaoka, R.; et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G42–G51. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nagata, N.; Shimakami, T.; Shirakura, T.; Matsui, C.; Ni, Y.; Zhuge, F.; Xu, L.; Chen, G.; Nagashimada, M.; et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci. Rep. 2020, 10, 815. [Google Scholar] [CrossRef]

| Variables | ALT <22 U/L (n = 30) | ALT ≥22 U/L (n = 33) | p-Value |
|---|---|---|---|
| Age, years | 68.9 ± 7.0 | 67.5 ± 7.8 | 0.482 |
| Male, n (%) | 23 (76.7) | 30 (90.9) | 0.172 |
| Body mass index, kg/m2 | 25.5 ± 3.9 | 26.1 ± 2.9 | 0.453 |
| Smoking, n (%) | 6 (20.0) | 7 (21.2) | 1.000 |
| Drinking, n (%) | 18 (60.0) | 26 (78.8) | 0.169 |
| Medical history, n (%) | |||
| Diabetes mellitus | 8 (26.7) | 5 (15.2) | 0.353 |
| Dyslipidemia | 11 (36.7) | 13 (39.4) | 1.000 |
| Chronic kidney disease | 0 (0.0) | 2 (6.1) | 0.493 |
| Liver disease | 1 (3.3) | 2 (6.1) | 1.000 |
| Stroke | 1 (3.3) | 1 (3.0) | 1.000 |
| Heart failure | 2 (6.7) | 0 (0.0) | 0.223 |
| Coronary artery disease | 1 (3.3) | 4 (12.1) | 0.357 |
| Non-valvular atrial disease | 1 (3.3) | 3 (9.1) | 0.614 |
| Antihypertensives, n (%) | |||
| ACEi | 2 (6.7) | 1 (3.0) | 0.601 |
| ARB | 19 (63.3) | 26 (78.8) | 0.264 |
| CCB | 16 (53.3) | 19 (57.6) | 0.803 |
| Beta-blocker | 4 (13.3) | 8 (24.2) | 0.344 |
| Diuretic | 7 (23.3) | 8 (24.2) | 1.000 |
| Other | 1 (3.3) | 1 (3.0) | 1.000 |
| Antidiabetic therapy, n (%) | 8 (26.7) | 5 (15.2) | 0.353 |
| Morning home SBP, mmHg | 136.2 ± 15.5 | 133.1 ± 12.2 | 0.396 |
| Morning home DBP, mmHg | 86.0 ± 12.6 | 81.2 ± 11.4 | 0.129 |
| Morning home HR, beats/min | 69.9 ± 10.3 | 67.8 ± 11.0 | 0.463 |
| CAVI, unit | 8.9 ± 1.0 | 9.3 ± 1.6 | 0.236 |
| baPWV, m/sec | 18.7 ± 2.3 | 21.1 ± 8.6 | 0.132 |
| ALT, U/L | 15.3 ± 4.3 | 35.8 ± 17.6 | <0.001 |
| AST, U/L | 21.0 ± 6.1 | 31.2 ± 11.6 | <0.001 |
| Uric acid, mg/dL | 7.6 ± 1.0 | 7.8 ± 1.3 | 0.331 |
| Creatinine, mg/dL | 0.90 ± 0.25 | 0.92 ± 0.19 | 0.752 |
| eGFR, mL/min/1.73 m2 | 64.9 ± 18.6 | 64.7 ± 14.0 | 0.967 |
| hs-CRP, ng/mL | 773 (428, 1500) | 1100 (375, 1910) | 0.995 |
| NT-pro BNP, pg/mL | 79 (45, 153) | 51 (27, 139) | 0.271 |
| UACR, mg/g·Cr | 20.6 (11.2, 42.4) | 12.4 (7.9, 49.2) | 0.405 |
| Cystatin-C, mg/L | 1.07 ± 0.21 | 1.04 ± 0.23 | 0.680 |
| Variables | ALT <30 U/L (n = 47) | ALT ≥30U/L (n = 16) | p-Value |
|---|---|---|---|
| Age, years | 69.2 ± 6.5 | 65.2 ± 9.2 | 0.121 |
| Male, n (%) | 38 (80.9) | 15 (93.8) | 0.429 |
| Body mass index, kg/m2 | 25.5 ± 3.3 | 26.7 ± 3.6 | 0.277 |
| Smoking, n (%) | 10 (21.3) | 3 (18.8) | 1.000 |
| Drinking, n (%) | 33 (70.2) | 11 (68.8) | 1.000 |
| Medical history, n (%) | |||
| Diabetes mellitus | 10 (21.3) | 3 (18.8) | 1.000 |
| Dyslipidemia | 18 (38.3) | 6 (37.5) | 1.000 |
| Chronic kidney disease | 2 (4.3) | 0 (0.0) | 1.000 |
| Liver disease | 3 (6.4) | 0 (0.0) | 0.564 |
| Stroke | 1 (2.1) | 1 (6.3) | 0.446 |
| Heart failure | 2 (4.3) | 0 (0.0) | 1.000 |
| Coronary artery disease | 3 (6.4) | 2 (12.5) | 0.594 |
| Non-valvular atrial disease | 3 (6.4) | 1 (6.3) | 1.000 |
| Antihypertensives, n (%) | |||
| ACEi | 3 (6.4) | 0 (0.0) | 0.564 |
| ARB | 31 (66.0) | 14 (87.5) | 0.121 |
| CCB | 24 (51.1) | 11 (68.8) | 0.257 |
| Beta-blocker | 7 (14.9) | 5 (31.3) | 0.162 |
| Diuretic | 11 (23.4) | 4 (25.0) | 1.000 |
| Other | 2 (4.3) | 0 (0.0) | 1.000 |
| Antidiabetic therapy, n (%) | 10 (21.3) | 3 (18.8) | 1.000 |
| Morning home SBP, mmHg | 135.0 ± 14.0 | 133.2 ± 13.6 | 0.641 |
| Morning home DBP, mmHg | 83.7 ± 11.5 | 82.5 ± 14.0 | 0.766 |
| Morning home HR, beats/min | 68.8 ± 11.2 | 69.1 ± 9.1 | 0.916 |
| CAVI, unit | 9.0 ± 1.2 | 9.4 ± 1.8 | 0.409 |
| baPWV, m/sec | 19.5 ± 5.7 | 21.5 ± 8.3 | 0.369 |
| ALT, U/L | 18.7 ± 5.8 | 47.8 ± 18.9 | <0.001 |
| AST, U/L | 23.4 ± 7.5 | 35.0 ± 14.0 | 0.005 |
| Uric acid, mg/dL | 7.7 ± 1.0 | 7.7 ± 1.7 | 0.952 |
| Creatinine, mg/dL | 0.91 ± 0.24 | 0.93 ± 0.18 | 0.768 |
| eGFR, mL/min/1.73 m2 | 64.5 ± 16.9 | 65.7 ± 14.7 | 0.792 |
| hs-CRP, ng/mL | 802 (363, 1840) | 1140 (424, 1985) | 0.670 |
| NT-pro BNP, pg/mL | 66 (36, 143) | 50 (21, 159) | 0.554 |
| UACR, mg/g·Cr | 15.9 (8.2, 49.2) | 15.0 (8.7, 46.9) | 0.969 |
| Cystatin-C, mg/L | 1.05 ± 0.19 | 1.07 ± 0.30 | 0.753 |
| Variables | ALT <40 U/L (n = 55) | ALT ≥40 U/L (n = 8) | p-Value |
|---|---|---|---|
| Age, years | 68.6 ± 7.2 | 65.3 ± 8.8 | 0.332 |
| Male, n (%) | 46 (83.6) | 7 (87.5) | 1.000 |
| Body mass index, kg/m2 | 25.5 ± 3.3 | 28.0 ± 3.3 | 0.074 |
| Smoking, n (%) | 11 (20.0) | 2 (25.0) | 0.665 |
| Drinking, n (%) | 40 (72.7) | 4 (50.0) | 0.229 |
| Medical history, n (%) | |||
| Diabetes mellitus | 11 (20.0) | 2 (25.0) | 0.665 |
| Dyslipidemia | 20 (36.4) | 4 (50.0) | 0.467 |
| Chronic kidney disease | 2 (3.6) | 0 (0.0) | 1.000 |
| Liver disease | 3 (5.5) | 0 (0.0) | 1.000 |
| Stroke | 1 (1.8) | 1 (12.5) | 0.240 |
| Heart failure | 2 (3.6) | 0 (0.0) | 1.000 |
| Coronary artery disease | 5 (9.1) | 0 (0.0) | 1.000 |
| Non-valvular atrial disease | 3 (5.5) | 1 (12.5) | 0.427 |
| Antihypertensives, n (%) | |||
| ACEi | 3 (5.5) | 0 (0.0) | 1.000 |
| ARB | 38 (69.1) | 7 (87.5) | 0.421 |
| CCB | 28 (50.9) | 7 (87.5) | 0.066 |
| Beta-blocker | 11 (20.0) | 1 (12.5) | 1.000 |
| Diuretic | 13 (23.6) | 2 (25.0) | 1.000 |
| Other | 2 (3.6) | 0 (0.0) | 1.000 |
| Antidiabetic therapy, n (%) | 11 (20.0) | 2 (25.0) | 0.665 |
| Morning home SBP, mmHg | 133.9 ± 14.1 | 138.6 ± 11.6 | 0.325 |
| Morning home DBP, mmHg | 82.8 ± 11.3 | 87.0 ± 17.0 | 0.522 |
| Morning home HR, beats/min | 68.1 ± 10.6 | 74.4 ± 9.3 | 0.136 |
| CAVI, unit | 9.0 ± 1.2 | 9.7 ± 2.3 | 0.417 |
| baPWV, m/sec | 19.4 ± 5.4 | 24.2 ± 11.0 | 0.257 |
| ALT, U/L | 21.0 ± 7.9 | 61.1 ± 18.6 | <0.001 |
| AST, U/L | 23.9 ± 7.3 | 43.3 ± 15.1 | 0.008 |
| Uric acid, mg/dL | 7.7 ± 1.1 | 7.9 ± 2.0 | 0.806 |
| Creatinine, mg/dL | 0.91 ± 0.23 | 0.93 ± 0.21 | 0.859 |
| eGFR, mL/min/1.73 m2 | 64.7 ± 16.2 | 65.3 ± 17.8 | 0.942 |
| hs-CRP, ng/mL | 802 (375, 1930) | 1140 (433, 1575) | 0.942 |
| NT-pro BNP, pg/mL | 61 (33, 143) | 62 (13, 159) | 0.613 |
| UACR, mg/g·Cr | 14.7 (8.2, 42.4) | 42.7 (13.6, 256.8) | 0.148 |
| Cystatin-C, mg/L | 1.04 ± 0.19 | 1.18 ± 0.36 | 0.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujishima, Y.; Nishizawa, H.; Kawachi, Y.; Nakamura, T.; Akari, S.; Ono, Y.; Fukuda, S.; Kita, S.; Maeda, N.; Hoshide, S.; et al. The Effects of Topiroxostat, a Selective Xanthine Oxidoreductase Inhibitor, on Arterial Stiffness in Hyperuricemic Patients with Liver Dysfunction: A Sub-Analysis of the BEYOND-UA Study. Biomedicines 2023, 11, 674. https://doi.org/10.3390/biomedicines11030674
Fujishima Y, Nishizawa H, Kawachi Y, Nakamura T, Akari S, Ono Y, Fukuda S, Kita S, Maeda N, Hoshide S, et al. The Effects of Topiroxostat, a Selective Xanthine Oxidoreductase Inhibitor, on Arterial Stiffness in Hyperuricemic Patients with Liver Dysfunction: A Sub-Analysis of the BEYOND-UA Study. Biomedicines. 2023; 11(3):674. https://doi.org/10.3390/biomedicines11030674
Chicago/Turabian StyleFujishima, Yuya, Hitoshi Nishizawa, Yusuke Kawachi, Takashi Nakamura, Seigo Akari, Yoshiyuki Ono, Shiro Fukuda, Shunbun Kita, Norikazu Maeda, Satoshi Hoshide, and et al. 2023. "The Effects of Topiroxostat, a Selective Xanthine Oxidoreductase Inhibitor, on Arterial Stiffness in Hyperuricemic Patients with Liver Dysfunction: A Sub-Analysis of the BEYOND-UA Study" Biomedicines 11, no. 3: 674. https://doi.org/10.3390/biomedicines11030674
APA StyleFujishima, Y., Nishizawa, H., Kawachi, Y., Nakamura, T., Akari, S., Ono, Y., Fukuda, S., Kita, S., Maeda, N., Hoshide, S., Shimomura, I., & Kario, K. (2023). The Effects of Topiroxostat, a Selective Xanthine Oxidoreductase Inhibitor, on Arterial Stiffness in Hyperuricemic Patients with Liver Dysfunction: A Sub-Analysis of the BEYOND-UA Study. Biomedicines, 11(3), 674. https://doi.org/10.3390/biomedicines11030674






