Human Plasma Xanthine Oxidoreductase Activity in Cardiovascular Disease: Evidence from a Population-Based Study
Abstract
:1. Introduction
2. XOR and the Pathway by Which It Acts
3. XOR Activity and Vascular Injury
4. Plasma XOR Activity and Cardiovascular Events in Patients with CVD
5. Plasma XOR Activity and Cardiovascular Events in the General Population
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2Fe/S | two iron–sulphur centres |
| AHF | acute heart failure |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| BNP | type B natriuretic peptide |
| Btn | butyrophilin1A1 |
| CHF | chronic heart failure |
| CI | confidence interval |
| CKD | chronic kidney disease |
| COX-2 | cyclooxygenase-2 |
| CVD | cardiovascular disease |
| cGMP | cyclic guanosine monophosphate |
| e− | electron |
| eGFR | estimated glomerular filtration |
| FAD | flavin adenine dinucleotide |
| H2O2 | hydrogen peroxide |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HF | heart failure |
| HFpEF | ejection fraction heart failure |
| HOMA-R | homeostasis model assessment of insulin resistance |
| hXOR | human xanthine oxidoreductase gene |
| LDL | low-density lipoprotein |
| MAP | mean arterial pressure |
| MACEs | major adverse cardiovascular events |
| Moco | molybdopterin cofactor containing a molybdenum atom |
| NAD+ | nicotinamide adenine dinucleotide |
| NADH | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NO | nitric oxide |
| OR | odds ratio |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SCD | sickle cell disease |
| SOD | superoxide dismutase |
| UA | uric acid |
| XDH | xanthine dehydrogenase |
| XO | xanthine oxidase |
| XOR | xanthine oxidoreductase |
References
- Kisker, C.; Schindelin, H.; Rees, D.C. Molybdenum-COFACTOR–CONTAINING ENZYMES: Structure and mechanism. Annu. Rev. Biochem. 1997, 66, 233–267. [Google Scholar] [CrossRef]
- Meneshian, A.; Bulkley, G.B. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 2002, 9, 161–175. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bolognesi, A.; Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta 2014, 1842, 1502–1517. [Google Scholar] [CrossRef]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Kushiyama, A.; Okubo, H.; Sakoda, H.; Kikuchi, T.; Fujishiro, M.; Sato, H.; Kushiyama, S.; Iwashita, M.; Nishimura, F.; Fukushima, T.; et al. Xanthine oxidoreductase is involved in macrophage foam cell formation and atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 291–298. [Google Scholar] [CrossRef]
- Parks, D.A.; Granger, D.N. Xanthine oxidase: Biochemistry, distribution and physiology. Acta. Physiol. Scand. Suppl. 1986, 548, 87–99. [Google Scholar]
- Liu, X.; Lin, W.M.; Yan, X.H.; Chen, X.H.; Hoidal, J.R.; Xu, P. Improved method for measurement of human plasma xanthine oxidoreductase activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 785, 101–114. [Google Scholar] [CrossRef]
- Beckman, J.S.; Parks, D.A.; Pearson, J.D.; Marshall, P.A.; Freeman, B.A. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic. Biol. Med. 1989, 6, 607–615. [Google Scholar] [CrossRef]
- Shamma’a, M.H.; Nasrallah, S.M.; al-Khalidi, U.A. Serum xanthine oxidase. An experience with 2000 patients. Am. J. Dig. Dis. 1973, 18, 15–22. [Google Scholar] [CrossRef]
- Murase, T.; Nampei, M.; Oka, M.; Ashizawa, N.; Matsumoto, K.; Miyachi, A.; Nakamura, T. Xanthine oxidoreductase activity assay in tissues using stable isotope-labeled substrate and liquid chromatography high-resolution mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1008, 189–197. [Google Scholar] [CrossRef]
- Murase, T.; Nampei, M.; Oka, M.; Miyachi, A.; Nakamura, T. A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope-labeled xanthine and LC/TQMS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1039, 51–58. [Google Scholar] [CrossRef]
- Murase, T.; Oka, M.; Nampei, M.; Miyachi, A.; Nakamura, T. A highly sensitive assay for xanthine oxidoreductase activity using a combination of [13 C2, 15N2]xanthine and liquid chromatography/triple quadrupole mass spectrometry. J. Labelled. Comp. Radiopharm. 2016, 59, 214–220. [Google Scholar] [CrossRef]
- Kanbay, M.; Segal, M.; Afsar, B.; Kang, D.H.; Rodriguez-Iturbe, B.; Johnson, R.J. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart 2013, 99, 759–766. [Google Scholar] [CrossRef]
- McManaman, J.L.; Bain, D.L. Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. J. Biol. Chem. 2002, 277, 21261–21268. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase in Drug Metabolism: Beyond a Role as a Detoxifying Enzyme. Curr. Med. Chem. 2016, 23, 4027–4036. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bortolotti, M.; Bolognesi, A.; Polito, L. Pro-Aging Effects of Xanthine Oxidoreductase Products. Antioxidants 2020, 9, 839. [Google Scholar] [CrossRef]
- Nishino, T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J. Biochem. 1994, 116, 1–6. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Nishino, T.; Okamoto, K.; Matsumura, T.; Eger, B.T.; Pai, E.F.; Nishino, T. Unique amino acids cluster for switching from the dehydrogenase to oxidase form of xanthine oxidoreductase. Proc. Natl. Acad. Sci. USA 2003, 100, 8170–8175. [Google Scholar] [CrossRef]
- Nishino, T.; Okamoto, K.; Kawaguchi, Y.; Matsumura, T.; Eger, B.T.; Pai, E.F.; Nishino, T. The C-terminal peptide plays a role in the formation of an intermediate form during the transition between xanthine dehydrogenase and xanthine oxidase. FEBS J. 2015, 282, 3075–3090. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Jesionowska, A.; Klaudyna, S.; Katarzyna, S.; Dominika, M.; Dominika, P.; Marta, U.; Dołęgowska, B. Xanthine oxidoreductase reference values in platelet-poor plasma and platelets in healthy volunteers. Oxid. Med. Cell Longev. 2015, 2015, 341926. [Google Scholar] [CrossRef]
- McKelvey, T.G.; Höllwarth, M.E.; Granger, D.N.; Engerson, T.D.; Landler, U.; Jones, H.P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am. J. Physiol. 1988, 254, G753–G760. [Google Scholar] [CrossRef]
- Battelli, M.G.; Abbondanza, A.; Stirpe, F. Effects of hypoxia and ethanol on xanthine oxidase of isolated rat hepatocytes: Conversion from D to O form and leakage from cells. Chem. Biol. Interact. 1992, 83, 73–84. [Google Scholar] [CrossRef]
- Aslan, M.; Ryan, T.M.; Adler, B.; Townes, T.M.; Parks, D.A.; Thompson, J.A.; Tousson, A.; Gladwin, M.T.; Patel, R.P.; Tarpey, M.M.; et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc. Natl. Acad. Sci. USA 2001, 98, 15215–15220. [Google Scholar] [CrossRef]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: Insights regarding where, when and how. Nitric Oxide 2013, 34, 19–26. [Google Scholar] [CrossRef]
- Johnson, R.J.; Lanaspa, M.A.; Gaucher, E.A. Uric acid: A danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: Evolutionary considerations. Semin. Nephrol. 2011, 31, 394–399. [Google Scholar] [CrossRef]
- Furuhashi, M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef]
- Xu, P.; LaVallee, P.; Hoidal, J.R. Repressed expression of the human xanthine oxidoreductase gene. E-box and TATA-like elements restrict ground state transcriptional activity. J. Biol. Chem. 2000, 275, 5918–5926. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2016, 5, 546–557. [Google Scholar] [CrossRef]
- Monks, J.; Dzieciatkowska, M.; Bales, E.S.; Orlicky, D.J.; Wright, R.M.; McManaman, J.L. Xanthine oxidoreductase mediates membrane docking of milk-fat droplets but is not essential for apocrine lipid secretion. J. Physiol. 2016, 594, 5899–5921. [Google Scholar] [CrossRef]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox. Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Lima, W.G.; Martins-Santos, M.E.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef]
- Neogi, T.; George, J.; Rekhraj, S.; Struthers, A.D.; Choi, H.; Terkeltaub, R.A. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis. Rheum. 2012, 64, 327–338. [Google Scholar] [CrossRef]
- Garattini, E.; Mendel, R.; Romão, M.J.; Wright, R.; Terao, M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: Structure, genetics, regulation, function and pathophysiology. Biochem. J. 2003, 372, 15–32. [Google Scholar] [CrossRef]
- Lin, J.; Xu, P.; LaVallee, P.; Hoidal, J.R. Identification of proteins binding to E-Box/Ku86 sites and function of the tumor suppressor SAFB1 in transcriptional regulation of the human xanthine oxidoreductase gene. J. Biol. Chem. 2008, 283, 29681–29689. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: A leading actor in cardiovascular disease drama. Redox. Biol. 2021, 48, 102195. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Li, J.M.; Shah, A.M. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1014–R1030. [Google Scholar] [CrossRef]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.; Estevez, A.; Chumley, P.; Aslan, M.; Marklund, S.; Parks, D.A.; Freeman, B.A. Binding of xanthine oxidase to vascular endothelium: Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999, 274, 4985–4994. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef]
- Daiber, A.; Andreadou, I.; Oelze, M.; Davidson, S.M.; Hausenloy, D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021, 163, 325–343. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- Patetsios, P.; Song, M.; Shutze, W.P.; Pappas, C.; Rodino, W.; Ramirez, J.A.; Panetta, T.F. Identification of uric acid and xanthine oxidase in atherosclerotic plaque1. Am. J. Cardiol. 2001, 88, 188–191. [Google Scholar] [CrossRef]
- Ganji, M.; Nardi, V.; Prasad, M.; Jordan, K.L.; Bois, M.C.; Franchi, F.; Zhu, X.Y.; Tang, H.; Young, M.D.; Lerman, L.O.; et al. Carotid plaques from symptomatic patients are characterized by local increase in xanthine oxidase expression. Stroke 2021, 52, 2792–2801. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.K.; Kelly, A.S.; Metzig, A.M.; Steinberger, J.; Johnson, L.A.A. Xanthine oxidase and cardiovascular risk in obese children. Child Obes. 2014, 10, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, Y.; Fujishima, Y.; Nishizawa, H.; Nakamura, T.; Akari, S.; Murase, T.; Saito, T.; Miyazaki, Y.; Nagao, H.; Fukuda, S.; et al. Increased plasma XOR activity induced by NAFLD/NASH and its possible involvement in vascular neointimal proliferation. JCI Insight 2021, 6, e144762. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Shirakabe, A.; Okazaki, H.; Shibata, Y.; Goda, H.; Shigihara, S.; Asano, K.; Tani, K.; Kiuchi, K.; Murase, T.; et al. Plasma xanthine oxidoreductase (XOR) activity in cardiovascular disease outpatients. Circ. Rep. 2020, 2, 104–112. [Google Scholar] [CrossRef]
- Landmesser, U.; Spiekermann, S.; Dikalov, S.; Tatge, H.; Wilke, R.; Kohler, C.; Harrison, D.G.; Hornig, B.; Drexler, H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: Role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 2002, 106, 3073–3078. [Google Scholar] [CrossRef]
- Okazaki, H.; Shirakabe, A.; Matsushita, M.; Shibata, Y.; Sawatani, T.; Uchiyama, S.; Tani, K.; Murase, T.; Nakamura, T.; Takayasu, T.; et al. Plasma xanthine oxidoreductase activity in patients with decompensated acute heart failure requiring intensive care. ESC Heart Fail. 2019, 6, 336–343. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Kinoshita, D.; Yokoyama, M.; Takahashi, T.; Toshima, T.; Sugai, T.; Murase, T.; Nakamura, T.; Nishiyama, S.; et al. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol. 2017, 228, 151–157. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, T.; Otaki, Y.; Shishido, T.; Murase, T.; Nakamura, T.; Kato, S.; Tamura, H.; Nishiyama, S.; Takahashi, H.; et al. Impact of plasma xanthine oxidoreductase activity in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 1735–1743. [Google Scholar] [CrossRef]
- Fujimura, Y.; Yamauchi, Y.; Murase, T.; Nakamura, T.; Fujita, S.I.; Fujisaka, T.; Ito, T.; Sohmiya, K.; Hoshiga, M.; Ishizaka, N. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS ONE 2017, 12, e0182699. [Google Scholar] [CrossRef]
- Nakatani, A.; Nakatani, S.; Ishimura, E.; Murase, T.; Nakamura, T.; Sakura, M.; Tateishi, Y.; Tsuda, A.; Kurajoh, M.; Mori, K.; et al. Xanthine oxidoreductase activity is associated with serum uric acid and glycemic control in hemodialysis patients. Sci. Rep. 2017, 7, 15416. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.R.; Struthers, A.D. Hyperuricemia and adverse outcomes in cardiovascular disease: Potential for therapeutic intervention. Am. J. Cardiovasc. Drugs 2003, 3, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Schillaci, G.; Reboldi, G.; Santeusanio, F.; Porcellati, C.; Brunetti, P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000, 36, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kang, H.J.; Kim, D.A.; Kim, M.J.; Ryu, E.S.; Lee, S.; Ryu, J.H.; Roncal, C.; Johnson, R.J.; Kang, D.H. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019, 33, 13334–13345. [Google Scholar] [CrossRef]
- Nakatani, S.; Ishimura, E.; Murase, T.; Nakamura, T.; Nakatani, A.; Toi, N.; Nishide, K.; Uedono, H.; Tsuda, A.; Kurajoh, M.; et al. Plasma xanthine oxidoreductase activity associated with glycemic control in patients with pre-dialysis chronic kidney disease. Kidney Blood Press Res. 2021, 46, 475–483. [Google Scholar] [CrossRef]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Yoshida, S.; Kurajoh, M.; Fukumoto, S.; Murase, T.; Nakamura, T.; Yoshida, H.; Hirata, K.; Inaba, M.; Emoto, M. Association of plasma xanthine oxidoreductase activity with blood pressure affected by oxidative stress level: MedCity21 health examination registry: MedCity. Sci. Rep. 2020, 10, 4437. [Google Scholar] [CrossRef]
- Furuhashi, M.; Matsumoto, M.; Tanaka, M.; Moniwa, N.; Murase, T.; Nakamura, T.; Ohnishi, H.; Saitoh, S.; Shimamoto, K.; Miura, T. Plasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ. J. 2018, 82, 1892–1899. [Google Scholar] [CrossRef]
- Kotozaki, Y.; Satoh, M.; Tanno, K.; Ohmomo, H.; Otomo, R.; Tanaka, F.; Nasu, T.; Taguchi, S.; Kikuchi, H.; Kobayashi, T.; et al. Plasma xanthine oxidoreductase activity is associated with a high risk of cardiovascular disease in a General Japanese population. Int. J. Environ. Res. Public Health 2021, 18, 1894. [Google Scholar] [CrossRef]
- Taguchi, S.; Nasu, T.; Satoh, M.; Kotozaki, Y.; Tanno, K.; Tanaka, F.; Asahi, K.; Ohmomo, H.; Kikuchi, H.; Kobayashi, T.; et al. Association between plasma xanthine oxidoreductase activity and the renal function in a general Japanese population: The Tohoku Medical Megabank community-based cohort study. Kidney Blood Press Res. 2022, 47, 722–728. [Google Scholar] [CrossRef]
- Amaya, Y.; Yamazaki, K.; Sato, M.; Noda, K.; Nishino, T.; Nishino, T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. Amino acid sequence of rat liver xanthine dehydrogenase and identification of the cleavage sites of the enzyme protein during irreversible conversion by trypsin. J. Biol. Chem. 1990, 265, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Suvorava, T.; Kojda, G. Reactive oxygen species as cardiovascular mediators: Lessons from endothelial-specific protein overexpression mouse models. Biochim. Biophys. Acta 2009, 1787, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Maeda, N.; Shimomura, I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens. Res. 2022, 45, 635–640. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Bolognesi, A. Chronic kidney disease: Which role for xanthine oxidoreductase activity and products? Pharmacol. Res. 2022, 184, 106407. [Google Scholar] [CrossRef]
- Furuhashi, M.; Koyama, M.; Higashiura, Y.; Murase, T.; Nakamura, T.; Matsumoto, M.; Sakai, A.; Ohnishi, H.; Tanaka, M.; Saitoh, S.; et al. Differential regulation of hypoxanthine and xanthine by obesity in a general population. J. Diabetes Investig. 2020, 11, 878–887. [Google Scholar] [CrossRef]
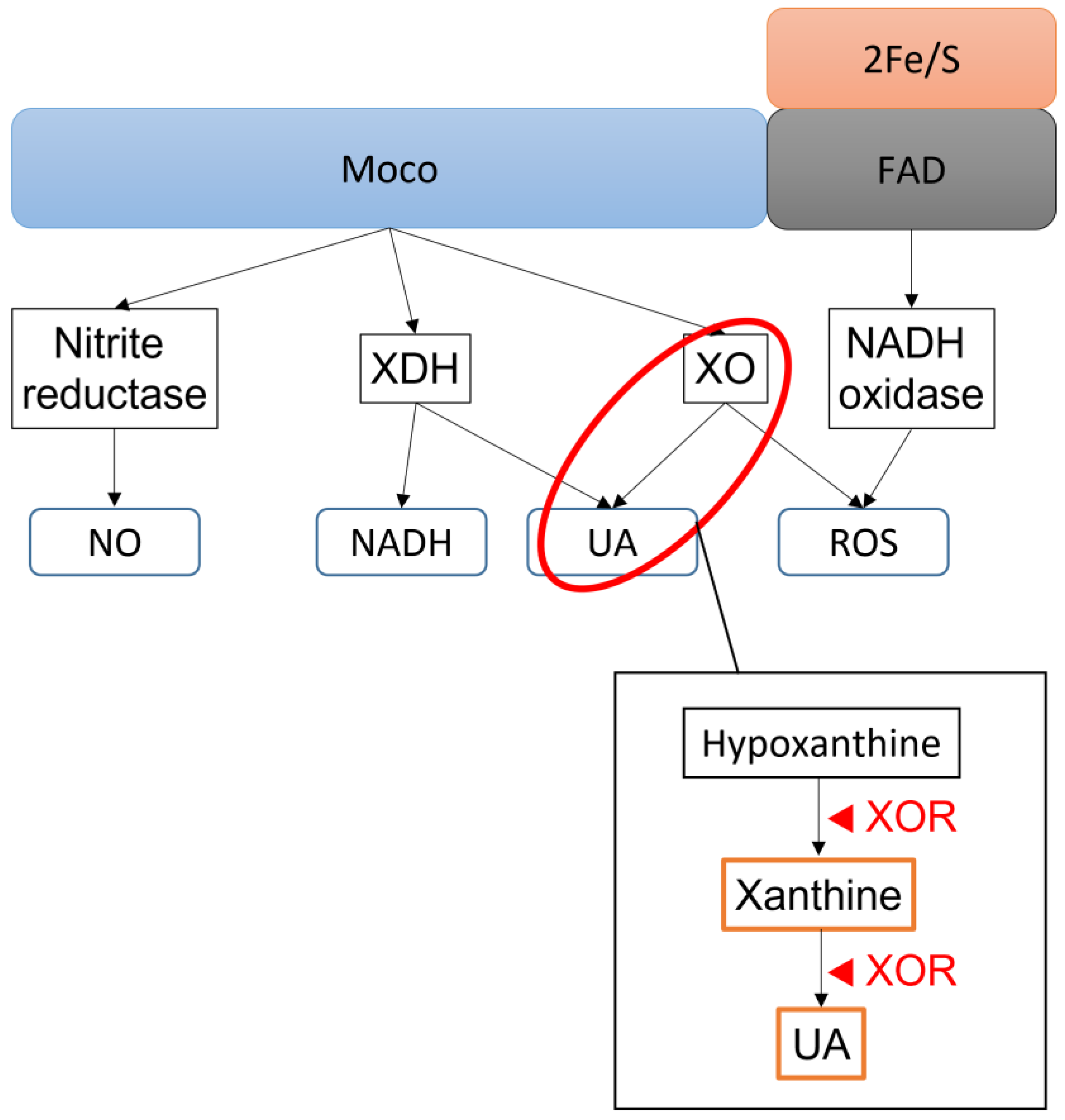
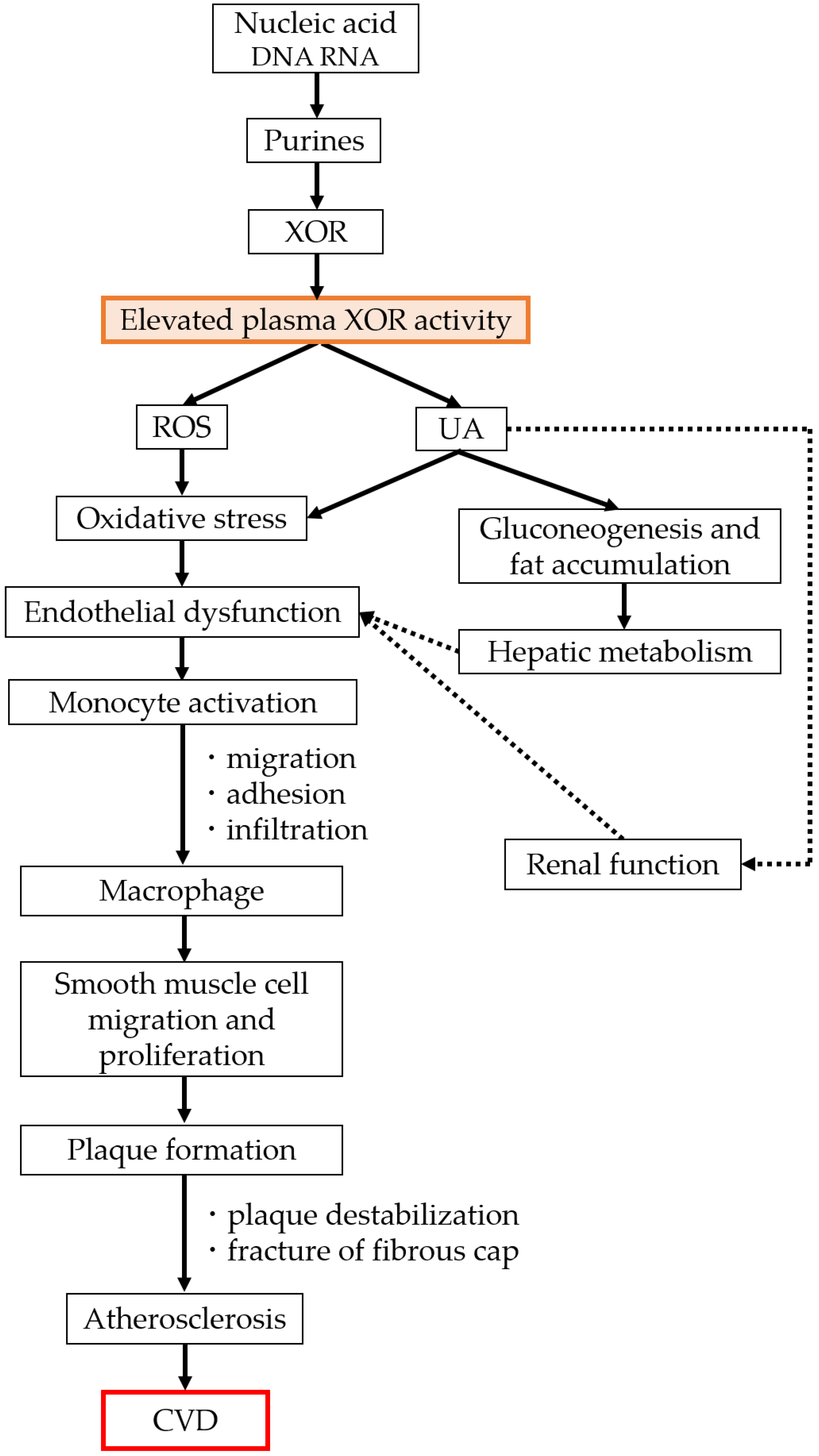
| Authors | Year | Study Group | Results |
|---|---|---|---|
| Tam H.K. et al. [52] | 2014 | 42 children and adolescents | A 3.8-fold increase in plasma XOR activity in obese children compared to healthy-weight children (p < 0.001). Plasma XOR activity was correlated with BMI z-score (r = 0.41), waist circumference (r = 0.41), HDL cholesterol (r = −0.32), oxidized LDL (r = 0.57), adiponectin (r = −0.53), and monocyte chemotactic protein-1 (r = −0.59). |
| Kawachi Y. et al. [53] | 2021 | 12 morbidly obese patients who underwent bariatric surgery | One year after bariatric surgery, plasma XOR activity decreased significantly (p = 0.0001). Changes in AST and ALT, but not in BMI, had significant positive associations with changes in plasma XOR activity both 1 week (AST: r = 0.94; p < 0.0001, ALT: r = 0.91; p < 0.0001) and 1 year (AST: r = 0.76; p = 0.004, ALT: r = 0.80; p = 0.002) after the surgery. |
| Matsushita M. et al. [54] | 2020 | 301 outpatients with CVD | Obese cardiovascular patients with diabetes were independently associated with higher plasma XOR activity (OR, 2.683; 95% CI: 1.441–4.996). BMI was independently associated with high plasma XOR activity (OR, 1.340; 95% CI: 1.149–1.540). Plasma hydrogen peroxide was significantly higher in diabetes patients with high plasma XOR activity and obesity (>22 kg/m2) than in other patients (median, 2967 vs. 2725 relative fluorescence unit). |
| Landmesser U. et al. [55] | 2002 | 14 patients with CHF and 10 control participants | Patients with HF were found to have markedly reduced endothelium-associated SOD activity (5.0 ± 0.7 vs. 14.4 ± 2.6 U mL−1 min−1; p < 0.01). Patients with HF had over 200% more endothelium-bound XOR activity compared to controls (38 ± 10 vs. 12 ± 4 nmol O2·− μL−1; p < 0.05). |
| Okazaki H. et al. [56] | 2019 | 118 AHF patients and 231 controls who attended a cardiovascular outpatient clinic | Plasma XOR activity in the AHF group was significantly higher than that in the control group (104.0 vs. 45.2 pmol/h/mL; p < 0.001). Serum UA (per 1.0 mg/dL increase, OR: 1.28; 95% CI: 1.07–1.54; p = 0.008) and lactate levels (per 1.0 mmol/L increase, OR: 1.24; 95% CI: 1.04–1.48; p = 0.016) were independently associated with high plasma XOR activity during the acute phase of AHF. |
| Otaki Y. et al. [57] | 2017 | 440 patients with CHF and 44 control participants | Both high and low levels of XOR activity were significantly associated with cardiac events in patients with CHF after adjusting for confounding risk factors, including serum UA and loop diuretic use (both p < 0.0001). The cardiac event rate was significantly higher in patients with either high or low XOR activity (high XOR activity: p = 0.0007; low XOR activity: p = 0.0729). The net reclassification index was significantly improved by adding XOR activity to the basic risk factors (C-index = 0.807; p = 0.0006). |
| Watanabe K. et al. [58] | 2020 | 257 patients with HFpEF | High XOR activity was significantly associated with major adverse cardiovascular events (MACEs) after adjusting for confounding factors (HR: 3.6; 95% CI: 1.68–8.12; p < 0.001). High XOR activity was associated with MACEs, regardless of the hyperuricemia status (HR: 4.0 to 4.4; p < 0.001). |
| Fujimura Y. et al. [59] | 2017 | 270 patients with CVD without UA-lowering drug treatment | XOR activity was associated with BMI (p = 0.004), ALT (p < 0.001), HbA1c (p < 0.001) and renal function (p < 0.001). Compared with patients with the lowest XOR activity quartile, those with the higher three XOR activity quartiles more frequently had left ventricular hypertrophy (p = 0.031, by χ2 test). Plasma XOR activity showed a U-shaped association with low left ventricular ejection fraction (p = 0.071) and increased plasma B-type natriuretic peptide levels (p < 0.001), and these associations were independent of age, gender, BMI, ALT, HbA1C, serum UA, and CKD stage. |
| Nakatani A. et al. [60] | 2017 | 163 hemodialysis patients | Plasma glucose (r = 0.23, p = 0.003) and serum UA levels (r = 0.23, p = 0.003) correlated significantly and positively with plasma XOR activity. Diabetes (β = 0.16, p = 0.028) and plasma glucose (β = 0.30, p < 0.001) were significantly, independently, and positively associated with plasma XOR activity. Serum UA (r = 0.29, p = 0.007) significantly and positively correlated with plasma XOR activity in hemodialysis patients without diabetes; plasma glucose (r = 0.34, p = 0.003) and serum glycated albumin (r = 0.29, p = 0.015) significantly and positively correlated with plasma XOR activity in those with diabetes. Serum UA was significantly and independently associated with plasma XOR activity in those without diabetes (β = 0.20, p = 0.042). |
| Nakatani S. et al. [65] | 2021 | 118 pre-dialysis CKD patients | eGFR (β = 0.22, p = 0.028) and HbA1c (β = 0.33, p < 0.001) were significantly, independently, and positively associated with plasma XOR activity after adjusting for several confounders (R2 = 0.26, p < 0.001), as were eGFR (β = 0.28, p = 0.007) and plasma glucose (β = 0.25, p = 0.007) (R2 = 0.22, p < 0.001) Plasma XOR activity was significantly higher in CKD patients with diabetes than in those without diabetes (62.7 vs. 25.7 pmol/h/mL, p < 0.001). The association between glycemic control and plasma XOR activity was significant even in CKD patients without uric acid-lowering drug treatment (R2 = 0.44, p < 0.001). |
| Gondouin B. et al. [66] | 2015 | 51 CKD and 50 hemodialysis patients were compared to 38 matched healthy controls | XOR activity was negatively correlated with SOD (r = −0.2, p = 0.04) and positively correlated with malondialdehyde (r = 0.3, p = 0.004). XOR activity was an independent predictor of cardiovascular events in CKD and hemodialysis patients, regardless of UA levels (HR: 1.55; 95% CI: 1.09–2.19; p < 0.05). |
| Authors | Year | Study Group | Results |
|---|---|---|---|
| Yoshida S. et al. [67] | 2020 | 156 participants registered in the health examination registry | Plasma XOR activity, but not serum UA, was significantly associated with mean arterial pressure (MAP) (β = 0.21, p = 0.019). Plasma XOR activity was shown to be significantly and positively associated with MAP in patients with a lower derivative of reactive oxygen metabolite levels (β = 0.43, p < 0.001). |
| Furuhashi M. et al. [68] | 2018 | 627 participants registered in a population-based cohort | Plasma XOR activity was significantly higher in males than in females (43 vs. 32 pmol/h/mL plasma, p = 0.002), and habitual smoking was associated with elevation of activity (p < 0.05). BMI (r = 0.32, p < 0.001), waist circumference (r = 0.29, p < 0.001), levels of liver enzymes, including alanine transaminase (r = 0.69, p < 0.001), UA (r = 0.25, p < 0.001), triglycerides (r = 0.31, p < 0.001), and HOMA-IR (r = 0.24, p < 0.001), were independent predictors of plasma XOR activity after adjusting for age and gender. |
| Kotozaki Y. et al. [69] | 2021 | 1631 participants registered in a community-based cohort | Plasma XOR activity was significantly higher in males than in females (43.7 vs. 31.6 pmol/h/mL plasma, p < 0.001). Plasma XOR activity was independently associated with BMI (β = 0.26, p < 0.001), diabetes (β = 0.09, p < 0.001), dyslipidemia (β = 0.08, p = 0.001), and UA (β = 0.13, p < 0.001). The highest quartile of plasma XOR activity was associated with a high risk for CVD (Framingham risk score ≥15) after adjusting for baseline characteristics (OR, 2.93; 95% CI: 1.16–7.40). The area under the receiver operating characteristic curve of the Framingham risk score with XOR activity was 0.81 (p = 0.008). |
| Taguchi S. et al. [70] | 2022 | 4248 participants registered in a community-based cohort | Blood pressure, BMI, UA, LDL cholesterol, and HbA1c were highest in the highest plasma XOR quartile (all p < 0.001). Plasma XOR activity was significantly higher in the subgroup with CKD stage G3 and G4 (G1 vs. G2 vs. G3–G4: 44.8 ± 40.5 vs. 52.0 ± 42.9 vs. 54.1 ± 43.9 pmol/h/mL, p = 0.02). The odds ratios (95% CIs) per 1 pmol/h/mL increase in XOR activity with CKD stage G1 as a reference were 1.37 (1.13–1.73) in G2 and 1.51 (1.30–1.84) in G3–G4. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotozaki, Y.; Satoh, M.; Nasu, T.; Tanno, K.; Tanaka, F.; Sasaki, M. Human Plasma Xanthine Oxidoreductase Activity in Cardiovascular Disease: Evidence from a Population-Based Study. Biomedicines 2023, 11, 754. https://doi.org/10.3390/biomedicines11030754
Kotozaki Y, Satoh M, Nasu T, Tanno K, Tanaka F, Sasaki M. Human Plasma Xanthine Oxidoreductase Activity in Cardiovascular Disease: Evidence from a Population-Based Study. Biomedicines. 2023; 11(3):754. https://doi.org/10.3390/biomedicines11030754
Chicago/Turabian StyleKotozaki, Yuka, Mamoru Satoh, Takahito Nasu, Kozo Tanno, Fumitaka Tanaka, and Makoto Sasaki. 2023. "Human Plasma Xanthine Oxidoreductase Activity in Cardiovascular Disease: Evidence from a Population-Based Study" Biomedicines 11, no. 3: 754. https://doi.org/10.3390/biomedicines11030754
APA StyleKotozaki, Y., Satoh, M., Nasu, T., Tanno, K., Tanaka, F., & Sasaki, M. (2023). Human Plasma Xanthine Oxidoreductase Activity in Cardiovascular Disease: Evidence from a Population-Based Study. Biomedicines, 11(3), 754. https://doi.org/10.3390/biomedicines11030754







