Histological Type, Cytotoxic T Cells and Macrophages in the Tumor Microenvironment Affect the PD-L1 Status of Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Immunohistochemistry
2.3. Immunofluorescence
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinicopathological Characteristics
3.2. Correlation between PD-L1 Status of Gastric Cancer and Clinicopathological Parameters
3.3. Correlation of IFN-γ Expression in Gastric Cancer with Clinicopathological Parameters
3.4. Analysis of Overall Survival of Patients Who Underwent Gastrectomy Due to GC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Telese, A.; Marasco, G.; Bazzoli, F.; Zagari, R.M. Gastric cancer prevention strategies: A global perspective. J. Gastroenterol. Hepatol. 2020, 35, 1495–1502. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libanio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, W.J.; Wang, Z.Q. Cancer Stem Cells and the Tumor Microenvironment in Gastric Cancer. Front. Oncol. 2021, 11, 803974. [Google Scholar] [CrossRef]
- Tajaldini, M.; Saeedi, M.; Amiriani, T.; Amiriani, A.H.; Sedighi, S.; Mohammad Zadeh, F.; Dehghan, M.; Jahanshahi, M.; Zanjan Ghandian, M.; Khalili, P.; et al. Cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs); where do they stand in tumorigenesis and how they can change the face of cancer therapy? Eur. J. Pharmacol. 2022, 928, 175087. [Google Scholar] [CrossRef]
- Larionova, I.; Cherdyntseva, N.; Liu, T.; Patysheva, M.; Rakina, M.; Kzhyshkowska, J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 2019, 8, 1596004. [Google Scholar] [CrossRef] [Green Version]
- Yaping, W.; Zhe, W.; Zhuling, C.; Ruolei, L.; Pengyu, F.; Lili, G.; Cheng, J.; Bo, Z.; Liuyin, L.; Guangdong, H.; et al. The soldiers needed to be awakened: Tumor-infiltrating immune cells. Front. Genet. 2022, 13, 988703. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, 1498–1512.e1495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020, 11, 622509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, C.; Song, W.; Li, J.; Zhao, G.; Cao, H. PD-L1 Expression and CD8(+) T Cell Infiltration Predict a Favorable Prognosis in Advanced Gastric Cancer. J. Immunol. Res. 2018, 2018, 4180517. [Google Scholar] [CrossRef] [Green Version]
- Fuertes Marraco, S.A.; Neubert, N.J.; Verdeil, G.; Speiser, D.E. Inhibitory Receptors Beyond T Cell Exhaustion. Front. Immunol. 2015, 6, 310. [Google Scholar] [CrossRef]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [Green Version]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Simundza, I.; Krnic, D.; Juricic, J.; Benzon, B.; Simundza, R.; Stanicic, I.M.; Capkun, V.; Vukojevic, K.; Glavina Durdov, M. Expression of PD-L1 Is Associated with Inflammatory Microenvironment in Surgical Specimens of Non-Small Cell Lung Cancer. J. Pers. Med. 2021, 11, 767. [Google Scholar] [CrossRef]
- Mashukov, A.; Shapochka, D.; Seleznov, O.; Kobyliak, N.; Falalyeyeva, T.; Kirkilevsky, S.; Yarema, R.; Sulaieva, O. Histological differentiation impacts the tumor immune microenvironment in gastric carcinoma: Relation to the immune cycle. World J. Gastroenterol. 2021, 27, 5259–5271. [Google Scholar] [CrossRef]
- Koh, J.; Ock, C.Y.; Kim, J.W.; Nam, S.K.; Kwak, Y.; Yun, S.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Kim, W.H.; et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget 2017, 8, 26356–26367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Zhu, Y.; Jiang, J.; Zhao, J.; Zhang, X.G.; Xu, N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006, 108, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lai, Y.; Sun, L.; Zhang, X.; Liu, R.; Feng, G.; Zhou, L.; Jia, L.; Huang, X.; Kang, Q.; et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum. Pathol. 2016, 55, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, K.; Chen, Z.; Chen, L.; Guo, W.; Liao, P.; Rotroff, D.; Knepper, T.C.; Liu, Z.; Zhang, W.; et al. Immunoclassification characterized by CD8 and PD-L1 expression is associated with the clinical outcome of gastric cancer patients. Oncotarget 2018, 9, 12164–12173. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, S.; Sahnane, N.; Tibiletti, M.G.; Magnoli, F.; Vanoli, A.; Sessa, F.; Chiaravalli, A.M. EBV(+) and MSI Gastric Cancers Harbor High PD-L1/PD-1 Expression and High CD8(+) Intratumoral Lymphocytes. Cancers 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.S.; da Silva, E.; Coit, D.G.; Tang, L.H. Intratumoral Immune Response to Gastric Cancer Varies by Molecular and Histologic Subtype. Am. J. Surg. Pathol. 2019, 43, 851–860. [Google Scholar] [CrossRef]
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann. Surg. Oncol. 2019, 26, 876–883. [Google Scholar] [CrossRef]
- Oki, E.; Okano, S.; Saeki, H.; Umemoto, Y.; Teraishi, K.; Nakaji, Y.; Ando, K.; Zaitsu, Y.; Yamashita, N.; Sugiyama, M.; et al. Protein Expression of Programmed Death 1 Ligand 1 and HER2 in Gastric Carcinoma. Oncology 2017, 93, 387–394. [Google Scholar] [CrossRef]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Osaki, L.H.; Bockerstett, K.A.; Wong, C.F.; Ford, E.L.; Madison, B.B.; DiPaolo, R.J.; Mills, J.C. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J. Pathol. 2019, 247, 513–523. [Google Scholar] [CrossRef]
- Mao, X.C.; Yang, C.C.; Yang, Y.F.; Yan, L.J.; Ding, Z.N.; Liu, H.; Yan, Y.C.; Dong, Z.R.; Wang, D.X.; Li, T. Peripheral cytokine levels as novel predictors of survival in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 884592. [Google Scholar] [CrossRef]
- Guo, L.; Overholser, J.; Darby, H.; Ede, N.J.; Kaumaya, P.T.P. A newly discovered PD-L1 B-cell epitope peptide vaccine (PDL1-Vaxx) exhibits potent immune responses and effective anti-tumor immunity in multiple syngeneic mice models and (synergizes) in combination with a dual HER-2 B-cell vaccine (B-Vaxx). Oncoimmunology 2022, 11, 2127691. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Zauco, N.; Torres, J.; Gomez, A.; Camorlinga-Ponce, M.; Munoz-Perez, L.; Herrera-Goepfert, R.; Medrano-Guzman, R.; Giono-Cerezo, S.; Maldonado-Bernal, C. Circulating blood levels of IL-6, IFN-gamma, and IL-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer 2017, 17, 384. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Chiba, T.; Kondo, T.; Kanzaki, H.; Kanayama, K.; Ao, J.; Kojima, R.; Kusakabe, Y.; Nakamura, M.; Saito, T.; et al. Interferon-gamma induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol. Lett. 2020, 20, 2161–2168. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404.e399. [Google Scholar] [CrossRef] [Green Version]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Xu, C.; Yang, W.; Chi, Z.; Sheng, X.; Si, L.; Xie, Y.; Yu, J.; Wang, S.; Yu, R.; et al. Ratio of the interferon-gamma signature to the immunosuppression signature predicts anti-PD-1 therapy response in melanoma. NPJ Genom. Med. 2021, 6, 7. [Google Scholar] [CrossRef] [PubMed]
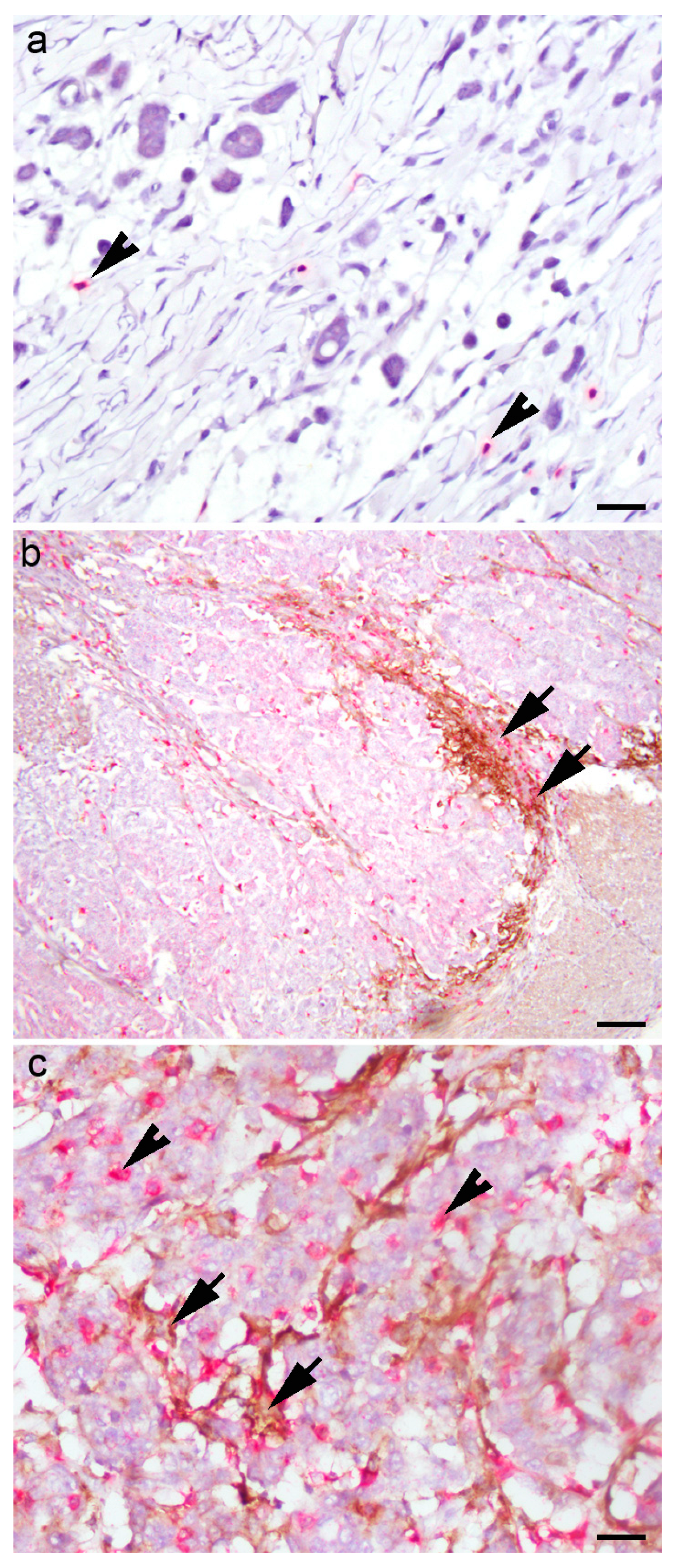
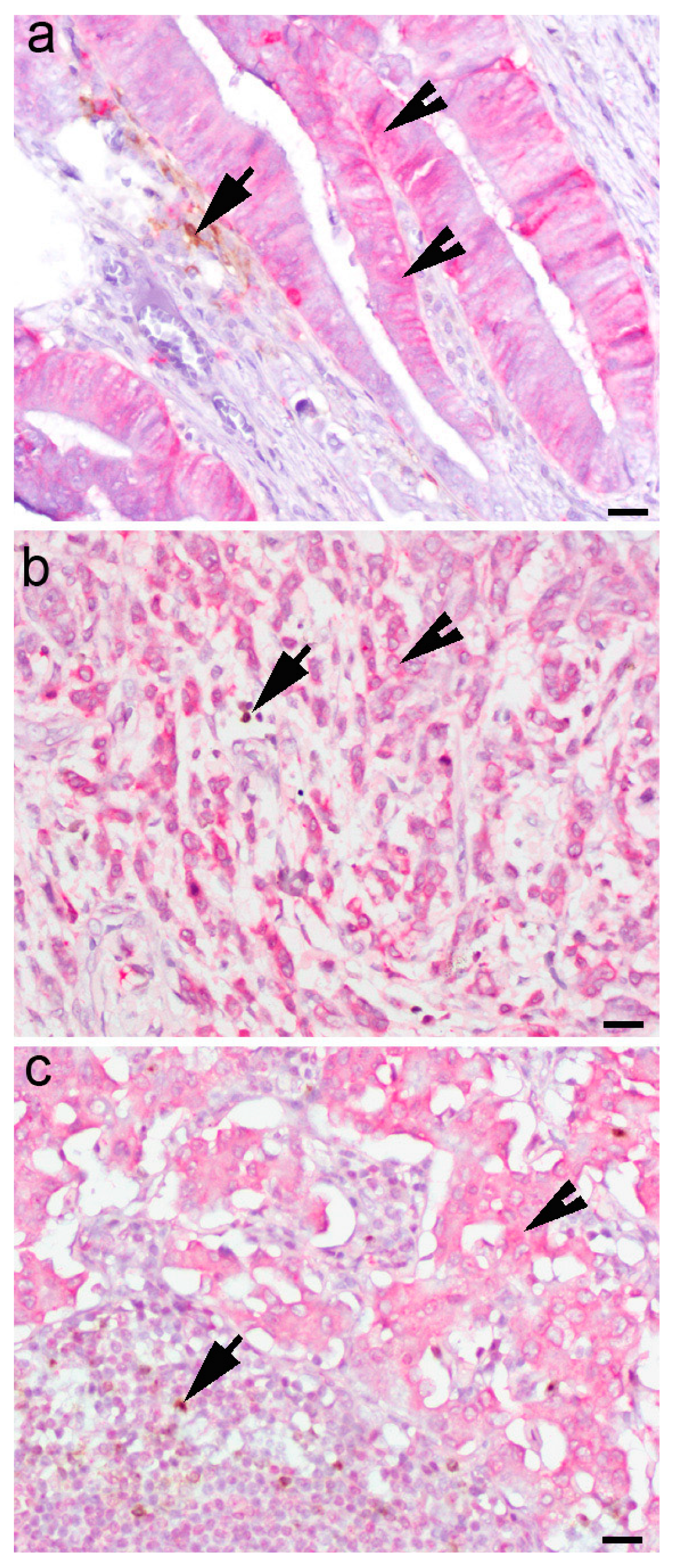




| Variable | N (%) | |
|---|---|---|
| Gender | male | 66 (68) |
| female | 34 (32) | |
| Histologic type | intestinal | 61 (63) |
| diffuse | 34 (35) | |
| mixed | 2 (2) | |
| Feature | polypoid | 16 (16.5) |
| fungoid | 40 (41.2) | |
| ulcerative | 17 (17.5) | |
| flat/infiltrative | 24 (24.7) | |
| Stage | IA | 10 (10.3) |
| IB | 4 (4.1) | |
| IIA | 14 (14.4) | |
| IIB | 17 (17.5) | |
| IIIA | 24 (24.7) | |
| IIIB | 25 (25.8) | |
| IVB | 3 (3.1) | |
| IFN-γ in tumor cells | negative | 60 (61.9) |
| low | 17 (17.5) | |
| moderate | 9 (9.3) | |
| strong | 11 (11.3) | |
| IFN-γ in metastasis | negative | 63 (64.9) |
| low | 14 (14.4) | |
| moderate | 10 (10.3) | |
| strong | 10 (10.3) | |
| CTLA-4+ in TI | M Q1-Q3; min–max | 0 (0–3; 0–35) |
| CTLA4++/IFNγ+ | 1 (1–3; 0–55) | |
| CTLA-4+ in IM | 0 (0–1; 0–26) | |
| CTLA4++/IFNγ+ | 0 (0–2; 0–74) | |
| CTLA-4+ in LN | 0 (0–1; 0–11) | |
| CTLA4++/IFNγ+ | 0 (0–1; 0–20) | |
| PD-L1 | negative | 66 (68) |
| low | 18 (18.6) | |
| moderate | 10 (10.3) | |
| strong | 3 (3.1) | |
| CD8+ T-cell | 22 (11–38; 3–186) | |
| macrophages | negative | 48 (49.5) |
| low | 23 (23.7) | |
| moderate | 13 (13.4) | |
| strong | 13 (13.4) |
| PD-L1 Status of Tumor | p | OR (95 CI) | p Log-Rank Test | ||
|---|---|---|---|---|---|
| Negative N = 66 (68.1%) | Positive N = 31 (31.9%) | ||||
| Age (years) | 73:64–79; 46–85 | (76:67–82; 51–87) | 0.181† | ||
| Gender | |||||
| m | 47 (71) | 19 (61) | 0.457 * | ||
| f | 19 (29) | 12 (39) | |||
| Histologic type | |||||
| intestinal | 38 (58) | 25 (81) | 0.046 * | (3.1; 1.1–8.5) | 0.030 |
| diffuse | 28 (42) | 6 (19) | |||
| feature | 0.239 * | ||||
| polypoid | 9 (13.6) | 7 (22.6) | |||
| fungating | 25 (37.9) | 15 (48.4) | |||
| ulcerated | 12 (18.2) | 5 (16.1) | |||
| flat/infiltrating | 20 (30.3) | 4 (12.9) | |||
| Stage | 0.057 * | ||||
| IA, IB | 11 (17) | 3 (10) | |||
| IIA, IIB | 16 (24) | 15 (48) | |||
| IIIA, IIIB, IVB | 39 (59) | 13 (42) | |||
| CD8+ T cells | 15:9–27; 3–118 | (39:27–56; 3–186) | <0.001 † | (9.8; 3.6–26) | 0.001 |
| ≥28.5 | 51 (77) | 8 (26) | |||
| <28.5 | 15 (23) | 23 (74) | |||
| IFN-γ in tumor cells | 0.181 * | ||||
| negative | 40 (60.6) | 20 (64.5) | |||
| low | 9 (13.6) | 8 (25) | |||
| moderate | 7 (10.6) | 2 (6.5) | |||
| strong | 10 (15.2) | 1 (3.2) | |||
| INF-γ in metastasis | 0.122 * | ||||
| negative | 60 (60.6) | 23 (74.2) | |||
| low | 8 (12.1) | 6 (19.4) | |||
| moderate | 9 (13.6) | 1 (3.2) | |||
| strong | 9 (13.6) | 1 (3.2) | |||
| Macrophages | <0.001 * | (13.5; 4.2–43) | |||
| negative | 44 (67) | 4 (13) | - | ||
| low, moderate, strong | 22 (33) | 27 (87) | |||
| CTLA-4 in TI * | (0:0–4; 0–35) | (0:0–2; 0–20) | 0.159 † | ||
| CTLA-4 | 0.188 * | ||||
| negative | 36 (54) | 22 (71) | |||
| positive | 30 (46) | 9 (29) | |||
| CTLA-4 in IM * | 0:0–1; 0–26 | (0:0–0; 0–7) | 0.197 † | ||
| CTLA-4 | |||||
| negative | 45 (68) | 25(81) | 0.301 * | ||
| positive | 21 (32) | 6(19) | |||
| CTLA-4 lymph node | 0:0–1; 0–11 | (0:0–0; 0–4) | 0.292 † | ||
| CTLA-4 | |||||
| negative | 40 (61) | 15 (48) | 0.361 * | ||
| positive | 26 (39) | 16 (52) | |||
| Variable | OR (95%CI) | p |
|---|---|---|
| Histologic type | 0.429 (0.12–1.5) | 0.182 |
| intestinal | ||
| diffuse | ||
| Macrophage infiltration | 9.8(2.7–35) | <0.001 |
| no | ||
| mild to strong | ||
| CD8+ T cells | 7 (2.3–21) | <0.001 |
| ≥28.5 | ||
| >28.5 | ||
| Stage | 0.86 (0.38–2) | 0.731 |
| IA, IB | ||
| IIA, IIB | ||
| IIIA, IIIB, IVB |
| Lymph Node | Gastric Cancer | p | OR (95%CI) | p Log-Rank Test | |
|---|---|---|---|---|---|
| IFN-γ-Negative (N = 60) | IFN-γ-Positive (N = 37) | ||||
| CTLA-4+ lymphocytes | 0 (0–1; 0–10) | 1 (0–2; 0–11) | 0.027 | ||
| CTLA-4+ lymphocytes | 0.059 | 2.4 (1.1–5.6) | 0.037 | ||
| negative | 39 (65) | 16 (43) | |||
| positive | 21 (35) | 21 (57) | |||
| Area CTLA-4/IFN-γ Lymphocytes | PD-L1 Tumor Status | p | |
|---|---|---|---|
| Negative | Positive | ||
| TI | 0.638 | ||
| −/− | 24 (36.4) | 15 (48.4) | |
| +/− | 16 (24.2) | 5 (16.1) | |
| −/+ | 12 (18) | 6 (19.4) | |
| +/+ | 14 (21.2) | 5 (16.1) | |
| 0.612 | |||
| TM | |||
| −/− | 28 (42.4) | 17 (54.8) | |
| +/− | 12 (18.) | 3 (9.7) | |
| −/+ | 18 (27.3) | 8 (25.8) | |
| +/+ | 8 (12.1) | 3 (9.7) | |
| LN | 0.069 | ||
| −/− | 28 (42.4) | 10 (32.3) | |
| +/− | 10 (15.2) | 12 (38.7) | |
| −/+ | 12 (18.2) | 5 (16.1) | |
| +/+ | 16 (24.2) | 4 (12.9) | |
| Area | CTLA4/IFN-γ Lymphocytes | IFN-γ Tumor Cells | p | |
|---|---|---|---|---|
| No (60) | Yes (37) | |||
| TI | <0.001 | |||
| −/− | 39 (65) | 0 | ||
| +/− | 21 (35) | 0 | ||
| −/+ | 0 | 18 (49) | ||
| +/+ | 0 | 19(51) | ||
| TM | <0.001 | |||
| −/− | 45 (75) | 0 | ||
| +/− | 15 (25) | 0 | ||
| −/+ | 0 | 26 (70) | ||
| +/+ | 0 | 11 (30) | ||
| LN | <0.001 | |||
| −/− | 37 (61.7) | 1 (2.7) | ||
| −/+ | 19 (31.7) | 3 (8.1) | ||
| +/− | 2 (3.3) | 15 (40.5) | ||
| +/+ | 2 (3.3) | 18 (28.6) | ||
| Variable | General Survival (95%CI) | Median Overall Survival (95%CI) | LR; p |
|---|---|---|---|
| CTLA4+ cells, TA | 0.569; 0.451 | ||
| negative | 29 (4) (22–37) | 17.5 (2.4; 13–22) | |
| positive | 35 (5.5; 24–46) | 14.5 (9; 1–31) | |
| CTLA4+ cells, TI | 0.001; 0.976 | ||
| negative | 31.6 (3.7) (24–39) | 17.5 (4) (9.7–25) | |
| positive | 32 (6.5) (19.5–45) | 11.3 (6.8) (1–25) | |
| CTLA4+ cell, LN | 2.2; 0.137 | ||
| negative | 26 (3.7 (19–33)) | 14.5 (3.8) (7.1–22) | |
| positive | 37 (5) (27–47) | 22(6) (9.6–34) | |
| Gender | 0.818; 0.366 | ||
| male | 29 (3.7) (22–37) | 16 (4) (7.6–25) | |
| female | 35 (6) (24–47) | 17.5 (7.5) (3–32) | |
| Histologic type | 0.166; 0.684 | ||
| intestinal/mixed | 33 (4) (25–41) | 16 (3) (10–22) | |
| diffuse | 27 (4) (19–36) | 19 (7) (6–32) | |
| Macrophages | 2.3; 0.503 | ||
| polypoid | 21 (5.6) (10–32) | 11 (2) (7–15) | |
| fungate | 35 (5) (25–45) | 22 (6) (9–35) | |
| ulcerative | 23 (5) (13–34) | 15 (7) (1.6–29) | |
| flat/infiltrating | 36 (7) (22–49) | 19 (8) (3–35) | |
| Disease stage | 10.9; 0.004 | ||
| IA, IB | 55.8 (7) (42–69) | ||
| IIA, IIB | 28.3 (6) (17–39) | 15.7 (9) (1–33) | |
| IIIA, IIIB, IVB | 25.6 (4) (18–33) | 13 (2) (9–18) | |
| CD8 + T cells (mean) | 0.967; 0.326 | ||
| ≤28.5 | 29 (4) (21–37) | 14.5 (3) (8–20) | |
| >28.5 | 36 (5) (25.5–46) | 24 (11) (2–46) | |
| IFN-γ in tumor cells | 3.8; 0.286 | ||
| no | 32.4 (4) (24–40) | 18 (4) (10–26) | |
| low | 24 (6) (12–36) | 13.5 (6) (2–25) | |
| moderate | 17 (5) (6.6–28) | 14.5 (11) (0–36) | |
| strong | 47 (12) (24–70) | ||
| IFN-γ in metastasis | 2.5; 0.476 | ||
| no | 32.5 (4) (25–40) | 22 (4) (14–29) | |
| low | 24 (7) (9.6–38) | 11 (5) (1.5–20) | |
| moderate | 19.9 (7) (6–33) | 7 (7) (0–21) | |
| strong | 43 (12) (19–67) | 11 | |
| Macrophages | 0.667; 0.881 | ||
| no | 32 (4.6) (23–41) | 18.6 (5) (8–29) | |
| sparse | 33 (6) (20–45) | 16 (3.6) (8.7–23) | |
| moderate | 26 (8) (11–41) | 16 (10) (0–36) | |
| strong | 25 (6) (13–38) | 11 (14) (0–38) | |
| Macrophages | 0.09; 0.41 | ||
| no | 32 (4.6) (23–41) | 18.6 (5) (8–29) | |
| sparse to strong | 31 (4) (22–39) | 16 (3) (9–22) | |
| PD-L1 | 0.599; 0.439 | ||
| negative | 29 (4) (22–37) | 14.5 (4) (7–22) | |
| positive | 36 (6) (25–47) | 30 (9) (12–48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanović, T.; Božić, D.; Benzon, B.; Čapkun, V.; Vukojević, K.; Glavina Durdov, M. Histological Type, Cytotoxic T Cells and Macrophages in the Tumor Microenvironment Affect the PD-L1 Status of Gastric Cancer. Biomedicines 2023, 11, 709. https://doi.org/10.3390/biomedicines11030709
Ivanović T, Božić D, Benzon B, Čapkun V, Vukojević K, Glavina Durdov M. Histological Type, Cytotoxic T Cells and Macrophages in the Tumor Microenvironment Affect the PD-L1 Status of Gastric Cancer. Biomedicines. 2023; 11(3):709. https://doi.org/10.3390/biomedicines11030709
Chicago/Turabian StyleIvanović, Tomislav, Dorotea Božić, Benjamin Benzon, Vesna Čapkun, Katarina Vukojević, and Merica Glavina Durdov. 2023. "Histological Type, Cytotoxic T Cells and Macrophages in the Tumor Microenvironment Affect the PD-L1 Status of Gastric Cancer" Biomedicines 11, no. 3: 709. https://doi.org/10.3390/biomedicines11030709
APA StyleIvanović, T., Božić, D., Benzon, B., Čapkun, V., Vukojević, K., & Glavina Durdov, M. (2023). Histological Type, Cytotoxic T Cells and Macrophages in the Tumor Microenvironment Affect the PD-L1 Status of Gastric Cancer. Biomedicines, 11(3), 709. https://doi.org/10.3390/biomedicines11030709






