Could Local Application of Hypoxia Inducible Factor 1-α Enhancer Deferoxamine Be Promising for Preventing of Medication-Related Osteonecrosis of the Jaw?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care and Procedures
2.2. Histological Evaluation
2.2.1. Histopathological and Histomorphometric Examination
2.2.2. Immunohistochemical Staining and Evaluation
- 0
- (−): 0–10% staining immunopositivity
- 1
- (+): 10–25% staining immunopositivity
- 2
- (++): 25–50% staining immunopositivity
- 3
- (+++): 50–70% staining immunopositivity
- 4
- (++++): >75% staining immunopositivity
2.3. Statistical Evaluation
3. Results
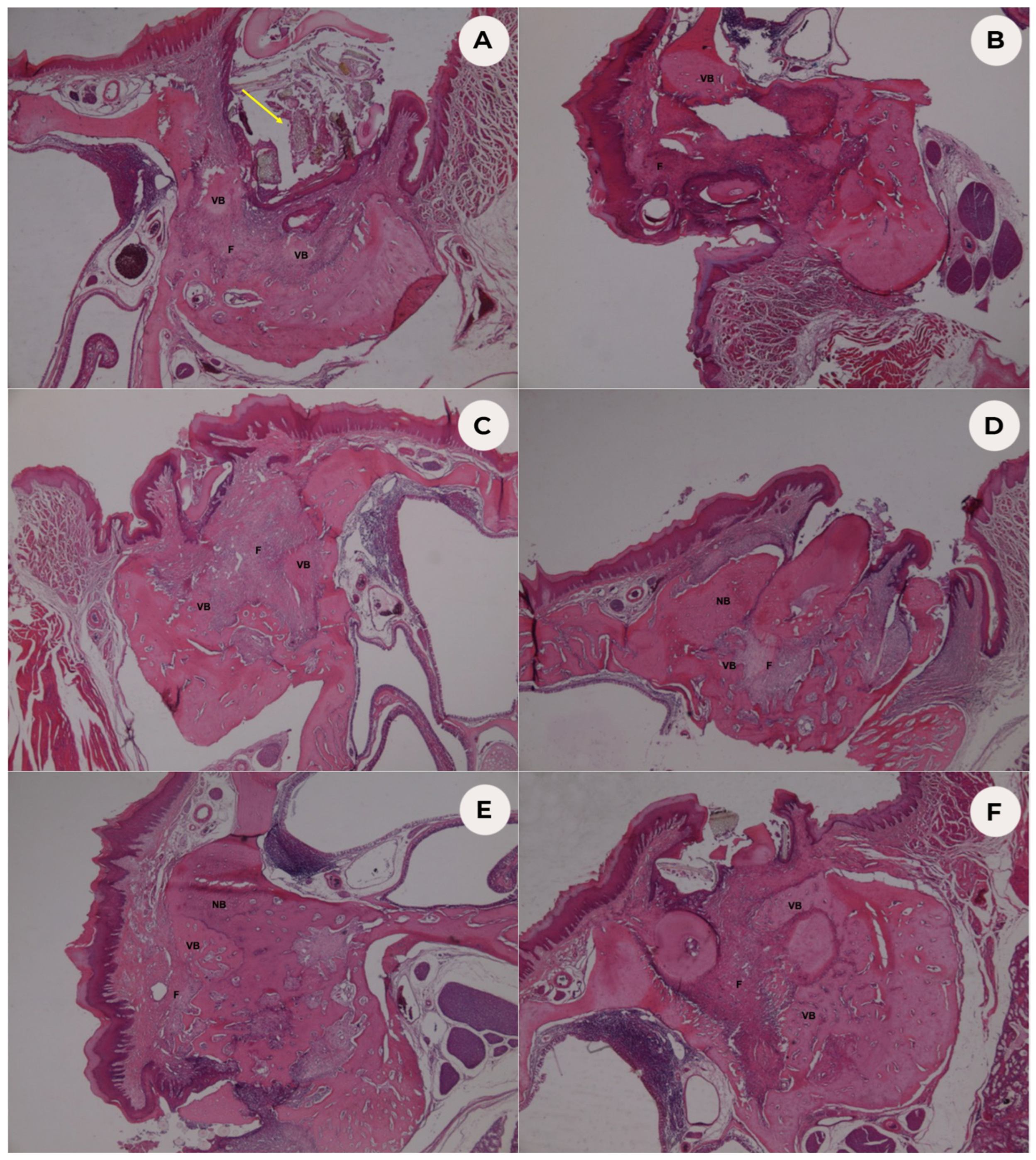
3.1. Histopathological Analysis
3.2. Histomorphometric Analysis
3.3. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nussbaum, S.R.; Younger, J.; Vandepol, C.J.; Gagel, R.F.; Zubler, M.A.; Chapman, R.; Henderson, I.C.; Mallette, L.E. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: Comparison of 30-, 60-, and 90-mg dosages. Am. J. Med. 1993, 95, 297–304. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Kapitola, J.; Zák, J. Effect of pamidronate on bone blood flow in oophorectomized rats. Physiol. Res. 1998, 47, 237–240. [Google Scholar]
- Santini, D.; Vincenzi, B.; Avvisati, G.; Dicuonzo, G.; Battistoni, F.; Gavasci, M.; Salerno, A.; Denaro, V.; Tonini, G. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin. Cancer Res. 2002, 8, 1080–1084. [Google Scholar] [CrossRef]
- Vincenzi, B.; Santini, D.; Rocci, L.; Tonini, G. Bisphosphonates: New antiangiogenic molecules in cancer treatment? Ann. Oncol. 2003, 14, 806–807. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Farberg, A.S.; Jing, X.L.; Monson, L.A.; Donneys, A.; Tchanque-Fossuo, C.N.; Deshpande, S.S.; Buchman, S.R. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone 2012, 50, 1184–1187. [Google Scholar] [CrossRef] [Green Version]
- Donneys, A.; Weiss, D.M.; Deshpande, S.S.; Ahsan, S.; Tchanque-Fossuo, C.N.; Sarhaddi, D.; Levi, B.; Goldstein, S.A.; Buchman, S.R. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone 2013, 52, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef]
- Donneys, A.; Yang, Q.; Forrest, M.L.; Nelson, N.S.; Zhang, T.; Ettinger, R.; Ranganathan, K.; Snider, A.; Deshpande, S.S.; Cohen, M.S.; et al. Implantable hyaluronic acid-deferoxamine conjugate prevents nonunions through stimulation of neovascularization. NPJ Regen. Med. 2019, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Tamari, T.; Elimelech, R.; Cohen, G.; Cohen, T.; Doppelt, O.; Eskander-Hashoul, L.; Zigdon-Giladi, H. Endothelial Progenitor Cells inhibit jaw osteonecrosis in a rat model: A major adverse effect of bisphosphonate therapy. Sci. Rep. 2019, 9, 18896. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Hamlet, S.; Vaquette, C.; Petcu, E.B.; Ramamurthy, P.; Ivanovski, S. Local delivery of hydrogel encapsulated vascular endothelial growth factor for the prevention of medication-related osteonecrosis of the jaw. Sci. Rep. 2021, 11, 23371. [Google Scholar] [CrossRef]
- Dayisoylu, E.H.; Üngör, C.; Tosun, E.; Ersöz, S.; Kadioglu Duman, M.; Taskesen, F.; Senel, F.Ç. Does an alkaline environment prevent the development of bisphosphonate-related osteonecrosis of the jaw? An experimental study in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 329–334. [Google Scholar] [CrossRef]
- Yalcin-Ulker, G.M.; Cumbul, A.; Duygu-Capar, G.; Uslu, Ü.; Sencift, K. Preventive Effect of Phosphodiesterase Inhibitor Pentoxifylline Against Medication-Related Osteonecrosis of the Jaw: An Animal Study. J. Oral Maxillofac. Surg. 2017, 75, 2354–2368. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Rissanen, T.T.; Vajanto, I.; Hartikainen, J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J. Am. Coll. Cardiol. 2007, 49, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Landesberg, R.; Woo, V.; Cremers, S.; Cozin, M.; Marolt, D.; Vunjak-Novakovic, G.; Kousteni, S.; Raghavan, S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 2011, 1218, 62–79. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wang, Z.; Han, W.; Li, H. Zoledronate induces autophagic cell death in human umbilical vein endothelial cells via Beclin-1 dependent pathway activation. Mol. Med. Rep. 2016, 14, 4747–4754. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Yang, S.S.; Kim, C.S.; Lee, J. Zoledronate suppresses VEGF-induced capillary tube formation and inhibits expression of interferon-induced transmembrane protein-1 in human umbilical vein endothelial cells. Int. J. Mol. Med. 2018, 41, 2879–2884. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.; Bonjean, K.; Ruetz, S.; Bellahcène, A.; Devy, L.; Foidart, J.M.; Castronovo, V.; Green, J.R. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J. Pharmacol. Exp. Ther. 2002, 302, 1055–1061. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Edwards, S.; Sankaralingam, A.; Evans, B.A.; Elford, C.; Frost, M.L.; Fogelman, I.; Hampson, G. The effect of nitrogen containing bisphosphonates, zoledronate and alendronate, on the production of pro-angiogenic factors by osteoblastic cells. Cytokine 2015, 71, 154–160. [Google Scholar] [CrossRef]
- Gkouveris, I.; Hadaya, D.; Soundia, A.; Bezouglaia, O.; Chau, Y.; Dry, S.M.; Pirih, F.Q.; Aghaloo, T.L.; Tetradis, S. Vasculature submucosal changes at early stages of osteonecrosis of the jaw (ONJ). Bone 2019, 123, 234–245. [Google Scholar] [CrossRef]
- Balooch, G.; Balooch, M.; Nalla, R.K.; Schilling, S.; Filvaroff, E.H.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O.; Derynck, R.; Alliston, T. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc. Natl. Acad. Sci. USA 2005, 102, 18813–18818. [Google Scholar] [CrossRef] [Green Version]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Ruiz, C.; García-Martínez, O. Bisphosphonate Modulation of the Gene Expression of Different Markers Involved in Osteoblast Physiology: Possible Implications in Bisphosphonate-Related Osteonecrosis of the Jaw. Int. J. Med. Sci. 2018, 15, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Göhringer, I.; Muller, C.L.S.; Cunha, E.J.; Passoni, G.N.S.; Vieira, J.S.; Zielak, J.C.; Scariot, R.; Deliberador, T.M.; Giovanini, A.F. Would Be Prophylactic Administrations of Low Concentration of Alendronate an Alternative for Improving the Craniofacial Bone Repair? A Preliminary Study Focused in the Period of Cellular Differentiation and Tissue Organization. J. Craniofac. Surg. 2017, 28, 1869–1873. [Google Scholar] [CrossRef]
- Kim, S.; Williams, D.W.; Lee, C.; Kim, T.; Arai, A.; Shi, S.; Li, X.; Shin, K.H.; Kang, M.K.; Park, N.H.; et al. IL-36 Induces Bisphosphonate-Related Osteonecrosis of the Jaw-Like Lesions in Mice by Inhibiting TGF-β-Mediated Collagen Expression. J. Bone Miner. Res. 2017, 32, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Kim, S.J.; Kim, M.R. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: A prospective feasibility study. Br. J. Oral Maxillofac. Surg. 2014, 52, 854–859. [Google Scholar] [CrossRef]
- Pelaz, A.; Junquera, L.; Gallego, L.; García-Consuegra, L.; Junquera, S.; Gómez, C. Alternative treatments for oral bisphosphonate-related osteonecrosis of the jaws: A pilot study comparing fibrin rich in growth factors and teriparatide. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e320-6. [Google Scholar] [CrossRef]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Giudice, A.; Barone, S.; Giudice, C.; Bennardo, F.; Fortunato, L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 390–403. [Google Scholar] [CrossRef]
- Poxleitner, P.; Steybe, D.; Kroneberg, P.; Ermer, M.A.; Yalcin-Ülker, G.M.; Schmelzeisen, R.; Voss, P.J. Tooth extractions in patients under antiresorptive therapy for osteoporosis: Primary closure of the extraction socket with a mucoperiosteal flap versus application of platelet-rich fibrin for the prevention of antiresorptive agent-related osteonecrosis of the jaw. J. Craniomaxillofac. Surg. 2020, 48, 444–451. [Google Scholar]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte- and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef]
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.B.; Pereira, E.S.B.M.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 3150. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Bielecki, T.; Dohan Ehrenfest, D.M. The Future of Platelet Concentrates in Sports Medicine: Platelet-Rich Plasma, Platelet-Rich Fibrin, and the Impact of Scaffolds and Cells on the Long-term Delivery of Growth Factors. Oper. Tech. Sports Med. 2011, 19, 190–197. [Google Scholar] [CrossRef]
- Dor, Y.; Djonov, V.; Abramovitch, R.; Itin, A.; Fishman, G.I.; Carmeliet, P.; Goelman, G.; Keshet, E. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 2002, 21, 1939–1947. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, C.R.; Banfi, A.; Glazer, N.L.; Thurston, G.; Springer, M.L.; Kraft, P.E.; McDonald, D.M.; Blau, H.M. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Investig. 2004, 113, 516–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tafuro, S.; Ayuso, E.; Zacchigna, S.; Zentilin, L.; Moimas, S.; Dore, F.; Giacca, M. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc. Res. 2009, 83, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Semenza, G.L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood 1993, 82, 3610–3615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Wan, C.; Ramaswamy, G.; Mavalli, M.; Wang, Y.; Duvall, C.L.; Deng, L.F.; Guldberg, R.E.; Eberhart, A.; Clemens, T.L.; et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J. Orthop. Res. 2009, 27, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.M.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Gerstenfeld, L.C.; Einhorn, T.A.; et al. Role of hypoxia inducible factor-1 alpha pathway in bone regeneration. J. Musculoskelet. Neuronal Interact. 2008, 8, 323–324. [Google Scholar]
- Chung, J.H.; Kim, Y.S.; Noh, K.; Lee, Y.M.; Chang, S.W.; Kim, E.C. Deferoxamine promotes osteoblastic differentiation in human periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J. Periodontal Res. 2014, 49, 563–573. [Google Scholar] [CrossRef]
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2016, 104, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Bonham, C.A.; Rodrigues, M.; Galvez, M.; Trotsyuk, A.; Stern-Buchbinder, Z.; Inayathullah, M.; Rajadas, J.; Gurtner, G.C. Deferoxamine can prevent pressure ulcers and accelerate healing in aged mice. Wound Repair Regen. 2018, 26, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Januszyk, M.; Maan, Z.N.; Whittam, A.J.; Hu, M.S.; Walmsley, G.G.; Dong, Y.; Khong, S.M.; Longaker, M.T.; Gurtner, G.C. Comparison of the Hydroxylase Inhibitor Dimethyloxalylglycine and the Iron Chelator Deferoxamine in Diabetic and Aged Wound Healing. Plast. Reconstr. Surg. 2017, 139, 695e–706e. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snider, A.E.; Lynn, J.V.; Urlaub, K.M.; Donneys, A.; Polyatskaya, Y.; Nelson, N.S.; Ettinger, R.E.; Gurtner, G.C.; Banaszak Holl, M.M.; Buchman, S.R. Topical Deferoxamine Alleviates Skin Injury and Normalizes Atomic Force Microscopy Patterns Following Radiation in a Murine Breast Reconstruction Model. Ann. Plast. Surg. 2018, 81, 604–608. [Google Scholar] [CrossRef]
- Minegaki, T.; Koiki, S.; Douke, Y.; Yamane, C.; Suzuki, A.; Mori, M.; Tsujimoto, M.; Nishiguchi, K. Augmentation of the cytotoxic effects of nitrogen-containing bisphosphonates in hypoxia. J. Pharm. Pharmacol. 2018, 70, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Sun, H.; Li, Q.; Lu, R.; Zou, X.; Yu, K.; Li, X.; Shu, Y.; Zhao, Y. Zoledronic Acid Inhibits Angiogenesis Through Promoting HIF-1α Protein Degradation in Human Umbilical Vein Endothelial Cells. J. Biomater. Tissue Eng. 2016, 6, 745–753. [Google Scholar] [CrossRef]
- Trebec-Reynolds, D.P.; Voronov, I.; Heersche, J.N.; Manolson, M.F. VEGF-A expression in osteoclasts is regulated by NF-kappaB induction of HIF-1alpha. J. Cell Biochem. 2010, 110, 343–351. [Google Scholar] [PubMed]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Léniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef] [Green Version]
- Park, S.M.; Li, Q.; Ryu, M.O.; Nam, A.; An, J.H.; Yang, J.I.; Kim, S.M.; Song, W.J.; Youn, H.Y. Preconditioning of canine adipose tissue-derived mesenchymal stem cells with deferoxamine potentiates anti-inflammatory effects by directing/reprogramming M2 macrophage polarization. Vet. Immunol. Immunopathol. 2020, 219, 109973. [Google Scholar] [CrossRef] [PubMed]
- Hellwig-Bürgel, T.; Stiehl, D.P.; Wagner, A.E.; Metzen, E.; Jelkmann, W. Review: Hypoxia-inducible factor-1 (HIF-1): A novel transcription factor in immune reactions. J. Interferon Cytokine Res. 2005, 25, 297–310. [Google Scholar] [CrossRef]
- Paschalidi, P.; Gkouveris, I.; Soundia, A.; Kalfarentzos, E.; Vardas, E.; Georgaki, M.; Kostakis, G.; Erovic, B.M.; Tetradis, S.; Perisanidis, C.; et al. The role of M1 and M2 macrophage polarization in progression of medication-related osteonecrosis of the jaw. Clin. Oral Investig. 2021, 25, 2845–2857. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Liu, W.; Lei, Y.; Wu, T.; Zhang, S.; Guo, Y.; Liu, Y.; Chen, D.; Yuan, Q.; Wang, Y. Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xie, R.; Hu, E.; Qian, P.; Lu, B.; Lan, G.; Lu, F. Protein-reduced gold nanoparticles mixed with gentamicin sulfate and loaded into konjac/gelatin sponge heal wounds and kill drug-resistant bacteria. Int. J. Biol. Macromol. 2020, 148, 921–931. [Google Scholar] [CrossRef]

| SS | ZA | |
|---|---|---|
| (0.1 mg/kg 3 Times per Week IP) | (0.1 mg/kg 3 Times per Week IP) | |
| Weeks | ||
| 1 | Groups I, II, and III | Groups IV, V, and VI |
| 2 | Groups I, II, and III | Groups IV, V, and VI |
| 3 | Groups I, II, and III | Groups IV, V, and VI |
| 4 | Groups I, II, and III | Groups IV, V, and VI |
| 5 | Groups I, II, and III | Groups IV, V, and VI |
| 6 | Groups I, II, and III | Groups IV, V, and VI |
| 7 | Groups I, II, and III | Groups IV, V, and VI |
| 8 | Groups I, II, and III | Groups IV, V, and VI |
| MAXILLARY MOLAR EXTRACTION | ||
| Procedure | ||
| Empty Socket Group I (Control) Group IV (ZA) | GS Group II (GS) Group V (ZA-GS) | GS/DFO Group III (GS/DFO) Group VI (ZA-GS/DFO) |
| 16 | EUTHANASIA | |
| Group I | Group II | Group III | Group IV | Group V | Group VI | Test Statistics | p * | |
|---|---|---|---|---|---|---|---|---|
| Control | GS | GS/DFO | ZA | ZA-GS | ZA-GS/DFO | |||
| Inflammation | ||||||||
| Absent | 2 (33.3) | 5 (83.3) | 3 (50) | 1 (16.7) | 1 (16.7) | 4 (66.7) | 22 | 0.108 |
| Mild | 3 (50) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 2 (33.3) | 2 (33.3) | ||
| Moderate | 1 (16.7) | 0 (0) | 1 (16.7) | 1 (16.7) | 3 (50) | 0 (0) | ||
| Severe | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) | ||
| Necrosis | ||||||||
| No | 6 (100) a | 6 (100) a | 6 (100) a | 0 (0) b | 2 (33.3) ab | 4 (66.7) ab | 24 | <0.001 |
| Yes | 0 (0) a | 0 (0) a | 0 (0) a | 6 (100) b | 4 (66.7) ab | 2 (33.3) ab |
| New Bone Formation Rate (%) | ||
|---|---|---|
| Mean ± SD | Median (Minimum–Maximum) | |
| Group I Control | 43.57 ± 18.66 ab | 46.2 (16.3–62.9) |
| Group II GS | 44.49 ± 12.05 ab | 41 (31.3–60.3) |
| Group III GS/DFO | 54.15 ± 16.95 b | 50.4 (37.3–84) |
| Group IV ZA | 22.57 ± 6.22 a | 24.2 (12.5–28.6) |
| Group V ZA-GS | 29.48 ± 7.22 a | 29.6 (20.3–36.6) |
| Group VI ZA-GS/DFO | 41.12 ± 14.74 ab | 38.4 (25–68) |
| F = 4.269 | ||
| p | 0.005 | |
| Mean ± SD | Median (Minimum–Maximum) | Test Statistics | p * | |
|---|---|---|---|---|
| Group I (Control) | 1.33 ± 0.52 | 1.00 (1.00–2.00) a | 27.117 | <0.001 |
| Group II (GS) | 1.50 ± 0.55 | 1.50 (1.00–2.00) a | ||
| Group III (GS/DFO) | 3.50 ± 0.55 | 3.50 (3.00–4.00) b | ||
| Group IV (ZA) | 2.50 ± 0.55 | 2.50 (2.00–3.00) ab | ||
| Group V (ZA-GS) | 2.17 ± 0.41 | 2.00 (2.00–3.00) ab | ||
| Group VI (ZA-GS/DFO) | 3.33 ± 0.52 | 3.00 (3.00–4.00) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin-Ülker, G.M.; Günbatan, M.; Duygu, G.; Soluk-Tekkesin, M.; Özcakir-Tomruk, C. Could Local Application of Hypoxia Inducible Factor 1-α Enhancer Deferoxamine Be Promising for Preventing of Medication-Related Osteonecrosis of the Jaw? Biomedicines 2023, 11, 758. https://doi.org/10.3390/biomedicines11030758
Yalcin-Ülker GM, Günbatan M, Duygu G, Soluk-Tekkesin M, Özcakir-Tomruk C. Could Local Application of Hypoxia Inducible Factor 1-α Enhancer Deferoxamine Be Promising for Preventing of Medication-Related Osteonecrosis of the Jaw? Biomedicines. 2023; 11(3):758. https://doi.org/10.3390/biomedicines11030758
Chicago/Turabian StyleYalcin-Ülker, Gül Merve, Murat Günbatan, Gonca Duygu, Merva Soluk-Tekkesin, and Ceyda Özcakir-Tomruk. 2023. "Could Local Application of Hypoxia Inducible Factor 1-α Enhancer Deferoxamine Be Promising for Preventing of Medication-Related Osteonecrosis of the Jaw?" Biomedicines 11, no. 3: 758. https://doi.org/10.3390/biomedicines11030758
APA StyleYalcin-Ülker, G. M., Günbatan, M., Duygu, G., Soluk-Tekkesin, M., & Özcakir-Tomruk, C. (2023). Could Local Application of Hypoxia Inducible Factor 1-α Enhancer Deferoxamine Be Promising for Preventing of Medication-Related Osteonecrosis of the Jaw? Biomedicines, 11(3), 758. https://doi.org/10.3390/biomedicines11030758







