Machine Learning: Using Xception, a Deep Convolutional Neural Network Architecture, to Implement Pectus Excavatum Diagnostic Tool from Frontal-View Chest X-rays
Abstract
:1. Introduction
Convolutional Neural Networks (CNN) and Medical Image Detection
2. Materials and Methods
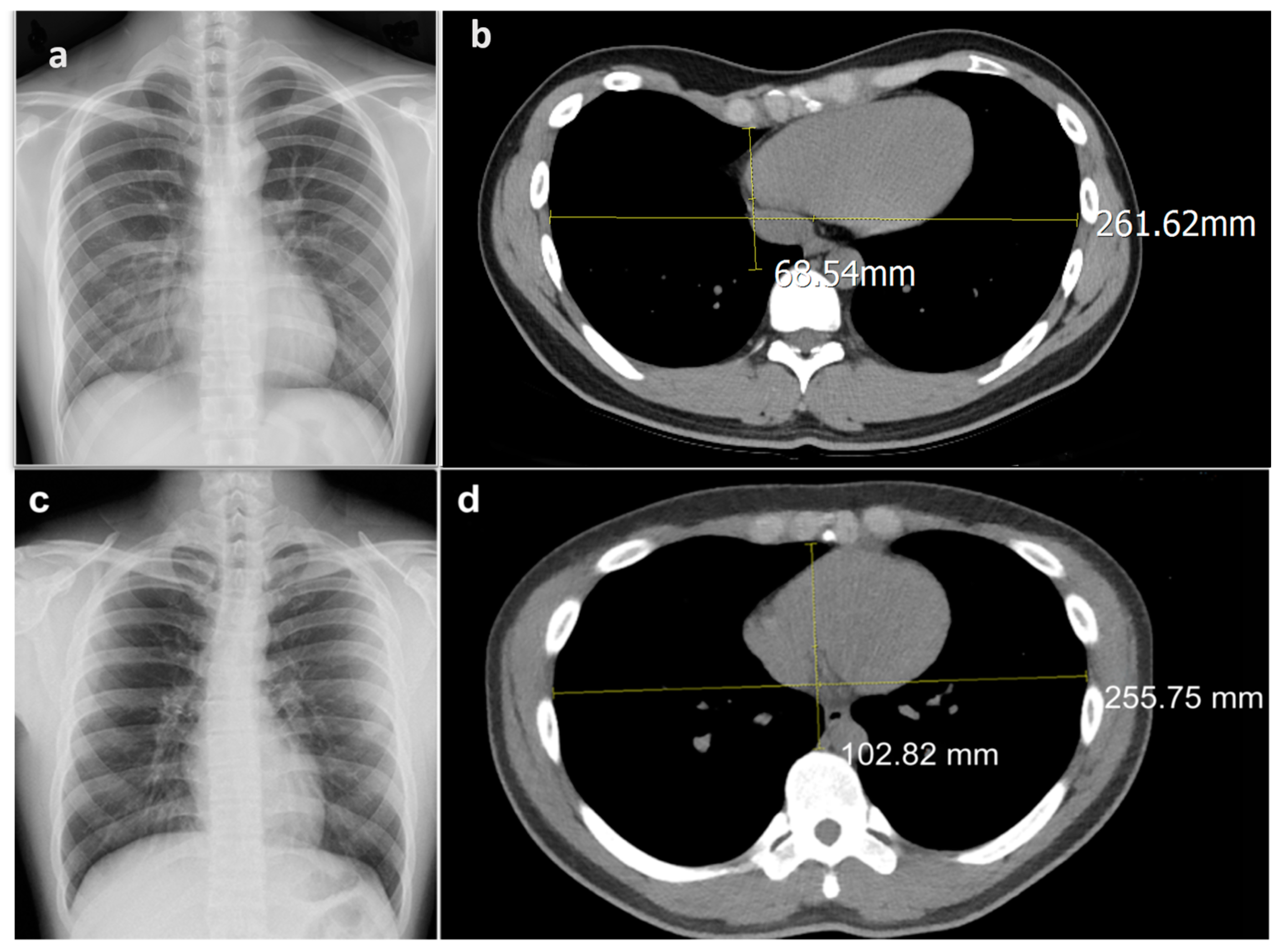
2.1. Data Set
2.2. Model Development
2.3. Model Evaluation
2.4. Statistical Analyses
3. Results
Model Performance
4. Discussion
Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biavati, M.; Kozlitina, J.; Alder, A.C.; Foglia, R.; McColl, R.W.; Peshock, R.M.; Kelly, R.E., Jr.; Kim Garcia, C. Prevalence of pectus excavatum in an adult population-based cohort estimated from radiographic indices of chest wall shape. PLoS ONE 2020, 15, e0232575. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewski, D.E.; Velazco, C.S.; Pulivarthi, V.; Arsanjani, R.; Obermeyer, R.J. Cardiopulmonary Function in Thoracic Wall Deformities: What Do We Really Know? Eur. J. Pediatr. Surg. 2018, 28, 327–346. [Google Scholar] [CrossRef]
- Kelly, R.E., Jr.; Obermeyer, R.J.; Nuss, D. Diminished pulmonary function in pectus excavatum: From denying the problem to finding the mechanism. Ann. Cardiothorac. Surg. 2016, 5, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Lo, P.C.; Tzeng, I.S.; Hsieh, M.S.; Yang, M.C.; Wei, B.C.; Cheng, Y.L. The Nuss procedure for pectus excavatum: An effective and safe approach using bilateral thoracoscopy and a selective approach to use multiple bars in 296 adolescent and adult patients. PLoS ONE 2020, 15, e0233547. [Google Scholar] [CrossRef] [PubMed]
- Neviere, R.; Montaigne, D.; Benhamed, L.; Catto, M.; Edme, J.L.; Matran, R.; Wurtz, A. Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. Eur. J. Cardiothorac. Surg. 2011, 40, e77–e82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, J.A., Jr.; Kramer, S.S.; Lietman, S.A. Use of CT scans in selection of patients for pectus excavatum surgery: A preliminary report. J. Pediatr. Surg. 1987, 22, 904–906. [Google Scholar] [CrossRef]
- Ward, R.; Carroll, W.D.; Cunningham, P.; Ho, S.A.; Jones, M.; Lenney, W.; Thompson, D.; Gilchrist, F.J. Radiation dose from common radiological investigations and cumulative exposure in children with cystic fibrosis: An observational study from a single UK centre. BMJ Open 2017, 7, e017548. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Carvalho, P.E.; da Silva, M.V.; Rodrigues, O.R.; Cataneo, A.J. Surgical interventions for treating pectus excavatum. Cochrane Database Syst. Rev. 2014, 10, CD008889. [Google Scholar] [CrossRef]
- LeCun, Y.; Jackel, L.D.; Bottou, L.; Cortes, C.; Denker, J.S.; Drucker, H.; Guyon, I.; Muller, U.A.; Sackinger, E.; Simard, P. Learning algorithms for classification: A comparison on handwritten digit recognition. Neural Netw. Stat. Mech. Perspect. 1995, 261, 2. [Google Scholar]
- Soffer, S.; Ben-Cohen, A.; Shimon, O.; Amitai, M.M.; Greenspan, H.; Klang, E. Convolutional Neural Networks for Radiologic Images: A Radiologist’s Guide. Radiology 2019, 290, 590–606. [Google Scholar] [CrossRef]
- Majkowska, A.; Mittal, S.; Steiner, D.F.; Reicher, J.J.; McKinney, S.M.; Duggan, G.E.; Eswaran, K.; Cameron Chen, P.H.; Liu, Y.; Kalidindi, S.R.; et al. Chest Radiograph Interpretation with Deep Learning Models: Assessment with Radiologist-adjudicated Reference Standards and Population-adjusted Evaluation. Radiology 2020, 294, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Irvin, J.; Ball, R.L.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.P. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 2018, 15, e1002686. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Cai, S.; Huang, L.; Zhou, H.; Xie, L. Computer-aided diagnosis of pectus excavatum using CT images and deep learning methods. Sci. Rep. 2020, 10, 20294. [Google Scholar] [CrossRef] [PubMed]
- Chollet, F. Xception: Deep Learning with Depthwise Separable Convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 1251–1258, arXiv2016, arXiv:1610.02357. [Google Scholar]
- Bochkovskiy, A.; Wang, C.-Y.; Liao, H.-Y.M. YOLOv4: Optimal Speed and Accuracy of Object Detection. arXiv 2020, arXiv:2004.10934. [Google Scholar]
- Parker, C. An Analysis of Performance Measures for Binary Classifiers. In Proceedings of the 2011 IEEE 11th International Conference on Data Mining, Vancouver, BC, Canada, 11–14 December 2011; pp. 517–526. [Google Scholar]
- Hung, T.N.K.; Vy, V.P.T.; Tri, N.M.; Hoang, L.N.; Tuan, L.V.; Ho, Q.T.; Le, N.Q.K.; Kang, J.H. Automatic Detection of Meniscus Tears Using Backbone Convolutional Neural Networks on Knee MRI. J. Magn. Reson. Imaging 2022. [Google Scholar] [CrossRef]
- Le, N.Q.K.; Ho, Q.T. Deep transformers and convolutional neural network in identifying DNA N6-methyladenine sites in cross-species genomes. Methods 2022, 204, 199–206. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Lu, L.; Lu, Z.; Bagheri, M.; Summers, R.M. ChestX-ray8: Hospital-scale Chest X-ray Database and Benchmarks on Weakly-Supervised Classification and Localization of Common Thorax Diseases. arXiv 2017, arXiv:1705.02315. [Google Scholar]
- Irvin, J.; Rajpurkar, P.; Ko, M.; Yu, Y.; Ciurea-Ilcus, S.; Chute, C.; Marklund, H.; Haghgoo, B.; Ball, R.; Shpanskaya, K. Chexpert: A large chest radiograph dataset with uncertainty labels and expert comparison. In Proceedings of the AAAI Conference on Artificial Intelligence, Honolulu, HI, USA, 27 January–1 February 2019; pp. 590–597. [Google Scholar]
- Johnson, A.E.W.; Pollard, T.J.; Berkowitz, S.J.; Greenbaum, N.R.; Lungren, M.P.; Deng, C.-y.; Mark, R.G.; Horng, S. MIMIC-CXR, a de-identified publicly available database of chest radiographs with free-text reports. Sci. Data 2019, 6, 317. [Google Scholar] [CrossRef] [Green Version]
- Bustos, A.; Pertusa, A.; Salinas, J.-M.; de la Iglesia-Vayá, M. PadChest: A large chest x-ray image dataset with multi-label annotated reports. arXiv 2019, arXiv:1901.07441. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Irvin, J.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.; Shpanskaya, K.; et al. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv 2017, arXiv:1711.05225. [Google Scholar]
- Lakhani, P.; Sundaram, B. Deep learning at chest radiography: Automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 2017, 284, 574–582. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, S.; Hwang, E.J.; Lee, J.H.; Jin, K.N.; Lim, K.Y.; Vu, T.H.; Sohn, J.H.; Hwang, S.; Goo, J.M.; et al. Development and Validation of Deep Learning-based Automatic Detection Algorithm for Malignant Pulmonary Nodules on Chest Radiographs. Radiology 2019, 290, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.G.; Mielke, C.; Mongan, J. Automated detection of moderate and large pneumothorax on frontal chest X-rays using deep convolutional neural networks: A retrospective study. PLoS Med. 2018, 15, e1002697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, A.; Ellsworth, W.; Banerjee, O.; Ng, A.Y.; Rajpurkar, P. CheXtransfer: Performance and Parameter Efficiency of ImageNet Models for Chest X-Ray Interpretation. arXiv 2021, arXiv:2101.06871. [Google Scholar]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-validation. Encycl. Database Syst. 2009, 5, 532–538. [Google Scholar]
- Kuyama, H.; Uemura, S.; Yoshida, A. Recurrence of pectus excavatum in long-term follow-up after the Nuss procedure in young children based on the radiographic Haller index. J. Pediatr. Surg. 2020, 55, 2699–2702. [Google Scholar] [CrossRef]
- Gibreel, W.; Zendejas, B.; Joyce, D.; Moir, C.R.; Zarroug, A.E. Minimally Invasive Repairs of Pectus Excavatum: Surgical Outcomes, Quality of Life, and Predictors of Reoperation. J. Am. Coll. Surg. 2016, 222, 245–252. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Socher, R.; Li, L.; Kai, L.; Li, F.-F. ImageNet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar]
- Ouahabi, A. A review of wavelet denoising in medical imaging. In Proceedings of the 2013 8th International Workshop on Systems, Signal Processing and their Applications (WoSSPA) IEEE, Algiers, Algeria, 12–15 May 2013; pp. 19–26. [Google Scholar]
- Mahdaoui, A.E.; Ouahabi, A.; Moulay, M.S. Image Denoising Using a Compressive Sensing Approach Based on Regularization Constraints. Sensors 2022, 22, 2199. [Google Scholar] [CrossRef]



| Parameter | Values | Explanation |
|---|---|---|
| Arch | Xception | Architecture: Xception |
| Imgshape | 224 × 224 | Image shape: downsized image pixels |
| pooling | Global average | Pooling method after convoluted filter layers |
| LR | default | Learning rate |
| LR schedure | default | changes the learning rate during learning |
| Batch size | 32 | a number of samples processed everytime the model is updated |
| dropout | 0 | Dropout setting applied to fully connected layers |
| Augmentation (zoom, shear, rotation) | (0, 0, 0) | Image transformation to expand data size |
| optimizer | nadamax | Optimization algorithm used for training |
| Batch normalization | no | A layer inserted before the pooling layer |
| Characteristic | PE Data Set | N Data Set | p Value |
|---|---|---|---|
| Total No. of chest X-rays Mean examined age (y) | 774 23.4 ± 7.8 | 1253 41.0 ± 6.7 | <0.001 |
| Patients (n) Men (n) Women (n) | 520 440 (84.6%) 80 (15.4%) | 667 328 (49.2%) 339 (50.8%) | |
| Haller index, mean ± SD Men Women | 4 ± 1.2 | 2.5 ± 0.37 | <0.001 |
| Patients with (n) 1 chest X-ray 2 chest X-rays ≥3 chest X-rays | 378 (72.7%) 125(24.0%) 17 (3.3%) | 428 (64.2%) 102(15.2%) 137(20.6%) | |
| PE shape (n) symmetric Asymmetric Right site depression Left site depression | 244 (46.9%) 276 (53.1%) 176 (33.8%) 100 (19.3%) | 100 (100%) NA NA NA | |
| Scoliosis (n) # | 50 (9.6%) | 28 (4.2 %) |
| Accuracy (95% CI) | Precision | Recall | F1-Score | AUCOC (95% CI) |
|---|---|---|---|---|
| 0.973 (0.968–0.978) | 0.986 | 0.943 | 0.964 | 0.976 (0.962–0.990) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-J.; Tzeng, I.-S.; Huang, Y.-S.; Hsu, Y.-Y.; Wei, B.-C.; Hung, S.-T.; Cheng, Y.-L. Machine Learning: Using Xception, a Deep Convolutional Neural Network Architecture, to Implement Pectus Excavatum Diagnostic Tool from Frontal-View Chest X-rays. Biomedicines 2023, 11, 760. https://doi.org/10.3390/biomedicines11030760
Fan Y-J, Tzeng I-S, Huang Y-S, Hsu Y-Y, Wei B-C, Hung S-T, Cheng Y-L. Machine Learning: Using Xception, a Deep Convolutional Neural Network Architecture, to Implement Pectus Excavatum Diagnostic Tool from Frontal-View Chest X-rays. Biomedicines. 2023; 11(3):760. https://doi.org/10.3390/biomedicines11030760
Chicago/Turabian StyleFan, Yu-Jiun, I-Shiang Tzeng, Yao-Sian Huang, Yuan-Yu Hsu, Bo-Chun Wei, Shuo-Ting Hung, and Yeung-Leung Cheng. 2023. "Machine Learning: Using Xception, a Deep Convolutional Neural Network Architecture, to Implement Pectus Excavatum Diagnostic Tool from Frontal-View Chest X-rays" Biomedicines 11, no. 3: 760. https://doi.org/10.3390/biomedicines11030760
APA StyleFan, Y. -J., Tzeng, I. -S., Huang, Y. -S., Hsu, Y. -Y., Wei, B. -C., Hung, S. -T., & Cheng, Y. -L. (2023). Machine Learning: Using Xception, a Deep Convolutional Neural Network Architecture, to Implement Pectus Excavatum Diagnostic Tool from Frontal-View Chest X-rays. Biomedicines, 11(3), 760. https://doi.org/10.3390/biomedicines11030760







