Risk Stratification Model for Severe COVID-19 Disease: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Study Database
2.3. Outcomes
2.4. Model Development
2.5. Model Evaluation
3. Results
3.1. Study Population
3.2. Model Components
3.3. Final Model
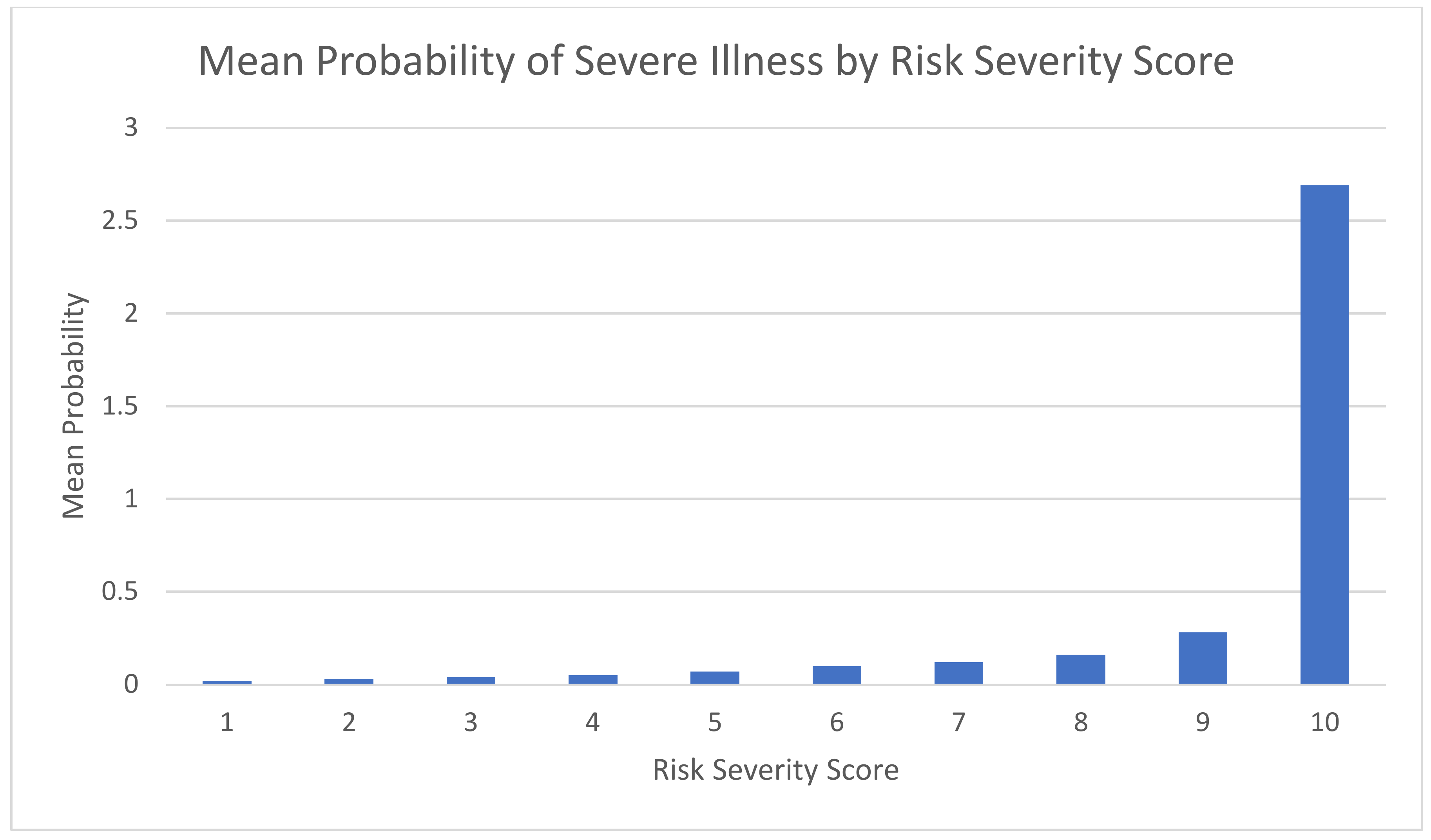
3.4. Model Accuracy
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, I.F.; Becker, A.D.; Grenfell, B.T.; Metcalf, C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat. Med. 2020, 26, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron-The Evolving COVID-19 Pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Tiwari, R.; Sarangi, A.K.; Islam, M.R.; Chakraborty, C.; Dhama, K. Omicron (B.1.1.529) variant of SARS-CoV-2: Concerns, challenges, and recent updates. J. Med. Virol. 2022, 94, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Leshem, E.; Wilder-Smith, A. COVID-19 vaccine impact in Israel and a way out of the pandemic. Lancet 2021, 397, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Understanding Risk|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/understanding-risk.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Findex.html (accessed on 18 February 2023).
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.A.; Debray, T.P.A.; et al. Prediction models for diagnosis and prognosis of COVID-19: Systematic review and critical appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Riesel, D.; Akriv, A.; Levy, J.; Finkel, U.; Yona, G.; Greenfeld, D.; Sheiba, S.; Somer, J.; Bachmat, E.; et al. Developing a COVID-19 mortality risk prediction model when individual-level data are not available. Nat. Commun. 2020, 11, 4439. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.; Fan, V.S.; Dominitz, J.A.; O’Hare, A.M.; Backus, L.I.; Locke, E.; Eastment, M.C.; Osborne, T.F.; Ioannou, N.G.; et al. Development of COVIDVax Model to Estimate the Risk of SARS-CoV-2-Related Death among 7.6 Million US Veterans for Use in Vaccination Prioritization. JAMA Netw. Open 2021, 4, e214347. [Google Scholar] [CrossRef] [PubMed]
- Experton, B.; Tetteh, H.A.; Lurie, N.; Walker, P.; Elena, A.; Hein, C.S.; Schwendiman, B.; Vincent, J.L.; Burrow, C.R. A Predictive Model for Severe COVID-19 in the Medicare Population: A Tool for Prioritizing Primary and Booster COVID-19 Vaccination. Biology 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.A.; Mehta, N.; Keogh, R.H.; Diaz-Ordaz, K.; Khunti, K.; Lyons, R.A.; Kee, F.; Sheikh, A.; Rahman, S.; et al. Risk prediction of COVID-19 related death and hospital admission in adults after COVID-19 vaccination: National prospective cohort study. BMJ 2021, 374, n2244. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Schäffer, A.A.; Merzon, E.; Green, I.; Magen, E.; Golan-Cohen, A.; Vinker, S.; Ruppin, E. A Calculator for COVID-19 Severity Prediction Based on Patient Risk Factors and Number of Vaccines Received. Microorganisms 2022, 10, 1238. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Alharahsheh, B.; Garg, N.; Guha, P. Evaluating risk stratification scoring systems to predict mortality in patients with COVID-19. BMJ Health Care Inform. 2021, 28, e100389. [Google Scholar] [CrossRef] [PubMed]
- Shapiro Ben David, S.; Cohen, D.; Karplus, R.; Irony, A.; Ofer-Bialer, G.; Potasman, I.; Greenfeld, O.; Azuri, J.; Ash, N. COVID-19 community care in Israel-a nationwide cohort study from a large health maintenance organization. J. Public Health 2021, 43, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Vela, E.; Carot-Sans, G.; Clèries, M.; Monterde, D.; Acebes, X.; Comella, A.; Eroles, L.G.; Coca, M.; Valero-Bover, D.; Sust, P.P.; et al. Development and validation of a population-based risk stratification model for severe COVID-19 in the general population. Sci. Rep. 2022, 12, 3277. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Rea, F.; Carle, F.; Scondotto, S.; Allotta, A.; Lepore, V.; D’Ettorre, A.; Tanzarella, C.; Vittori, P.; Abena, S.; et al. Stratification of the risk of developing severe or lethal COVID-19 using a new score from a large Italian population: A population-based cohort study. BMJ Open 2021, 11, e053281. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Li, Y.; Wang, W.; Ding, W.; Niu, Z.; Yang, X.; Wu, C. The Prediction Model of Risk Factors for COVID-19 Developing into Severe Illness Based on 1046 Patients with COVID-19. Emerg. Med. Int. 2021, 2021, 7711056. [Google Scholar] [CrossRef] [PubMed]
- Kertes, J.; Gez, S.B.; Saciuk, Y.; Supino-Rosin, L.; Stein, N.S.; Mizrahi-Reuveni, M.; Zohar, A.E. Effectiveness of mRNA BNT162b2 Vaccine 6 Months after Vaccination among Patients in Large Health Maintenance Organization, Israel. Emerg. Infect. Dis. 2022, 28, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.A.; Qamar, M.A.; Gilani, J.A.; Irfan, O.; Waqar, U.; Sajid, M.I.; Mahmood, S.F. The mystery of COVID-19 reinfections: A global systematic review and meta-analysis. Ann. Med. Surg. 2021, 72, 103130. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Category | n | % |
|---|---|---|---|
| Gender | % Male | 184,844 | 45.1 |
| Age group | <18 | 140,890 | 31.4 |
| 18–29 | 68,545 | 16.7 | |
| 30–44 | 83,561 | 20.4 | |
| 45–59 | 77,769 | 19.0 | |
| 60–74 | 30,921 | 7.5 | |
| 75+ | 8007 | 2.0 | |
| Socioeconomic Status | Low | 69,855 | 17.1 |
| Middle | 210,251 | 51.3 | |
| High | 129,587 | 31.6 | |
| No. COVID-19 illness episodes | % two or more | 31,053 | 7.6 |
| Vaccine Status | Never vaccinated | 110,286 | 26.9 |
| Vaccinated 6+ months ago | 37,004 | 9.0 | |
| Received 1–2 doses in last 6 months | 55,938 | 13.7 | |
| Received 3–4 doses in last 6 months | 206,465 | 50.4 | |
| Heart disease | % with illness | 17,746 | 4.3 |
| Diabetes | % with illness | 17,066 | 4.2 |
| HTN | % with illness | 37,503 | 9.2 |
| Cancer | % with illness | 11,160 | 2.7 |
| CKD | % with illness | 6694 | 1.6 |
| Immunosuppression status | % with disorder | 5215 | 1.3 |
| COPD | % with illness | 2681 | 0.7 |
| Complicated medical condition | % with illness | 1673 | 0.4 |
| Obesity | % with BMI ≥ 30 | 51,985 | 12.7 |
| Pregnancy | % 5+ months pregnant | 1886 | 0.5 |
| Lives in a nursing home | % live in a nursing home | 4176 | 1.0 |
| Characteristic | Category | N | OR | 95% CI |
|---|---|---|---|---|
| Gender | Female | 71,090 | 1 | |
| Male | 58,910 | 1.2 | 1.0–1.5 | |
| Age group | <18 | 44,709 | 1 | |
| 18–29 | 21,851 | 2.7 | 1.6–4.5 | |
| 30–44 | 26,528 | 2.2 | 1.3–3.7 | |
| 45–59 | 24,610 | 6.6 | 4.2–10.4 | |
| 60–74 | 9735 | 16.6 | 10.4–26.5 | |
| 75+ | 2567 | 70.4 | 42.8–115.9 | |
| Socioeconomic status | High | 41,152 | 1 | |
| Middle | 66,722 | 1.6 | 1.2–2.1 | |
| Low | 22,126 | 1.9 | 1.4–2.6 | |
| No. COVID-19 illness episodes | Once | 120,181 | 1 | |
| Two or more | 9819 | 0.3 | 0.2–0.5 | |
| Vaccine Status | Never vaccinated | 35,076 | 1 | |
| Vaccinated 6+ months ago | 11,803 | 0.6 | 0.4–0.8 | |
| Received 1–2 doses in last 6 months | 17,691 | 0.4 | 0.2–0.6 | |
| Received 3–4 doses in last 6 months | 65,430 | 0.2 | 0.1–0.2 | |
| Heart disease | No | 124,397 | 1 | |
| Yes | 5603 | 1.6 | 1.2–2.0 | |
| Diabetes | No | 124,629 | 1 | |
| Yes | 5371 | 1.4 | 1.1–1.8 | |
| HTN | No | 118,147 | 1 | |
| Yes | 11,853 | 1.5 | 1.1–1.9 | |
| Cancer | No | 126,420 | 1 | |
| Yes | 3580 | 1.5 | 1.1–2.0 | |
| CKD | No | 127,877 | 1 | |
| Yes | 2123 | 1.7 | 1.2–2.2 | |
| Immunosuppression status | No | 128,370 | 1 | |
| Yes | 1630 | 4.8 | 3.4–6.7 | |
| COPD | No | 129,152 | 1 | |
| Yes | 848 | 1.9 | 1.3–2.7 | |
| Complicated medical condition | No | 129,474 | 1 | |
| Yes | 526 | 2.5 | 1.3–4.6 | |
| Obesity | No | 113,457 | 1 | |
| Yes | 16,543 | 1.0 | 0.8–1.3 | |
| Lives in a nursing home | No | 128,673 | 1 | |
| Yes | 1327 | 2.5 | 1.9–3.4 | |
| Pregnancy | No/0–4 months pregnant | 118,147 | 1 | |
| 5+ months pregnant | 597 | 82.9 | 53.0–129.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizrahi Reuveni, M.; Kertes, J.; Shapiro Ben David, S.; Shahar, A.; Shamir-Stein, N.; Rosen, K.; Liran, O.; Bar-Yishay, M.; Adler, L. Risk Stratification Model for Severe COVID-19 Disease: A Retrospective Cohort Study. Biomedicines 2023, 11, 767. https://doi.org/10.3390/biomedicines11030767
Mizrahi Reuveni M, Kertes J, Shapiro Ben David S, Shahar A, Shamir-Stein N, Rosen K, Liran O, Bar-Yishay M, Adler L. Risk Stratification Model for Severe COVID-19 Disease: A Retrospective Cohort Study. Biomedicines. 2023; 11(3):767. https://doi.org/10.3390/biomedicines11030767
Chicago/Turabian StyleMizrahi Reuveni, Miri, Jennifer Kertes, Shirley Shapiro Ben David, Arnon Shahar, Naama Shamir-Stein, Keren Rosen, Ori Liran, Mattan Bar-Yishay, and Limor Adler. 2023. "Risk Stratification Model for Severe COVID-19 Disease: A Retrospective Cohort Study" Biomedicines 11, no. 3: 767. https://doi.org/10.3390/biomedicines11030767
APA StyleMizrahi Reuveni, M., Kertes, J., Shapiro Ben David, S., Shahar, A., Shamir-Stein, N., Rosen, K., Liran, O., Bar-Yishay, M., & Adler, L. (2023). Risk Stratification Model for Severe COVID-19 Disease: A Retrospective Cohort Study. Biomedicines, 11(3), 767. https://doi.org/10.3390/biomedicines11030767





