Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Preparation of the EMMPRIN Knocked-Down Cells
2.3. Cell Viability Assay
2.4. Real-Time PCR
2.5. Wester Blot Analysis
2.6. Sandwich ELISA
2.7. Wound Assay
2.8. Immunofluorescence (IF)
2.9. Statistical Analysis
3. Results
3.1. The Construction and Validation of the CT26 EMMPRIN-KD Cells
3.2. Macrophages Secrete Mediators That May Drive the Metastatic Outbreak
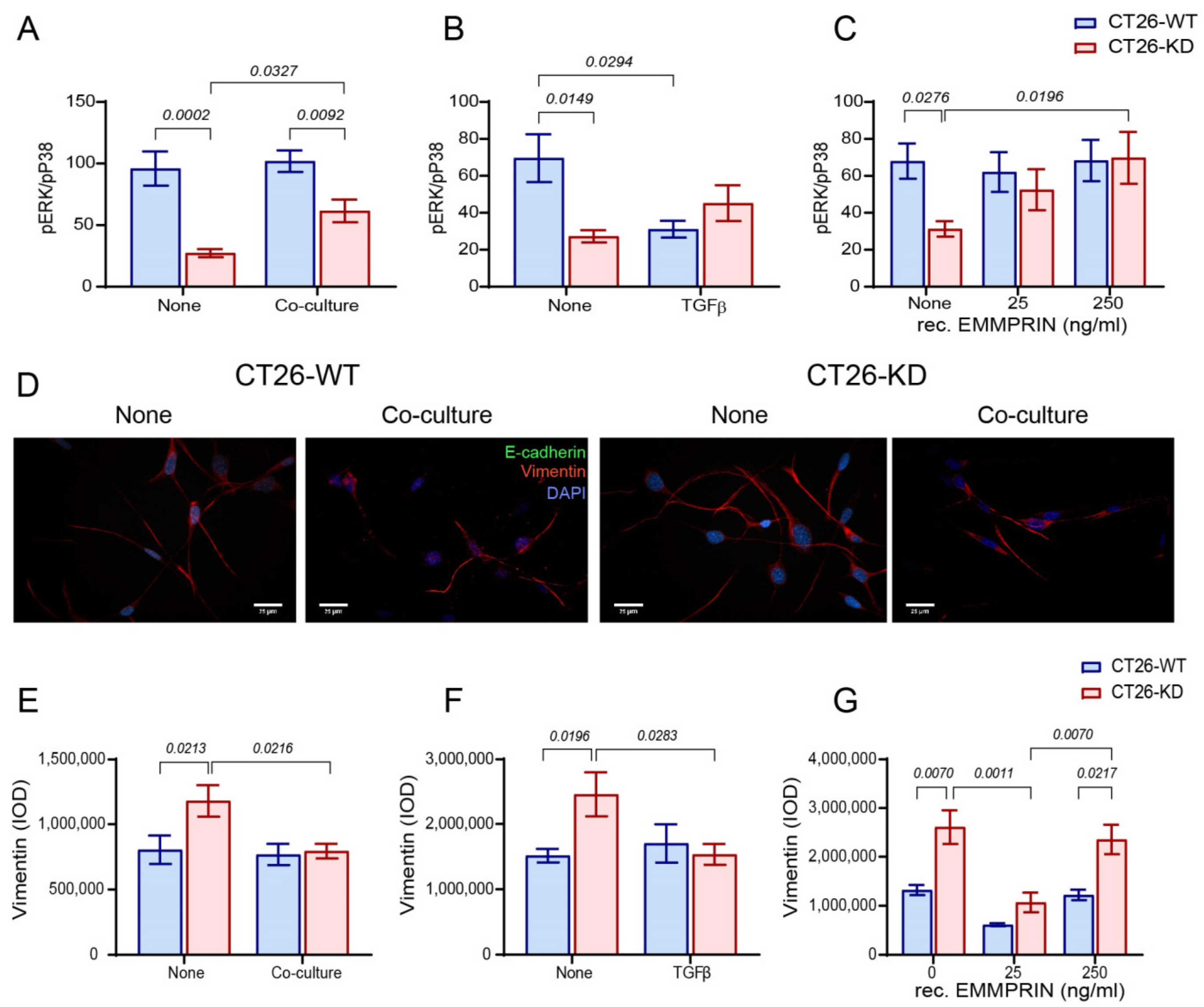
3.3. Reduced EMMPRIN Expression Enhances Markers of Dorancy
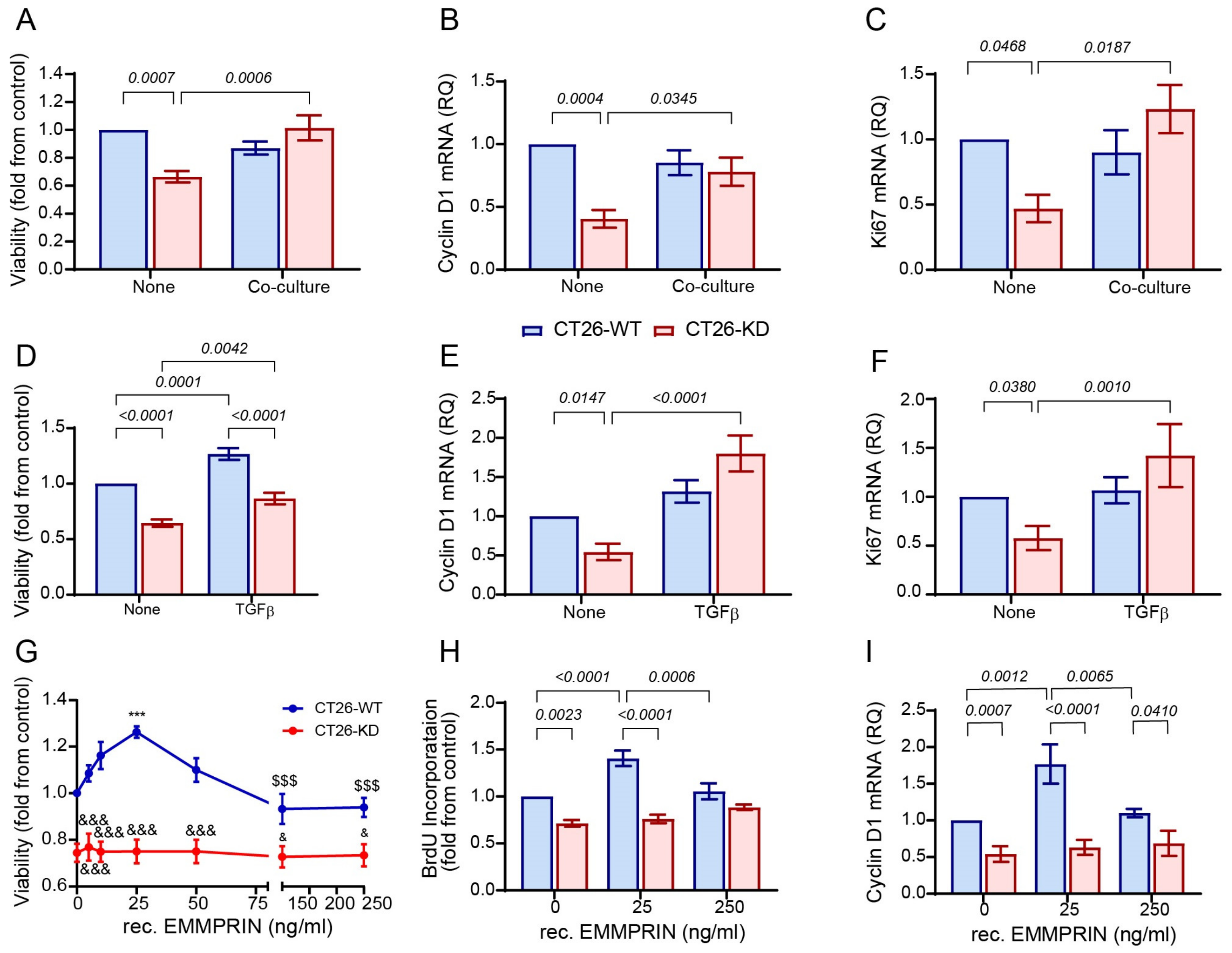
3.4. Reduced EMMPRIN Expression Inhibits Proliferation
3.5. Reduced EMMPRIN Expression Inhibits Angiogenesis
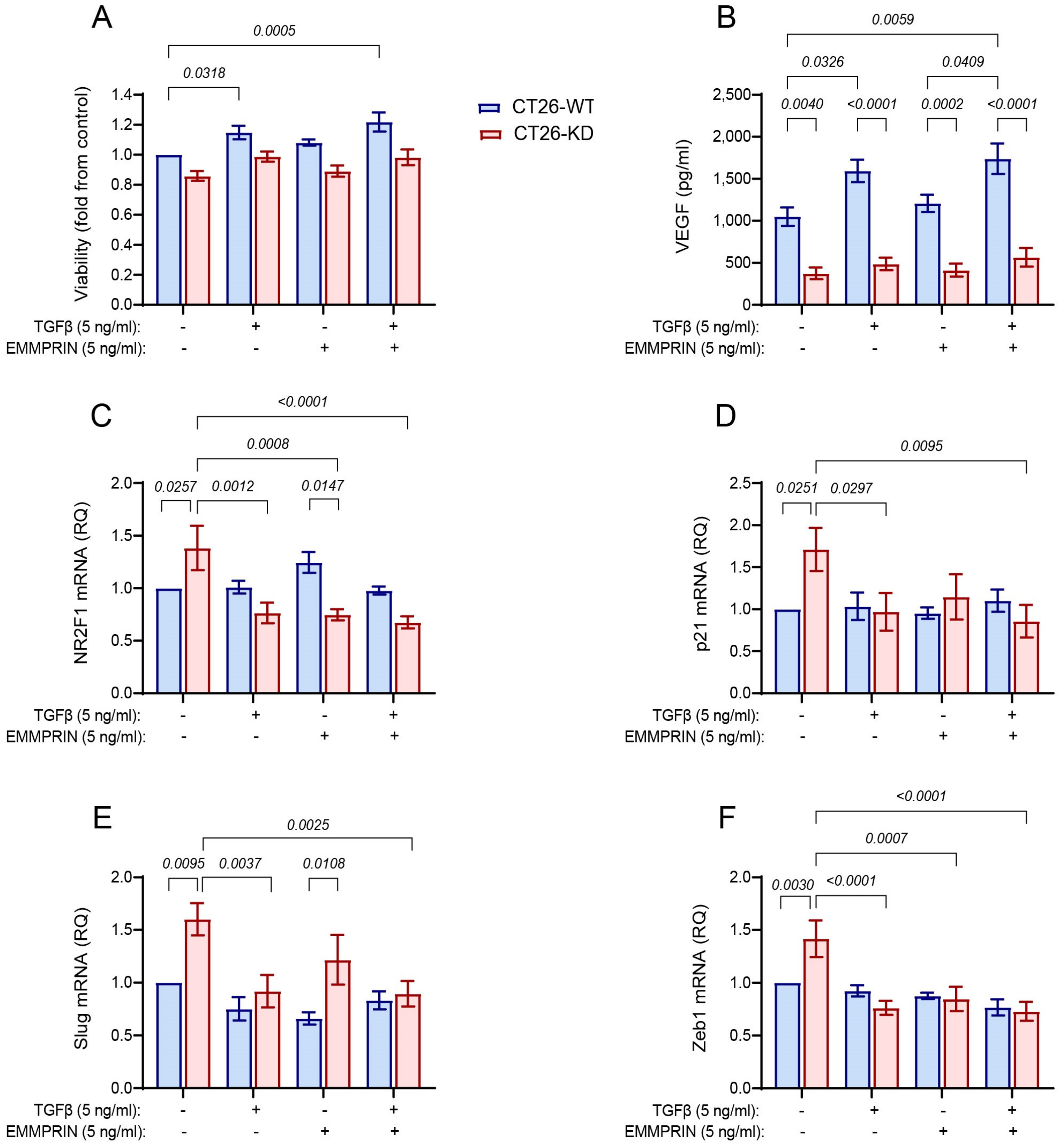
3.6. Combined Stimulation with TGFβ and EMMPRIN Is not Sufficient to Simulate the Co-Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Park, J.V.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The colorectal cancer tumor microenvironment and its impact on liver and lung metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef]
- Pretzsch, E.; Nieß, H.; Bösch, F.; Westphalen, C.B.; Jacob, S.; Neumann, J.; Werner, J.; Heinemann, V.; Angele, M.K. Age and metastasis—How age influences metastatic spread in cancer. Colorectal cancer as a model. Cancer Epidemiol. 2022, 77, 102112. [Google Scholar] [CrossRef]
- Aykut, B.; Lidsky, M.E. Colorectal Cancer Liver Metastases: Multimodal Therapy. Surg. Oncol. Clin. N. Am. 2022, 32, 119–141. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Calon, A.; Lonardo, E.; Batlle, E. Determinants of metastatic competency in colorectal cancer. Mol. Oncol. 2017, 11, 97–119. [Google Scholar] [CrossRef]
- Shaked, Y. Balancing efficacy of and host immune responses to cancer therapy: The yin and yang effects. Nat. Rev. Clin. Oncol. 2016, 13, 611–626. [Google Scholar] [CrossRef]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef]
- Chemi, F.; Mohan, S.; Guevara, T.; Clipson, A.; Rothwell, D.G.; Dive, C. Early Dissemination of Circulating Tumor Cells: Biological and Clinical Insights. Front. Oncol. 2021, 11, 672195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.P.Z.P.; Ribeiro, P.d.F.; Bruni-Cardoso, A. Sleeping beauty and the microenvironment enchantment: Microenvironmental regulation of the proliferation-quiescence decision in normal tissues and in cancer development. Front. Cell Dev. Biol. 2018, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-L.; Zhang, M.; Tang, Y.-L.; Liang, X.-H. Cancer cell dormancy: Mechanisms and implications of cancer recurrence and metastasis. OncoTargets Ther. 2017, 10, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Jehanno, C.; Vulin, M.; Richina, V.; Richina, F.; Bentires-Alj, M. Phenotypic plasticity during metastatic colonization. Trends Cell Biol. 2022, 32, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Bornes, L.; Belthier, G.; Van Rheenen, J. Epithelial-to-Mesenchymal Transition in the Light of Plasticity and Hybrid E/M States. J. Clin. Med. 2021, 10, 2403. [Google Scholar] [CrossRef] [PubMed]
- Imodoye, S.O.; Adedokun, K.A.; Muhammed, A.O.; Bello, I.O.; Muhibi, M.A.; Oduola, T.; Oyenike, M.A. Understanding the Complex Milieu of Epithelial-Mesenchymal Transition in Cancer Metastasis: New Insight Into the Roles of Transcription Factors. Front. Oncol. 2021, 11, 762817. [Google Scholar] [CrossRef]
- Aouad, P.; Zhang, Y.; De Martino, F.; Stibolt, C.; Ali, S.; Ambrosini, G.; Mani, S.A.; Maggs, K.; Quinn, H.M.; Sflomos, G.; et al. Epithelial-mesenchymal plasticity determines estrogen receptor positive breast cancer dormancy and epithelial reconversion drives recurrence. Nat. Commun. 2022, 13, 4975. [Google Scholar] [CrossRef]
- Balayan, V.; Guddati, A.K. Tumor Dormancy: Biologic and Therapeutic Implications. World J. Oncol. 2022, 13, 8–19. [Google Scholar] [CrossRef]
- Sauer, S.; Reed, D.R.; Ihnat, M.; Hurst, R.E.; Warshawsky, D.; Barkan, D. Innovative Approaches in the Battle against Cancer Recurrence: Novel Strategies to Combat Dormant Disseminated Tumor Cells. Front. Oncol. 2021, 11, 659963. [Google Scholar] [CrossRef]
- Khalil, B.D.; Sanchez, R.; Rahman, T.; Rodriguez-Tirado, C.; Moritsch, S.; Rodriguez Martinez, A.; Miles, B.; Farias, E.; Mezei, M.; Nobre, A.R.; et al. An NR2F1-specific agonist suppresses metastasis by inducing cancer cell dormancy. J. Exp. Med. 2021, 219, e20210836. [Google Scholar] [CrossRef]
- Sosa, M.S.; Parikh, F.; Maia, A.G.; Estrada, Y.; Bosch, A.; Bragado, P.; Ekpin, E.; George, A.; Zheng, Y.; Lam, H.M.; et al. NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nat. Commun. 2015, 6, 6170–6184. [Google Scholar] [CrossRef]
- Sosa, M.S. Dormancy programs as emerging antimetastasis therapeutic alternatives. Mol. Cell. Oncol. 2016, 3, 6–8. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Chen, X.; Han, X.; Dong, P. M2 macrophages induce EMT through the TGF-β/Smad2 signaling pathway. Cell Biol. Int. 2017, 41, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Xie, M.; Yu, H.; Chen, H. Intestinal dysbacteriosis activates tumor-associated macrophages to promote epithelial-mesenchymal transition of colorectal cancer. Innate Immun. 2018, 24, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Deng, Y.; Im, J.H.; Muschel, R.J.; Zou, Y.; Li, J.; Lang, R.A.; Pollard, J.W. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 2009, 4, e6562. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lin, J.C.; Hwang, W.L.; Kuo, Y.J.; Chen, H.K.; Tai, S.K.; Lin, C.C.; Yang, M.H. Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat. Commun. 2018, 9, 3763. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Aras, S.; Raza Zaidi, M. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Hänggi, K.; Ruffell, B. The immune microenvironment and cancer metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037424. [Google Scholar] [CrossRef]
- Amit-Cohen, B.C.; Rahat, M.M.; Rahat, M.A. Tumor cell-macrophage interactions increase angiogenesis through secretion of EMMPRIN. Front. Physiol. 2013, 4, 178. [Google Scholar] [CrossRef]
- Grass, G.D.; Toole, B.P. How, with whom and when: An overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity. Biosci. Rep. 2016, 36, e00283. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, P.; Jiang, J.L.; Zhang, Q.; Wu, Z.B.; Yao, X.Y.; Tang, H.; Lu, N.; Yang, Y.; Chen, Z.N. Involvement of CD147 in overexpression of MMP-2 and MMP-9 and enhancement of invasive potential of PMA-differentiated THP-1. BMC Cell Biol. 2005, 6, 25–35. [Google Scholar] [CrossRef]
- Landras, A.; De Moura, C.R.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers 2019, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Vetrivel, U.; Parameswaran, S.; Subramanian, K.K. Structural insights on druggable hotspots in CD147: A bull’s eye view. Life Sci. 2019, 224, 76–87. [Google Scholar] [CrossRef]
- Toole, B.P. The CD147-HYALURONAN Axis in Cancer. Anat. Rec. 2020, 303, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Ru, N.Y.; Wu, J.; Chen, Z.N.; Bian, H. HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal transition and hepatocellular carcinoma invasion. Cell Biol. Int. 2015, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, Q.; Wu, M.; Nie, C.; Xu, H.; Wang, L. CD147 promotes epithelial-mesenchymal transition of prostate cancer cells via the Wnt/β-catenin pathway. Exp. Ther. Med. 2020, 20, 3154–3160. [Google Scholar] [CrossRef]
- Gambotto, A.; Dworacki, G.; Cicinnati, V.; Kenniston, T.; Steitz, J.; Tüting, T.; Robbins, P.D.; DeLeo, A.B. Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: Identification of an H2-Kd-restricted CTL epitope. Gene Ther. 2000, 7, 2036–2040. [Google Scholar] [CrossRef]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3464. [Google Scholar] [CrossRef]
- Simanovich, E.; Brod, V.; Rahat, M.M.; Rahat, M.A. Function of miR-146a-5p in Tumor Cells As a Regulatory Switch between Cell Death and Angiogenesis: Macrophage Therapy Revisited. Front. Immunol. 2018, 8, 1931. [Google Scholar] [CrossRef]
- Jiang, H.; Ge, H.; Shi, Y.; Yuan, F.; Yue, H. CAFs secrete CXCL12 to accelerate the progression and cisplatin resistance of colorectal cancer through promoting M2 polarization of macrophages. Med. Oncol. Oncol. 2023, 40, 90. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Shintani, Y.; Fujiwara, A.; Kimura, T.; Kawamura, T.; Funaki, S.; Minami, M.; Okumura, M. IL-6 secreted from Cancer-Associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J. Thorac. Oncol. 2016, 11, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Magidey-Klein, K.; Cooper, T.J.; Kveler, K.; Normand, R.; Zhang, T.; Timaner, M.; Raviv, Z.; James, B.P.; Gazit, R.; Ronai, Z.A.; et al. IL-6 contributes to metastatic switch via the differentiation of monocytic-dendritic progenitors into prometastatic immune cells. J. Immunother. Cancer 2021, 9, e002856. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.S.; Avivar-Valderas, A.; Bragado, P.; Wen, H.C.; Aguirre-Ghiso, J.A. ERK1/2 and p38α/β signaling in tumor cell quiescence: Opportunities to control dormant residual disease. Clin. Cancer Res. 2011, 17, 5850–5857. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat. Rev. Cancer 2014, 14, 611–622. [Google Scholar] [CrossRef]
- Lee, J.H.; Massagué, J. TGF-β in developmental and fibrogenic EMTs. Semin. Cancer Biol. 2022, 86, 136–145. [Google Scholar] [CrossRef]
- Xue, V.W.; Chung, J.Y.F.; Córdoba, C.A.G.; Cheung, A.H.K.; Kang, W.; Lam, E.W.F.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M.K. Transforming growth factor-β: A multifunctional regulator of cancer immunity. Cancers 2020, 12, 3099. [Google Scholar] [CrossRef]
- Weidenfeld, K.; Barkan, D. EMT and stemness in tumor dormancy and outgrowth: Are they intertwined processes? Front. Oncol. 2018, 8, 381. [Google Scholar] [CrossRef]
- Nobre, A.R.; Dalla, E.; Yang, J.; Huang, X.; Wullkopf, L.; Risson, E.; Razghandi, P.; Anton, M.L.; Zheng, W.; Seoane, J.A.; et al. ZFP281 drives a mesenchymal-like dormancy program in early disseminated breast cancer cells that prevents metastatic outgrowth in the lung. Nat. Cancer 2022, 3, 1165–1180. [Google Scholar] [CrossRef]
- Ho, C.; Lin, S.; Tseng, L.; Hung, M.; Hung, S. Snail induces dormancy in disseminated luminal type A breast cancer through Src inhibition. Am. J. Cancer Res 2022, 12, 3932–3946. [Google Scholar]
- St-Pierre, Y.; Couillard, J.; Van Themsche, C. Regulation of MMP-9 gene expression for the development of novel molecular targets against cancer and inflammatory diseases. Expert Opin. Ther. Targets 2004, 8, 473–489. [Google Scholar] [CrossRef]
- Buyuk, B.; Jin, S.; Ye, K. Epithelial-to-Mesenchymal Transition Signaling Pathways Responsible for Breast Cancer Metastasis. Cell. Mol. Bioeng. 2022, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sosnoski, D.M.; Norgard, R.J.; Grove, C.D.; Foster, S.J.; Mastro, A.M. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin. Exp. Metastasis 2015, 32, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, M.; Li, Y.; Shang, Y.K.; Wang, S.J.; Meng, Y.; Wang, Z.; Li, Z.S.; Chen, H.; Chen, Z.N.; et al. Regulation of a TGF-β1-CD147 self-sustaining network in the differentiation plasticity of hepatocellular carcinoma cells. Oncogene 2016, 35, 5468–5479. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ru, N.-Y.; Zhang, Y.; Li, Y.; Wei, D.; Ren, Z.; Huang, X.-F.; Chen, Z.-N.; Bian, H. HAb18G/CD147 promotes epithelial–mesenchymal transition through TGF-β signaling and is transcriptionally regulated by Slug. Oncogene 2011, 30, 4410–4427. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Li, Y.; Du, D.; Liu, Y.; Yin, Y. CD147 induces up-regulation of vascular endothelial growth factor in U937-derived foam cells through PI3K/AKT pathway. Arch. Biochem. Biophys. 2016, 609, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Swierczak, A.; Pollard, J.W. Myeloid cells in metastasis. Cold Spring Harb. Perspect. Biol. 2020, 10, a038026. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Tang, Y.; Xiao, M.; Li, M.; Li, Q.; Yang, L.; Li, X.; Yang, Y.; Wang, Y. Silencing PARG decreases invasion in CT26 cells. Int. J. Clin. Exp. Pathol. 2019, 12, 3847–3854. [Google Scholar] [PubMed]
- Castle, J.C.; Loewer, M.; Boegel, S.; de Graaf, J.; Bender, C.; Tadmor, A.D.; Boisguerin, V.; Bukur, T.; Sorn, P.; Paret, C.; et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genom. 2014, 15, 190–200. [Google Scholar] [CrossRef]
- Suzuki, S.; Toyoma, S.; Tsuji, T.; Kawasaki, Y.; Yamada, T. CD147 mediates transforming growth factor-β1-induced epithelial-mesenchymal transition and cell invasion in squamous cell carcinoma of the tongue. Exp. Ther. Med. 2019, 17, 2855–2860. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Fok, K.L.; Tsang, L.L.; Ye, M.; Liu, J.; Li, F.; Zhao, A.Z.; Chan, H.C.; Chen, H. CD147 Induces Epithelial-to-Mesenchymal Transition by Disassembling Cellular Apoptosis Susceptibility Protein/E-Cadherin/β-Catenin Complex in Human Endometriosis. Am. J. Pathol. 2018, 188, 1597–1607. [Google Scholar] [CrossRef]
- Su, J.; Morgani, S.M.; David, C.J.; Wang, Q.; Er, E.E.; Huang, Y.H.; Basnet, H.; Zou, Y.; Shu, W.; Soni, R.K.; et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 2020, 577, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhou, M.; Peng, L.; Kong, S.; Miao, R.; Shi, Y.; Sheng, H.; Li, L. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7432–7441. [Google Scholar] [PubMed]
- Cai, S.; Li, N.; Bai, X.; Liu, L.; Banerjee, A.; Lavudi, K.; Zhang, X.; Zhao, J.; Venere, M.; Duan, W.; et al. ERK inactivation enhances stemness of NSCLC cells via promoting Slug-mediated epithelial-to-mesenchymal transition. Theranostics 2022, 12, 7051–7066. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Clair, J.M.-t.; Wang, G.; Luo, J.; Palma-Diaz, F.; Lai, C.; Elashoff, D.A.; Sharma, S.; Dubinett, S.M.; St. John, M. p38 MAPK mediates epithelial-mesenchymal transition by regulating p38IP and Snail in head and neck squamous cell carcinoma. Oral Oncol. 2016, 60, 81–89. [Google Scholar] [CrossRef]
- Guereño, M.; Delgado Pastore, M.; Lugones, A.C.; Cercato, M.; Todaro, L.; Urtreger, A.; Peters, M.G. Glypican-3 (GPC3) inhibits metastasis development promoting dormancy in breast cancer cells by p38 MAPK pathway activation. Eur. J. Cell Biol. 2020, 99, 151096. [Google Scholar] [CrossRef]
- Shantanam, S. MUELLER TGFβ2 dictates disseminated tumour cell fate in target organs through TGFβ-RIII and p38α/β signalling. Nat. Cell Biol. 2013, 15, 1351–1361. [Google Scholar]
- Belton, R.J.; Chen, L.; Mesquita, F.S.; Nowak, R.A. Basigin-2 is a cell surface receptor for soluble basigin ligand. J. Biol. Chem. 2008, 283, 17805–17814. [Google Scholar] [CrossRef]




| Gene Amplified | Forward Primer | Reversed Primer |
|---|---|---|
| EMMPRIN | 5′-TGGCCTTCACGCTCTTGAG | 5′-CAACGCCACTGCTGTTCAAA |
| Snail | 5′-TGTCTGCACGACCTGTGGAAAG | 5′-CTTCACATCCGAGTGGGTTTGG |
| Slug | 5′-TCTGTGGCAAGGCTTTCTCCAG | 5′-TGCAGATGTGCCCTCAGGTTTG |
| Twist1 | 5′-GATTCAGACCCTCAAACTGGCG | 5′-AGACGGAGAAGGCGTAGCTGAG |
| Zeb1 | 5′-ATTCAGCTACTGTGAGCCCTGC | 5′-CATTCTGGTCCTCCACAGTGGA |
| SOX2 | 5′-AACGGCAGCTACAGCATGATGC | 5′-CGAGCTGGTCATGGAGTTGTAC |
| NR2F1 | 5′-CCAACAGGAACTGTCCCATCGA | 5′-CCGTTTGTGAGTGCATACTGGC |
| p21 | 5′-TCGCTGTCTTGCACTCTGGTGT | 5′-CCAATCTGCGCTTGGAGTGATAG |
| p27 | 5-AGCAGTGTCCAGGGATGAGGAA | 5′-TTCTTGGGCGTCTGCTCCACAG |
| Cyclin D1 | 5′-GCAGAAGGAGATTGTGCCATCC | 5′-AGGAAGCGGTCCAGGTAGTTCA |
| c-Myc | 5′-TCGCTGCTGTCCTCCGAGTCC | 5′-GGTTTGCCTCTTCTCCACAGAC |
| Ki-67 | 5′-GAGGAGAAACGCCAACCAAGAG | 5′-TTTGTCCTCGGTGGCGTTATCC |
| PBGD | 5′-CAGTTTGAAATCATTGCTATGTCCA | 5′-CTCCAATCTTAGAGAGTGCAGTATC |
| GAPDH | 5′- CATCACTGCCACCCAGAAGACTG | 5′- ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feigelman, G.; Simanovich, E.; Brockmeyer, P.; Rahat, M.A. Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages. Biomedicines 2023, 11, 768. https://doi.org/10.3390/biomedicines11030768
Feigelman G, Simanovich E, Brockmeyer P, Rahat MA. Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages. Biomedicines. 2023; 11(3):768. https://doi.org/10.3390/biomedicines11030768
Chicago/Turabian StyleFeigelman, Gabriele, Elina Simanovich, Phillipp Brockmeyer, and Michal A. Rahat. 2023. "Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages" Biomedicines 11, no. 3: 768. https://doi.org/10.3390/biomedicines11030768
APA StyleFeigelman, G., Simanovich, E., Brockmeyer, P., & Rahat, M. A. (2023). Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages. Biomedicines, 11(3), 768. https://doi.org/10.3390/biomedicines11030768






_Amit_Rahat.jpg)

