Tissue Engineering and Targeted Drug Delivery in Cardiovascular Disease: The Role of Polymer Nanocarrier for Statin Therapy
Abstract
1. Introduction
2. Search Strategy
3. Statins: Summary
4. Drug Delivery Systems
4.1. Micelles
4.2. Liposomes
4.3. Polymeric Nanoparticles
4.4. Dendrimers
5. In Vivo Kinetics of the Carrier System with Nanoparticles
6. Biological and Clinical Effects
6.1. Bioavailability
6.2. Endothelial Dysfunction
6.3. Intimal Hyperplasia
6.4. Neoangiogenesis
6.5. Ischemia–Reperfusion Injury
6.6. Cardiac Regeneration
6.7. Remodeling in the Extracellular Matrix
6.8. Neointimal Growth and Reendothelialization
6.9. Anti-Inflammatory Target Therapy
7. Clinical Implications
7.1. Biological Evidence about Statins and Angiogenesis
7.2. Statins and Bleeding Risk
7.3. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbab-Zadeh, A.; Fuster, V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient. J. Am. Coll. Cardiol. 2019, 74, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.C.; Zhong, S.; Ou, J.S.; Tian, J.W. Application of targeted therapy strategies with nanomedicine delivery for atherosclerosis. Acta Pharmacol. Sin. 2021, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Zhang, J.; Zu, Y.; Dhanasekara, C.S.; Li, J.; Wu, D.; Fan, Z.; Wang, S. Detection and treatment of atherosclerosis using nanoparticles. WIREs Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Niedzielski, M.; Broncel, M.; Gorzelak-Pabiś, P.; Woźniak, E. New possible pharmacological targets for statins and ezetimibe. Biomed. Pharm. 2020, 129, 110388. [Google Scholar] [CrossRef]
- Musunuru, K. Treating Coronary Artery Disease: Beyond Statins, Ezetimibe, and PCSK9 Inhibition. Annu. Rev. Med. 2021, 72, 447–458. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, L.; Zhao, M.; Kong, F.; Lu, X.; Tang, C.; Yin, C. Dual targeted delivery of statins and nucleic acids by chitosan-based nanoparticles for enhanced antiatherosclerotic efficacy. Biomaterials 2022, 280, 121324. [Google Scholar] [CrossRef]
- Cheung, Y.; O’Brien, R.; Ekinci, E.I. What is new in lipid-lowering therapies in diabetes? Intern. Med. J. 2019, 49, 1472–1480. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clín. 2018, 150, 398–402. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling: Prevention of eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Wijaya, A.; Wang, Y.; Tang, D.; Zhong, Y.; Liu, B.; Yan, B.; Jiu, Q.; Wu, W.; Wang, G. A study of lovastatin and L-arginine co-loaded PLGA nanomedicine for enhancing nitric oxide production and eNOS expression. J. Mater. Chem. B 2022, 10, 607–624. [Google Scholar] [CrossRef]
- Groner, J.; Goepferich, A.; Breunig, M. Atherosclerosis: Conventional intake of cardiovascular drugs versus delivery using nanotechnology–A new chance for causative therapy? J. Control. Release 2021, 333, 536–559. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation 2014, 129, 45. [Google Scholar] [CrossRef]
- Iranshahy, M.; Banach, M.; Hasanpour, M.; Lavie, C.J.; Sahebkar, A. Killing the Culprit: Pharmacological Solutions to Get Rid of Cholesterol Crystals. Curr. Probl. Cardiol. 2022, 47, 101274. [Google Scholar] [CrossRef]
- Dayar, E.; Pechanova, O. Targeted Strategy in Lipid-Lowering Therapy. Biomedicines 2022, 10, 1090. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Strijkers, G.J.; van Tilborg, G.A.F.; Cormode, D.P.; Fayad, Z.A.; Nicolay, K. Nanoparticulate Assemblies of Amphiphiles and Diagnostically Active Materials for Multimodality Imaging. Acc. Chem. Res. 2009, 42, 904–914. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Morgan, M.T.; Carnahan, M.A.; Finkelstein, S.; Prata, C.A.H.; Degoricija, L.; Lee, S.; Grinstaff, M.W. Dendritic supramolecular assemblies for drug delivery. Chem. Commun. 2005, 4309. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Katsuki, S.; Matoba, T.; Koga, J.I.; Nakano, K.; Egashira, K. Anti-inflammatory Nanomedicine for Cardiovascular Disease. Front. Cardiovasc. Med. 2017, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Asai, T. Nanoparticle-Mediated Delivery of Anticancer Agents to Tumor Angiogenic Vessels. Biol. Pharm. Bull. 2012, 35, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Butler, A.E.; Badiee, A.; Sahebkar, A. Liposomal nanocarriers for statins: A pharmacokinetic and pharmacodynamics appraisal. J. Cell. Physiol. 2019, 234, 1219–1229. [Google Scholar] [CrossRef]
- Meola, T.R.; Abuhelwa, A.Y.; Joyce, P.; Clifton, P.; Prestidge, C.A. A safety, tolerability, and pharmacokinetic study of a novel simvastatin silica-lipid hybrid formulation in healthy male participants. Drug Deliv. Transl. Res. 2021, 11, 12. [Google Scholar] [CrossRef]
- Shaker, M.A.; Elbadawy, H.M.; Shaker, M.A. Improved solubility, dissolution, and oral bioavailability for atorvastatin-Pluronic® solid dispersions. Int. J. Pharm. 2020, 574, 118891. [Google Scholar] [CrossRef]
- Shaker, M.A.; Elbadawy, H.M.; Al Thagfan, S.S.; Shaker, M.A. Enhancement of atorvastatin oral bioavailability via encapsulation in polymeric nanoparticles. Int. J. Pharm. 2021, 592, 120077. [Google Scholar] [CrossRef]
- Ahmed, I.S.; El-Hosary, R.; Shalaby, S.; Abd-Rabo, M.M.; Elkhateeb, D.G.; Nour, S. PD-PK evaluation of freeze-dried atorvastatin calcium-loaded poly-ε-caprolactone nanoparticles. Int. J. Pharm. 2016, 504, 70–79. [Google Scholar] [CrossRef]
- Yao, G.T.; Song, L.P.; Xue, W.H.; Su, G.H.; Ning, A.H.; Wang, J. Nano-particle engineered atorvastatin delivery to support mesenchymal stem cell survival in infarcted myocardium. Saudi J. Biol. Sci. 2018, 25, 1016–1021. [Google Scholar] [CrossRef]
- Adeleke, O.A. Premium ethylcellulose polymer based architectures at work in drug delivery. Int. J. Pharm. X 2019, 1, 100023. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose–A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef]
- Taneja, G.; Sud, A.; Pendse, N.; Panigrahi, B.; Kumar, A.; Sharma, A.K. Nano-medicine and Vascular Endothelial Dysfunction: Options and Delivery Strategies. Cardiovasc. Toxicol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Molinaro, R.; Boada, C.; Del Rosal, G.M.; Hartman, K.A.; Corbo, C.; Andrews, E.D.; Toledano-Furman, N.E.; Cooke, J.P.; Tasciotti, E. Vascular Inflammation: A Novel Access Route for Nanomedicine. Methodist DeBakey Cardiovasc. J. 2016, 12, 169. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction: Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; Hemingway, H. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef]
- Broz, P.; Ben-Haim, N.; Grzelakowski, M.; Marsch, S.; Meier, W.; Hunziker, P. Inhibition of Macrophage Phagocytotic Activity by a Receptor-targeted Polymer Vesicle-based Drug Delivery Formulation of Pravastatin. J. Cardiovasc. Pharmacol. 2008, 51, 246–252. [Google Scholar] [CrossRef]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van der Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef]
- Buabeid, M.; Arafa, E.S.A.; Yaseen, H.S.; Umar, M.I.; Murtaza, G. Anti-inflammatory effect of simvastatin by impeding TNF-α and interleukin-1ß pathways: Antiangiogenic activity of simvastatin and simvastatin-loaded silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 208–217. [Google Scholar] [CrossRef]
- Duivenvoorden, R.; Tang, J.; Cormode, D.P.; Mieszawska, A.J.; Izquierdo-Garcia, D.; Ozcan, C.; Otten, M.J.; Zaidi, N.; Lobatto, M.E.; Van Rijs, S.M.; et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 2014, 5, 3065. [Google Scholar] [CrossRef]
- Katsuki, S.; Matoba, T.; Nakashiro, S.; Sato, K.; Koga, J.; Nakano, K.; Nakano, Y.; Egusa, S.; Sunagawa, K.; Egashira, K. Nanoparticle-Mediated Delivery of Pitavastatin Inhibits Atherosclerotic Plaque Destabilization/Rupture in Mice by Regulating the Recruitment of Inflammatory Monocytes. Circulation 2014, 129, 896–906. [Google Scholar] [CrossRef]
- Katsuki, S.; Koga, J.I.; Matoba, T.; Umezu, R.; Nakashiro, S.; Nakano, K.; Tsutsui, H.; Egashira, E. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Apoe−/− Mice. J. Atheroscler. Thromb. 2022, 29, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Rashidijahanabad, Z.; Ramadan, S.; Kauffman, N.; Parameswaran, N.; Zinn, K.R.; Qian, C.; Arora, R.; Agnew, D.; Huang, X. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 2020, 12, 9541–9556. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.; Yue, L.; Wan, J.B.; Lee, S.M.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Heinen, Y.; Stegemann, E.; Sansone, R.; Benedens, K.; Wagstaff, R.; Balzer, J.; Rassaf, T.; Lauer, T.; Kelm, M.; Heiss, C. Local Association Between Endothelial Dysfunction and Intimal Hyperplasia: Relevance in Peripheral Artery Disease. J. Am. Heart Assoc. 2015, 4, e001472. [Google Scholar] [CrossRef] [PubMed]
- Helkin, A.; Bruch, D.; Wilson, D.R.; Gruessner, A.C.; Bader, R.R.; Maier, K.G.; Gahtan, V. Intraluminal Delivery of Simvastatin Attenuates Intimal Hyperplasia After Arterial Injury. Vasc. Endovasc. Surg. 2019, 53, 379–386. [Google Scholar] [CrossRef]
- Sugimoto, M.; Yamanouchi, D.; Komori, K. Therapeutic approach against intimal hyperplasia of vein grafts through endothelial nitric oxide synthase/nitric oxide (eNOS/NO) and the Rho/Rho-kinase pathway. Surg. Today 2009, 39, 459–465. [Google Scholar] [CrossRef]
- Hide, D.; Gil, M.; Andrade, F.; Rafael, D.; Raurell, I.; Bravo, M.; Barberá, A.; Gracia-Sancho, J.; Vargas, V.; Augustin, S.; et al. Simvastatin-loaded polymeric micelles are more effective and less toxic than conventional statins in a pre-clinical model of advanced chronic liver disease. Nanomedicine 2020, 29, 12. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Norata, G.D.; Magnoni, M.; Camici, P.G. The Role of Monocytes and Macrophages in Human Atherosclerosis, Plaque Neoangiogenesis, and Atherothrombosis. Mediat. Inflamm. 2019, 7434376. [Google Scholar] [CrossRef]
- Dunmore, B.J.; McCarthy, M.J.; Naylor, A.R.; Brindle, N.P. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J. Vasc. Surg. 2007, 45, 155–159. [Google Scholar] [CrossRef]
- Kamba, T.; McDonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 2007, 96, 1788–1795. [Google Scholar] [CrossRef]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2103–2109. [Google Scholar] [CrossRef]
- Winter, P.M.; Caruthers, S.D.; Zhang, H.; Williams, T.A.; Wickline, S.A.; Lanza, G.M. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc. Imaging 2008, 1, 624–634. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Ovize, M.; Baxter, G.F.; Di Lisa, F.; Ferdinandy, P.; Garcia-Dorado, D.; Hausenloy, D.J.; Heusch, G.; Vinten-Johansen, J.; Yellon, D.M.; Schulz, R. Postconditioning and protection from reperfusion injury: Where do we stand? Position Paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2010, 87, 406–423. [Google Scholar] [CrossRef]
- Post, S.; Post, M.C.; van den Branden, B.J.; Eefting, F.D.; Goumans, M.J.; Stella, P.R.; van Es, H.W.; Wildbergh, T.X.; Rensing, B.J.; Doevendans, P.A. Doevendans Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo-controlled pilot trial. Catheter. Cardiovasc. Interv. 2012, 80, 756–765. [Google Scholar] [CrossRef]
- Nagaoka, K.; Matoba, T.; Mao, Y.; Egusa, S.; Tokutome, M.; Nagahama, R.; Nakano, K.; Sunagawa, K. A New Therapeutic Modality for Acute Myocardial Infarction: Nanoparticle-Mediated Delivery of Pitavastatin Induces Cardioprotection from Ischemia-Reperfusion Injury via Activation of PI3K/Akt Pathway and Anti-Inflammation in a Rat Model. PLoS ONE 2015, 10, e0132451. [Google Scholar] [CrossRef]
- Cianflone, E.; Cappetta, D.; Mancuso, T.; Sabatino, J.; Marino, F.; Scalise, M.; Albanese, M.; Salatino, A.; Parrotta, E.I.; Cuda, G.; et al. Statins Stimulate New Myocyte Formation After Myocardial Infarction by Activating Growth and Differentiation of the Endogenous Cardiac Stem Cells. Int. J. Mol. Sci. 2020, 21, 7927. [Google Scholar] [CrossRef]
- Yokoyama, R.; Ii, M.; Tabata, Y.; Hoshiga, M.; Ishizaka, N.; Asahi, M. Cardiac Regeneration by Statin-Polymer Nanoparticle-Loaded Adipose-Derived Stem Cell Therapy in Myocardial Infarction. Stem Cells Transl. Med. 2019, 8, 1055–1067. [Google Scholar] [CrossRef]
- Jumabay, M.; Zhang, R.; Yao, Y.; Goldhaber, J.I.; Boström, K.I. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc. Res. 2010, 85, 17–27. [Google Scholar] [CrossRef]
- Francis Stuart, S.D.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef]
- Mao, Y.; Koga, J.I.; Tokutome, M.; Ikeda, G.; Nakano, K.; Egashira, K. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Left Ventricular Remodeling After Acute Myocardial Infarction by Inhibiting Monocyte-Mediated Inflammation. Int. Heart J. 2017, 58, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Huang, X. Nanotechnology for Targeted Therapy of Atherosclerosis. Front. Pharmacol. 2021, 12, 12. [Google Scholar]
- Nakashiro, S.; Matoba, T.; Umezu, R.; Katsuki, S.; Nakano, K.; Sunagawa, K.; Egashira, K. Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE−/− Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 491–500. [Google Scholar] [CrossRef]
- Lampi, M.C.; Faber, C.J.; Huynh, J.; Bordeleau, F.; Zanotelli, M.R.; Reinhart-King, C.A. Simvastatin Ameliorates Matrix Stiffness-Mediated Endothelial Monolayer Disruption. PLoS ONE 2016, 11, e0147033. [Google Scholar] [CrossRef]
- Hasegawa, H. Can Statins Modify the Wound Healing Process After Myocardial Infarction? Int. Heart J. 2017, 58, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Yamashita, S.; Yoshino, S.; Kurose, S.; Morisaki, K.; Nakano, K.; Koga, J.; Furuyama, T.; Mori, M.; Egashira, K. Therapeutic Arteriogenesis/Angiogenesis for Peripheral Arterial Disease by Nanoparticle-Mediated Delivery of Pitavastatin into Vascular Endothelial Cells. Ann. Vasc. Dis. 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Reichert, K.; Pereira do Carmo, H.R.; Galluce Torina, A.; de Carvalho, D.D.Â.; Sposito, A.C.; Vilarinho, K.A.d.; Silveira-Filho, L.d.; de Oliveira, P.P.M. Atorvastatin Improves Ventricular Remodeling after Myocardial Infarction by Interfering with Collagen Metabolism. PLoS ONE 2016, 11, e0166845. [Google Scholar] [CrossRef]
- Spadaccio, C.; Mozetic, P.; Nappi, F.; Nenna, A.; Sutherland, F.; Trombetta, M.; Chello, M.; Rainer, A. Cells and extracellular matrix interplay in cardiac valve disease: Because age matters. Basic Res. Cardiol. 2016, 111, 16. [Google Scholar] [CrossRef]
- Montelione, N.; Catanese, V.; Nenna, A.; Jawabra, M.; Verghi, E.; Loreni, F.; Nappi, F.; Lusin, M.; Mastroianni, C.; Jiritano, F. The Diagnostic Value of Circulating Biomarkers and Role of Drug-Coated Balloons for In-Stent Restenosis in Patients with Peripheral Arterial Disease. Diagnostics 2022, 12, 2207. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Larobina, D.; Martuscelli, G.; Singh, S.S.A.; Chello, M.; Ambrosio, L. The Use of Bioactive Polymers for Intervention and Tissue Engineering: The New Frontier for Cardiovascular Therapy. Polymers 2021, 13, 446. [Google Scholar] [CrossRef]
- Spadaccio, C.; Antoniades, C.; Nenna, A.; Chung, C.; Will, R.; Chello, M.; Gaudino, M.F. Preventing treatment failures in coronary artery disease: What can we learn from the biology of in-stent restenosis, vein graft failure, and internal thoracic arteries? Cardiovasc. Res. 2020, 116, 505–519. [Google Scholar] [CrossRef]
- Hu, H.J.; Zhou, S.H.; Liu, Q.M. Blockade of mTOR pathway inhibition in the neointimal hyperplasia and promoting macrophage autophagy—Effect of statin-eluting stents to reduce in-stent restenosis. Int. J. Cardiol. 2015, 187, 31–32. [Google Scholar] [CrossRef]
- Chu, J.; Chen, L.; Mo, Z.; Bowlin, G.L.; Minden-Birkenmaier, B.A.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Wang, W.; Mo, X. An atorvastatin calcium and poly(L-lactide-co-caprolactone) core-shell nanofiber-covered stent to treat aneurysms and promote reendothelialization. Acta Biomater. 2020, 111, 102–117. [Google Scholar] [CrossRef]
- De Negri Atanasio, G.; Ferrari, P.F.; Baião, A.; Perego, P.; Sarmento, B.; Palombo, D.; Campardelli, R. Bevacizumab encapsulation into PLGA nanoparticles functionalized with immunouteroglobin-1 as an innovative delivery system for atherosclerosis. Int. J. Biol. Macromol. 2022, 221, 1618–1630. [Google Scholar] [CrossRef]
- Hu, P.P.; Luo, S.X.; Fan, X.Q.; Li, D.; Tong, X.Y. Macrophage-targeted nanomedicine for the diagnosis and management of atherosclerosis. Front. Pharmacol. 2022, 13, 1000316. [Google Scholar] [CrossRef]
- Leal, B.H.; Velasco, B.; Cambón, A.; Pardo, A.; Fernandez-Vega, J.; Arellano, L.; Al-Modlej, A.; Mosquera, V.X.; Bouzas, A.; Prieto, G. Combined Therapeutics for Atherosclerosis Treatment Using Polymeric Nanovectors. Pharmaceutics 2022, 14, 258. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and Nanoparticles for Statin Delivery: Current Use and Future Perspectives in Cardiovascular Disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef]
- Nenna, A.; Nappi, F.; Lusini, M.; Satriano, U.M.; Schilirò, D.; Spadaccio, C.; Chello, M. Effect of Statins on Platelet Activation and Function: From Molecular Pathways to Clinical Effects. Biomed. Res. Int. 2021, 2021, 6661847. [Google Scholar] [CrossRef]
- Nenna, A.; Spadaccio, C.; Prestipino, F.; Lusini, M.; Sutherland, F.W.; Beattie, G.W.; Petitti, T.; Nappi, F.; Chello, M. Effect of Preoperative Aspirin Replacement With Enoxaparin in Patients Undergoing Primary Isolated On-Pump Coronary Artery Bypass Grafting. Am. J. Cardiol. 2016, 117, 563–570. [Google Scholar] [CrossRef]
- Nenna, A.; Spadaccio, C.; Lusini, M.; Nappi, F.; Mastroianni, C.; Giacinto, O.; Pugliese, G.; Casacalen-da, A.; Barbato, R.; Barberi, F.; et al. Preoperative atorvastatin reduces bleeding and blood transfusions in patients undergoing elective isolated aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Lusini, M.; Spadaccio, C.; Spadaccio, C.; Nappi, F.; Prestipino, F.; Barbato, R.; Casacalenda, A.; Pugliese, G.; Barberi, F.; et al. Preoperative atorvastatin reduces bleeding and blood products use in patients undergoing on-pump coronary artery bypass grafting. J. Cardiovasc. Med. 2017, 18, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Nenna, A.; Rose, D.; Piccirillo, F.; Nusca, A.; Grigioni, F.; Chello, M.; Vlahakes, G.J. The Role of Angiogenesis and Arteriogenesis in Myocardial Infarction and Coronary Revascularization. J. Cardiovasc. Transl. Res. 2022, 15, 1024–1048. [Google Scholar] [CrossRef]
- Frick, M.; Dulak, J.; Cisowski, J.; Józkowicz, A.; Zwick, R.; Alber, H.; Dichtl, W.; Schwarzacher, S.P.; Pachinger, O.; Weidinger, F. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis 2003, 170, 229–236. [Google Scholar] [CrossRef]
- Alber, H.F.; Dulak, J.; Frick, M.; Dichtl, W.; Schwarzacher, S.P.; Pachinger, O.; Weidinger, F. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J. Am. Coll. Cardiol. 2002, 39, 1951–1955. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Fu, X.; Liu, Y.; Xu, Q.; Sang, L. Effect of simvastatin on expression of VEGF and TGF-beta1 in atherosclerotic animal model of type 2 diabetes mellitus. Exp. Ther. Med. 2018, 16, 2889–2894. [Google Scholar] [CrossRef]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef]
- Schaefer, C.A.; Kuhlmann, C.R.; Gast, C.; Weiterer, S.; Li, F.; Most, A.K.; Neumann, T.; Backenköhler, U.; Tillmanns, H.; Waldecker, B. Statins prevent oxidized low-density lipoprotein- and lysophosphatidylcholine-induced proliferation of human endothelial cells. Vascul. Pharmacol. 2004, 41, 67–73. [Google Scholar] [CrossRef]
- Muck, A.O.; Seeger, H.; Wallwiener, D. Class-specific pro-apoptotic effect of statins on human vascular endothelial cells. Z. Kardiol. 2004, 93, 398–402. [Google Scholar] [CrossRef]
- Newton, C.J.; Xie, Y.X.; Burgoyne, C.H.; Adams, I.; Atkin, S.L.; Abidia, A.; McCollum, P.T. Fluvastatin induces apoptosis of vascular endothelial cells: Blockade by glucocorticoids. Cardiovasc. Surg. 2003, 11, 52–60. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A.; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.A. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.; Qi, X.; Huang, C.; Hou, L. Cholesterol levels and risk of hemorrhagic stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1833–1839. [Google Scholar] [CrossRef]
- Ma, C.; Gurol, M.E.; Huang, Z.; Lichtenstein, A.H.; Wang, X.; Wang, Y.; Neumann, S.; Wu, S.; Gao, X. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: A prospective study. Neurology 2019, 93, e445–e457. [Google Scholar] [CrossRef]
- Ziff, O.J.; Banerjee, G.; Ambler, G.; Werring, D.J. Statins and the risk of intracerebral haemorrhage in patients with stroke: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 75–83. [Google Scholar] [CrossRef]
- Ribe, A.R.; Vestergaard, C.H.; Vestergaard, M.; Fenger-Grøn, M.; Pedersen, H.S.; Lietzen, L.W.; Brynningse, P.K.N. Statins and Risk of Intracerebral Haemorrhage in a Stroke-Free Population: A Nationwide Danish Propensity Score Matched Cohort Study. EClinicalMedicine 2019, 8, 78–84. [Google Scholar] [CrossRef]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Béjot, Y.; Cabrejo, L.; Cha, J.K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2020, 382, 9. [Google Scholar] [CrossRef]
- Rikala, M.; Hauta-Aho, M.; Helin-Salmivaara, A.; Lassila, R.; Korhonen, M.J.; Huupponen, R. Co-Prescribing of Potentially Interacting Drugs during Warfarin Therapy-A Population-Based Register Study. Basic Clin. Pharm. Toxicol. 2015, 117, 126–132. [Google Scholar] [CrossRef]
- Shin, D.; Yoon, D.; Lim, S.G.; Hong, J.M.; Park, R.W.; Lee, J.S. Comparison of the Risk of Gastrointestinal Bleeding among Different Statin Exposures with Concomitant Administration of Warfarin: Electronic Health Record-Based Retrospective Cohort Study. PLoS ONE 2016, 11, e0158130. [Google Scholar] [CrossRef]
- Schelleman, H.; Bilker, W.B.; Brensinger, C.M.; Wan, F.; Yang, Y.; Hennessy, S. Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. Am. J. Med. 2010, 123, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Van Rein, N.; Biedermann, J.S.; Bonafacio, S.M.; Kruip, M.J.; van der Meer, F.J.; Lijfering, W.M. Statin use decreases coagulation in users of vitamin K antagonists. Eur. J. Clin. Pharmacol. 2016, 72, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Badillo, R.; Schmidt, R.; Mortensen, E.M.; Frei, C.R.; Mansi, I. Statin therapy and gastrointestinal hemorrhage: A retrospective cohort study with propensity score-matching. Pharmacoepidemiol. Drug Saf. 2015, 24, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Atar, S.; Cannon, C.P.; Murphy, S.A.; Rosanio, S.; Uretsky, B.F.; Birnbaum, Y. Statins are associated with lower risk of gastrointestinal bleeding in patients with unstable coronary syndromes: Analysis of the Orbofiban in Patients with Unstable coronary Syndromes-Thrombolysis In Myocardial Infarction 16 (OPUS-TIMI 16) trial. Am. Heart J. 2006, 151, e971–e976. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.; Di Minno, M.N.; Mumoli, N.; Pomero, F.; Franchini, M.; Bellesini, M.; Lupoli, R.; Sabatini, S.; Borretta, V.; Bonfanti, C.; et al. Statin use and bleeding risk during vitamin K antagonist treatment for venous thromboembolism: A multicenter retrospective cohort study. Haematologica 2015, 100, e295–e298. [Google Scholar] [CrossRef]
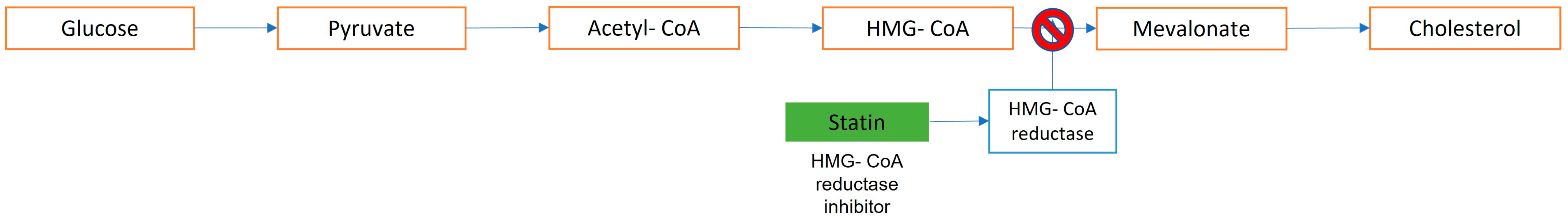
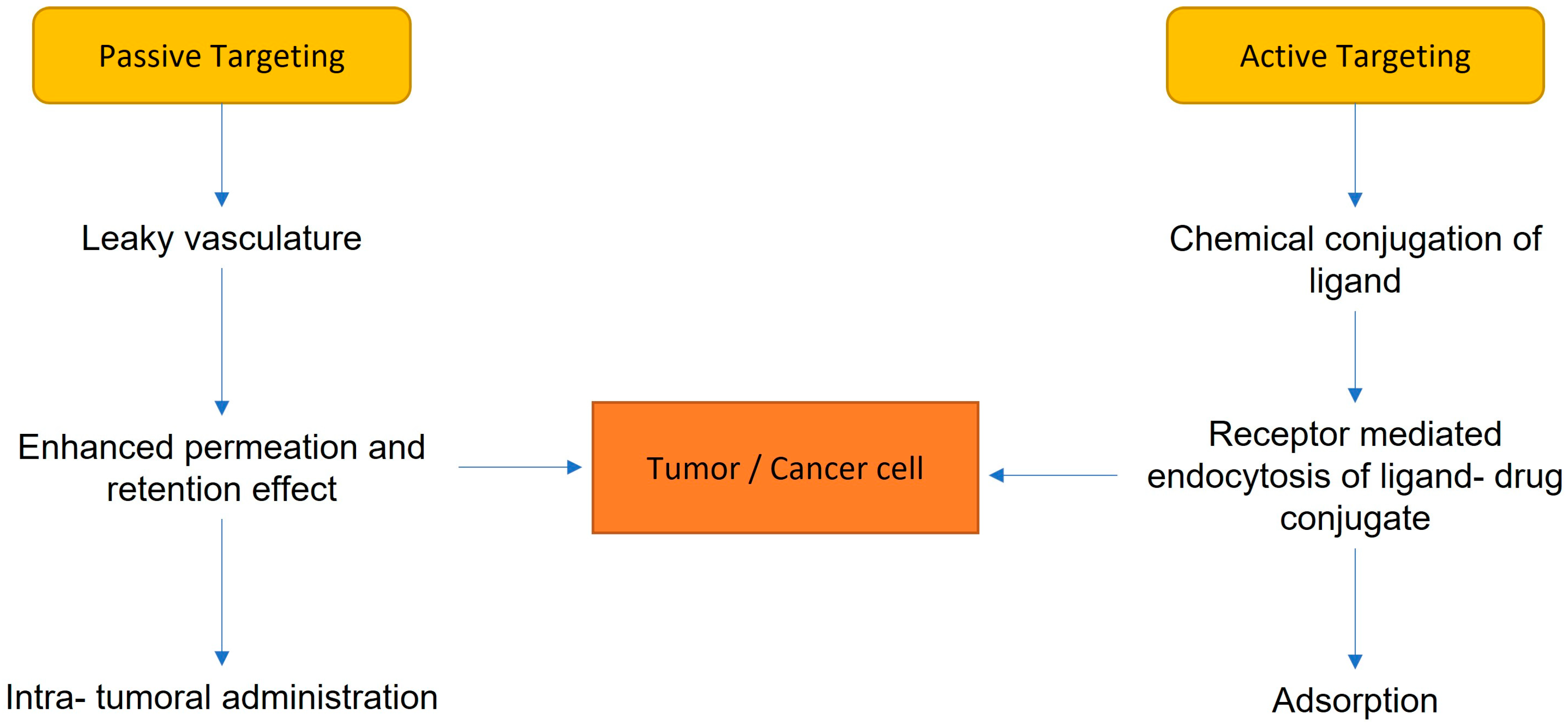


| Micelle | Liposome | Polymer Nanoparticle | Dendrimer | |
|---|---|---|---|---|
| Geometry | Vesicles formed from a monolayer of synthetic lipids or amphiphiles | Vesicles formed by double layers of phospholipids | Formation of macromolecular polymers | Highly branched macromolecules from a central nucleus |
| Size (nm) | 10–100 | 40–1000 | 20–1000 | 3–20 |
| Features | Incorporation of hydrophobic agents inside | Encapsulation of hydrophilic agents, incorporation of hydrophobic agents into the membrane | Incorporation of hydrophilic and hydrophobic agents with controlled release of embedded agents | Multivalent properties by exterior function groups |
| Parameter | Atorvastatin | Simvastatin | Rosuvastatin | Pitavastatin | Pravastatin |
|---|---|---|---|---|---|
| Prodrug | No | Yes | No | No | No |
| Hydrophilic | No | No | Yes | No | Yes |
| Fraction Absorbed (%) | 30 | 70 | Unknown | 80 | 34 |
| Bioavailability (%) | 12 | 5 | 20 | 80 | 18 |
| Active Metabolites | Yes | Yes | Yes | No | Yes |
| Half-Life (hours) | 15–30 | 2–3 | 20 | 11 | 1.5–2.5 |
| Hepatic Metabolism (%) | 70 | 78–87 | 63 | Unknown | 45–65 |
| Renal Metabolism (%) | 2 | 13 | 10 | 2 | 60 |
| PLGA | Poly-di-mehyl-siloxane Poly-2-2methyl-oxazoline | Nanoliposomes | Polysialic Acid–Polycaprolactone |
|---|---|---|---|
| ↑ Chemotactic proteins | ↓ Macrophage activation | ↓ Isoproterenol | ↓ Nitric oxide |
| ↑ Postischemic permeability | ↓ Oxidative burden | ↓ Fibrosis | ↓ Rho pathway |
| ↑ Growth factor | ↓ Inflammatory burden | ↓ Inflammation | |
| ↑ Micro/macrovascular angiogenesis | |||
| ↓ Monocyte mobilization |
| Drug | Carrier Type | Diameter (nm) | Targeted Cell Types/Receptors | Outcomes Compared to Free Drug | Superior Compared to Free Drug |
|---|---|---|---|---|---|
| Pitavastatin | PLGA | 196 | Alveolar macrophages, smooth muscle cells | eNOS ↑ (40%), NF-κB ↓ (60%) Smooth muscle cells ↓ Pulmonary hypertension ↓ Survival ↑ (20%) Chemotactic proteins ↑ Post ischemic permeability ↑ Growth factor ↑ Micro/macrovascular angiogenesis ↑ Monocyte mobilization ↓ | Yes |
| Simvastatin | PLGA | 233 | VCAM-1 | No | |
| Simvastatin | DSPC, DSPG, cholesterol | 164 | Macrophages, monocytes | Monocytes ↓ (24%) Stenosis ↓ (33%) | Yes |
| Simvastatin | rHDL | 26 | Macrophages, endothelial cells | Plaque area ↓ (36%), macrophages ↓ (84%) Inflammation ↓ Fibrosis ↓ | Yes |
| Pravastatin | PDMS/PMOXA | 97 | SR-A1 | LDL uptake ↓ | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montelione, N.; Loreni, F.; Nenna, A.; Catanese, V.; Scurto, L.; Ferrisi, C.; Jawabra, M.; Gabellini, T.; Codispoti, F.A.; Spinelli, F.; et al. Tissue Engineering and Targeted Drug Delivery in Cardiovascular Disease: The Role of Polymer Nanocarrier for Statin Therapy. Biomedicines 2023, 11, 798. https://doi.org/10.3390/biomedicines11030798
Montelione N, Loreni F, Nenna A, Catanese V, Scurto L, Ferrisi C, Jawabra M, Gabellini T, Codispoti FA, Spinelli F, et al. Tissue Engineering and Targeted Drug Delivery in Cardiovascular Disease: The Role of Polymer Nanocarrier for Statin Therapy. Biomedicines. 2023; 11(3):798. https://doi.org/10.3390/biomedicines11030798
Chicago/Turabian StyleMontelione, Nunzio, Francesco Loreni, Antonio Nenna, Vincenzo Catanese, Lucia Scurto, Chiara Ferrisi, Mohamad Jawabra, Teresa Gabellini, Francesco Alberto Codispoti, Francesco Spinelli, and et al. 2023. "Tissue Engineering and Targeted Drug Delivery in Cardiovascular Disease: The Role of Polymer Nanocarrier for Statin Therapy" Biomedicines 11, no. 3: 798. https://doi.org/10.3390/biomedicines11030798
APA StyleMontelione, N., Loreni, F., Nenna, A., Catanese, V., Scurto, L., Ferrisi, C., Jawabra, M., Gabellini, T., Codispoti, F. A., Spinelli, F., Chello, M., & Stilo, F. (2023). Tissue Engineering and Targeted Drug Delivery in Cardiovascular Disease: The Role of Polymer Nanocarrier for Statin Therapy. Biomedicines, 11(3), 798. https://doi.org/10.3390/biomedicines11030798









