Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
2.2. Exosome miRNAs’ Isolation and Quantitative PCR
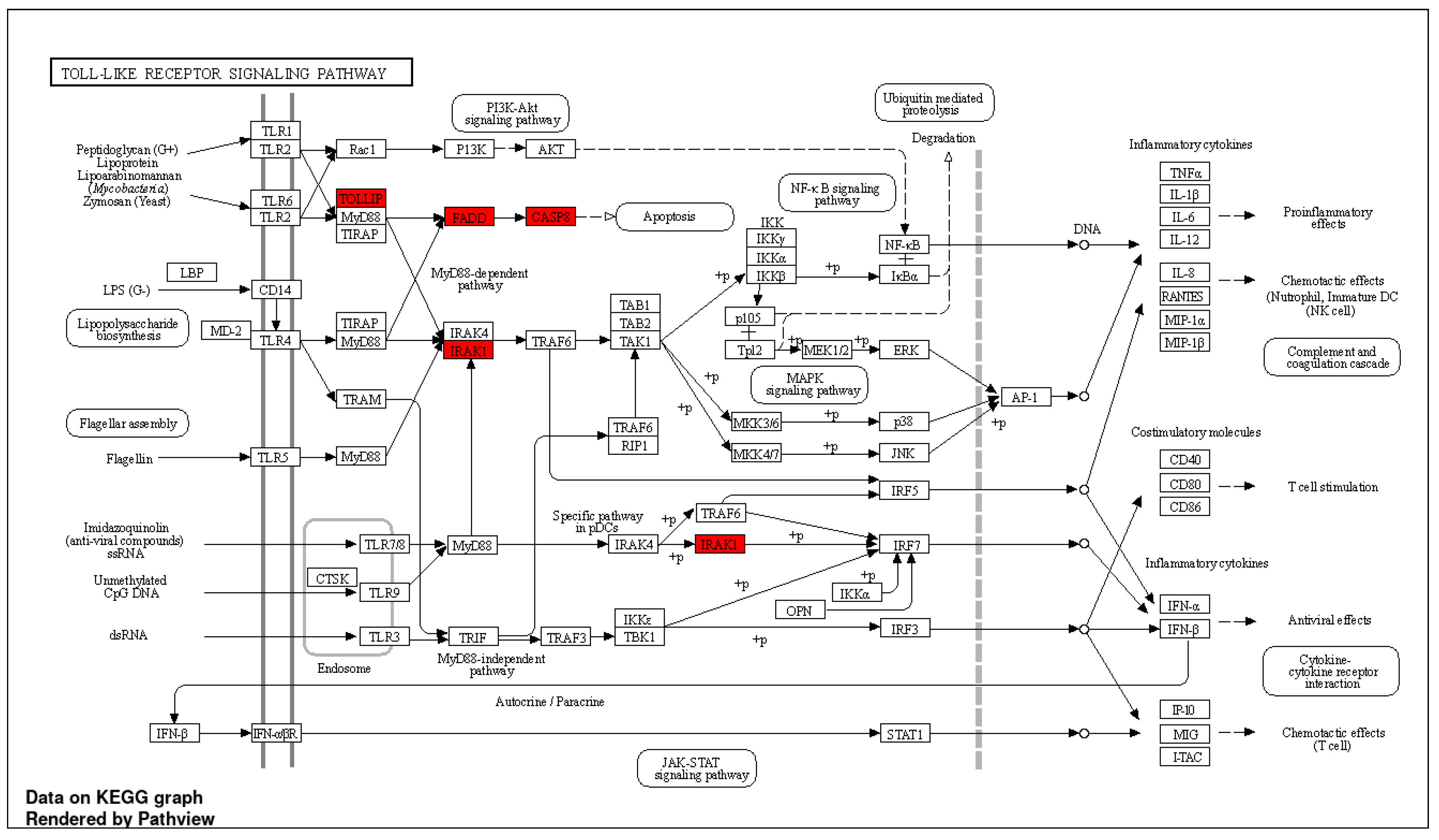
2.3. miRNA Target Prediction and Pathway Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
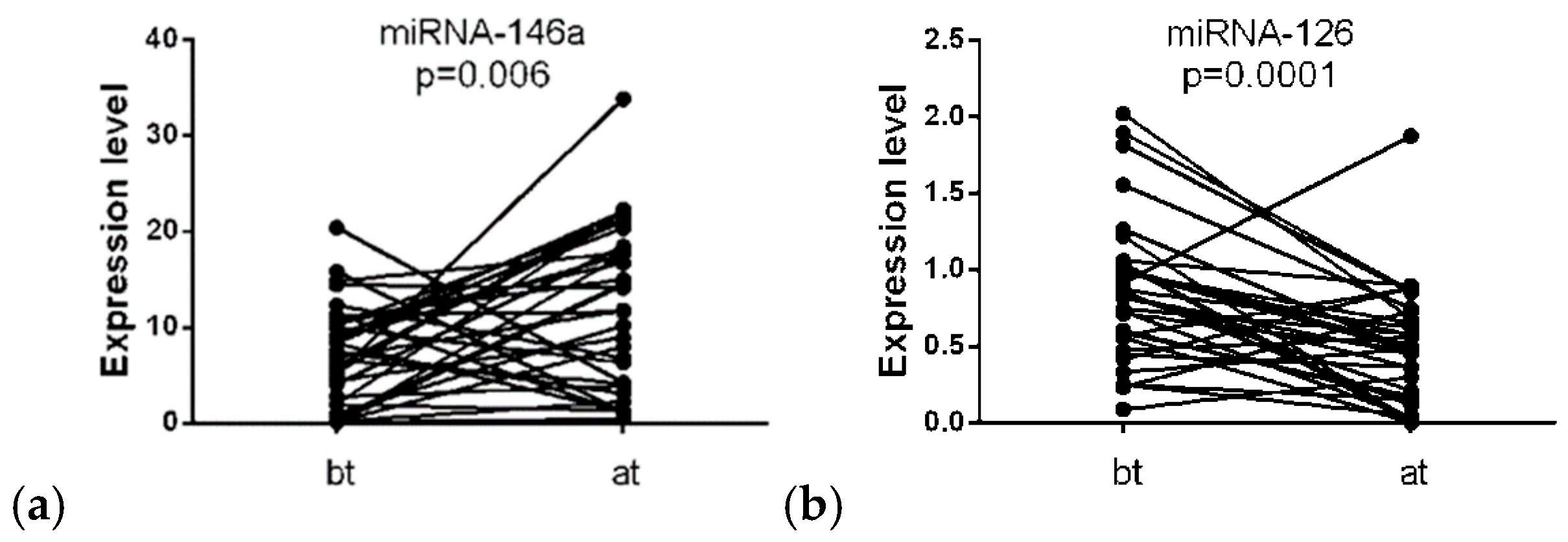
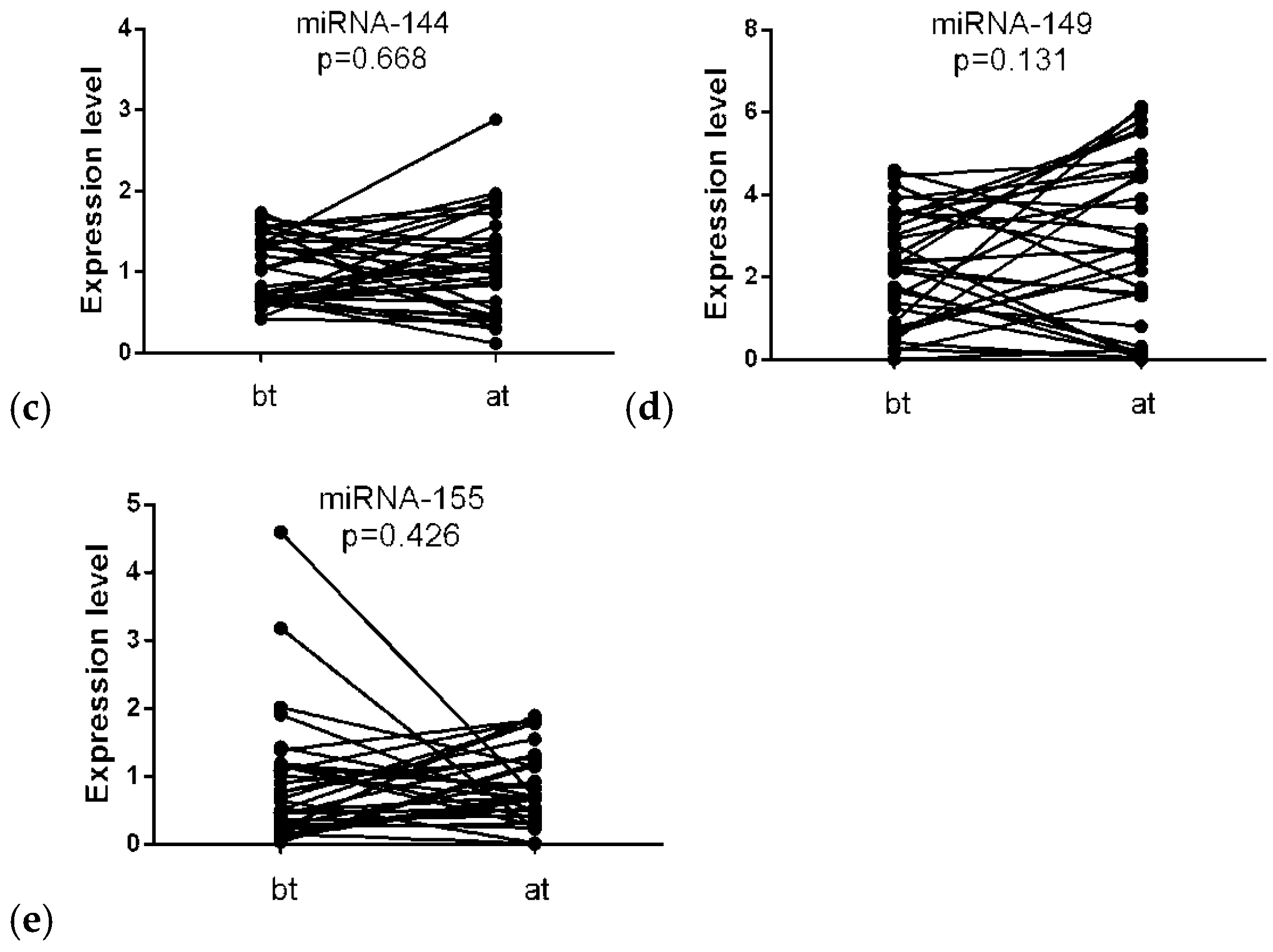
3.2. Differential Expression of miRNA
3.3. Association of miRNA-146a and miRNA-126 Expression Levels with Immune-Related Adverse Events
3.4. Association between miRNA-146a Expression and ccRCC Patient Overall Survival
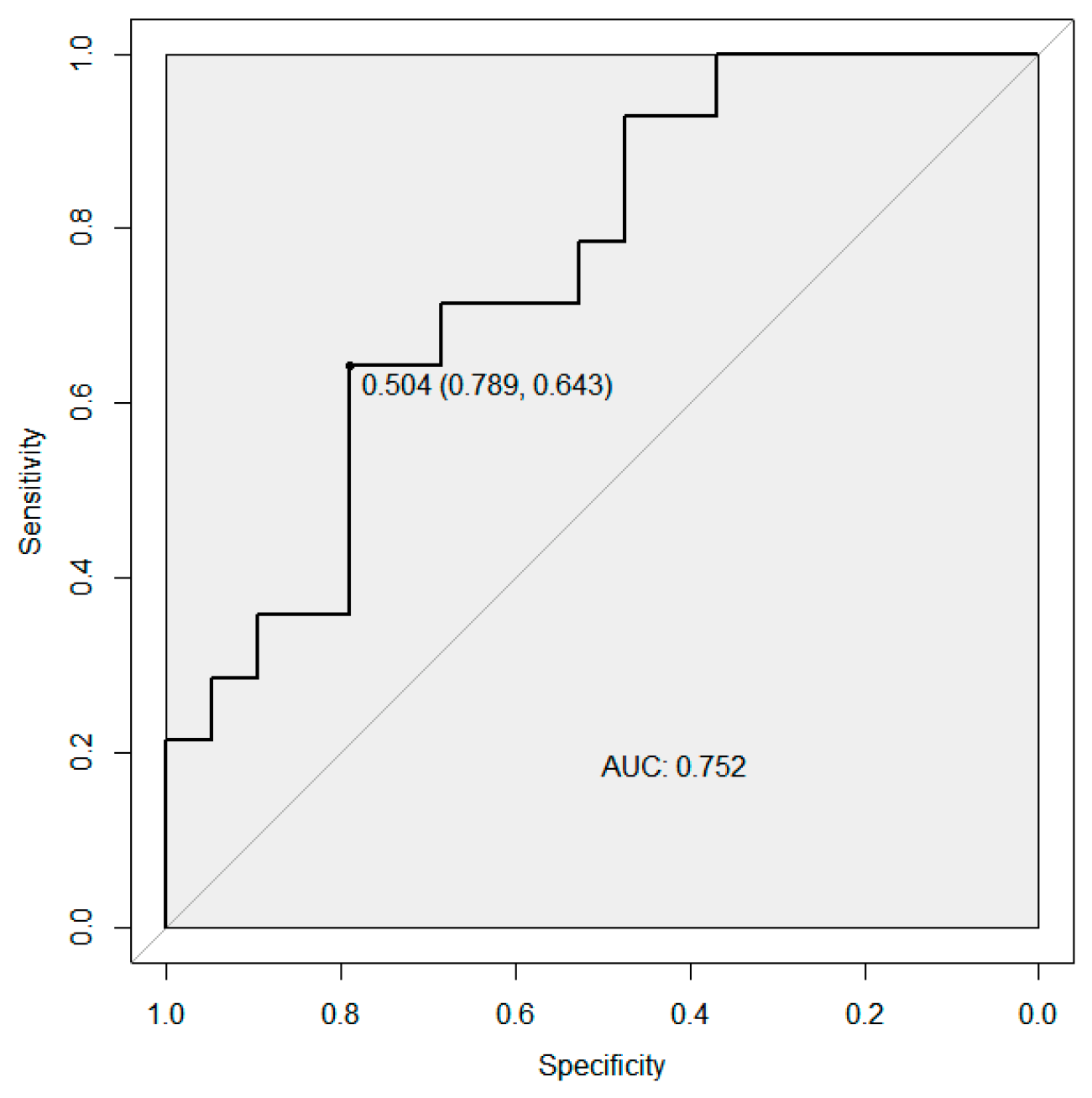
3.5. Logistic Regression and ROC Curve Analyses
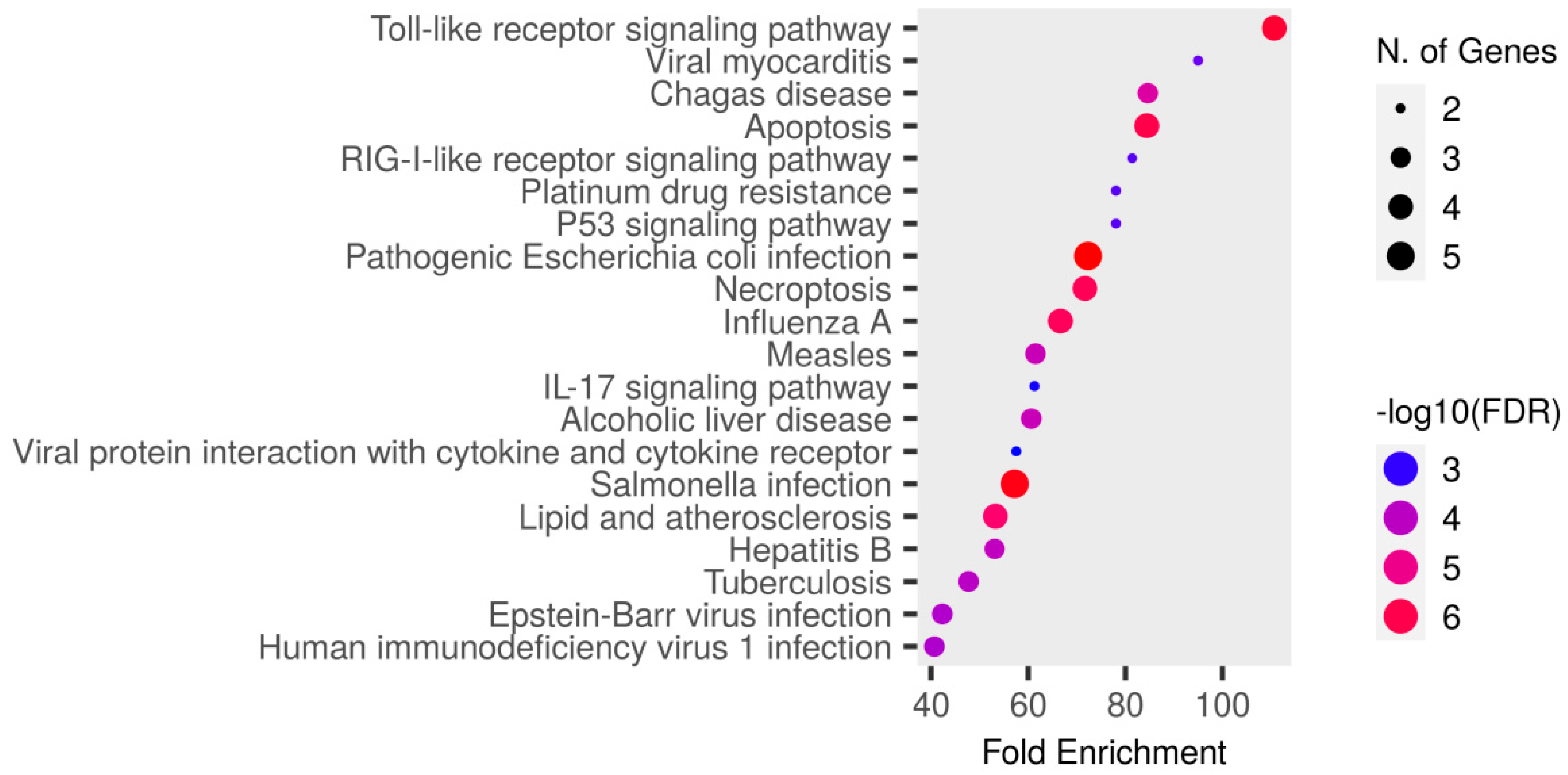
3.6. Pathway Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; Grivas, P. Emerging Role of Immunotherapy in Advanced Urothelial Carcinoma. Curr. Oncol. Rep. 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Mataraza, J.M.; Gotwals, P. Recent Advances in Immuno-Oncology and Its Application to Urological Cancers. BJU Int. 2016, 118, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Poprach, A.; Lakomý, R.; Büchler, T. Immunotherapy of Renal Cell Carcinoma. Klin. Onkol. 2017, 30, 3S55–3S61. [Google Scholar] [CrossRef] [PubMed]
- Sakamuri, D.; Glitza, I.C.; Betancourt Cuellar, S.L.; Subbiah, V.; Fu, S.; Tsimberidou, A.M.; Wheler, J.J.; Hong, D.S.; Naing, A.; Falchook, G.S.; et al. Phase I Dose-Escalation Study of Anti-CTLA-4 Antibody Ipilimumab and Lenalidomide in Patients with Advanced Cancers. Mol. Cancer Ther. 2018, 17, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Lang, E. The Most Recent Oncologic Emergency: What Emergency Physicians Need to Know About the Potential Complications of Immune Checkpoint Inhibitors. Cureus 2017, 9, e1774. [Google Scholar] [CrossRef]
- Ross, K.; Jones, R.J. Immune Checkpoint Inhibitors in Renal Cell Carcinoma. Clin. Sci. 2017, 131, 2627–2642. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Klyuchagina, Y.I.; Sokolova, Z.A.; Baryshnikova, M.A. Role of pd-1 receptor and its ligands pd-l1 and pd-l2 in cancer immunotherapy. Oncopediatrics 2017, 4, 49–55. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Uruga, H.; Mino-Kenudson, M. Predictive Biomarkers for Response to Immune Checkpoint Inhibitors in Lung Cancer: PD-L1 and Beyond. Virchows Arch. 2021, 478, 31–44. [Google Scholar] [CrossRef]
- Reed, T.; Schorey, J.; D’Souza-Schorey, C. Tumor-Derived Extracellular Vesicles: A Means of Co-Opting Macrophage Polarization in the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 746432. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, S.; Li, X.; Kim, B.Y.S.; Yang, Z.; Jiang, W. Extracellular Vesicles: An Emerging Nanoplatform for Cancer Therapy. Front. Oncol. 2021, 10, 606906. [Google Scholar] [CrossRef]
- Jabalee, J.; Towle, R.; Garnis, C. The Role of Extracellular Vesicles in Cancer: Cargo, Function, and Therapeutic Implications. Cells 2018, 7, 93. [Google Scholar] [CrossRef]
- Gupta, H.; Sahu, P.K.; Pattnaik, R.; Mohanty, A.; Majhi, M.; Mohanty, A.K.; Pirpamer, L.; Hoffmann, A.; Mohanty, S.; Wassmer, S.C. Plasma Levels of Hsa-MiR-3158-3p MicroRNA on Admission Correlate with MRI Findings and Predict Outcome in Cerebral Malaria. Clin. Transl. Med. 2021, 11, e396. [Google Scholar] [CrossRef]
- Ivanova, E.; Asadullina, D.; Rakhimov, R.; Izmailov, A.; Izmailov, A.; Gilyazova, G.; Galimov, S.; Pavlov, V.; Khusnutdinova, E.; Gilyazova, I. Exosomal MiRNA-146a Is Downregulated in Clear Cell Renal Cell Carcinoma Patients with Severe Immune-Related Adverse Events. Non-Coding RNA Res. 2022, 7, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). Protocol Development|CTEP. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 28 June 2022).
- Sethupathy, P.; Corda, B.; Hatzigeorgiou, A.G. TarBase: A Comprehensive Database of Experimentally Supported Animal MicroRNA Targets. RNA 2006, 12, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, Y.C.D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. MiRTarBase Update 2022: An Informative Resource for Experimentally Validated MiRNA–Target Interactions. Nucleic Acids Res. 2022, 50, D222. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C.; et al. STRING v9.1: Protein-Protein Interaction Networks, with Increased Coverage and Integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.T.; Riillo, C.; Scionti, F.; Grillone, K.; Polerà, N.; Caracciolo, D.; Arbitrio, M.; Tagliaferri, P.; Tassone, P. MiRNAs and LncRNAs as Novel Therapeutic Targets to Improve Cancer Immunotherapy. Cancers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Halvorsen, A.R.; Sandhu, V.; Sprauten, M.; Flote, V.G.; Kure, E.H.; Brustugun, O.T.; Helland, Å. Circulating MicroRNAs Associated with Prolonged Overall Survival in Lung Cancer Patients Treated with Nivolumab. Acta Oncol. 2018, 57, 1225–1231. [Google Scholar] [CrossRef]
- Peng, X.X.; Yu, R.Y.; Wu, X.; Wu, S.Y.; Pi, C.; Chen, Z.H.; Zhang, X.C.; Gao, C.Y.; Shao, Y.W.; Liu, L.; et al. Correlation of Plasma Exosomal MicroRNAs with the Efficacy of Immunotherapy in EGFR/ALK Wild-Type Advanced Non-Small Cell Lung Cancer. J. Immunother. Cancer 2020, 8, e000376. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Zalfa, F.; Iuliani, M.; Simonetti, S.; Manca, P.; Napolitano, A.; Tiberi, S.; Russano, M.; Citarella, F.; Foderaro, S.; et al. Large-Scale Profiling of Extracellular Vesicles Identified MiR-625-5p as a Novel Biomarker of Immunotherapy Response in Advanced Non-Small-Cell Lung Cancer Patients. Cancers 2022, 14, 2435. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.F.; et al. Predictive Role of Plasmatic Biomarkers in Advanced Non-Small Cell Lung Cancer Treated by Nivolumab. Oncoimmunology 2018, 7, e1452581. [Google Scholar] [CrossRef]
- Tartarone, A.; Lerose, R.; Tartarone, M.; Aieta, M. Potential Role of Tumor-Derived Exosomes in Non-Small-Cell Lung Cancer in the Era of Immunotherapy. Life 2022, 12, 2104. [Google Scholar] [CrossRef]
- Incorvaia, L.; Fanale, D.; Badalamenti, G.; Brando, C.; Bono, M.; De Luca, I.; Algeri, L.; Bonasera, A.; Corsini, L.R.; Scurria, S.; et al. A “Lymphocyte MicroRNA Signature” as Predictive Biomarker of Immunotherapy Response and Plasma PD-1/PD-L1 Expression Levels in Patients with Metastatic Renal Cell Carcinoma: Pointing towards Epigenetic Reprogramming. Cancers 2020, 12, 3396. [Google Scholar] [CrossRef]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Müller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a Regulates Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors. JCI Insight 2020, 5, e132334. [Google Scholar] [CrossRef]
- Waschbisch, A.; Atiya, M.; Linker, R.A.; Potapov, S.; Schwab, S.; Derfuss, T. Glatiramer Acetate Treatment Normalizes Deregulated MicroRNA Expression in Relapsing Remitting Multiple Sclerosis. PLoS ONE 2011, 6, e24604. [Google Scholar] [CrossRef] [PubMed]
- Saba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef]
- Huber, V.; Vallacchi, V.; Fleming, V.; Hu, X.; Cova, A.; Dugo, M.; Shahaj, E.; Sulsenti, R.; Vergani, E.; Filipazzi, P.; et al. Tumor-Derived MicroRNAs Induce Myeloid Suppressor Cells and Predict Immunotherapy Resistance in Melanoma. J. Clin. Investig. 2018, 128, 5517–5530. [Google Scholar] [CrossRef] [PubMed]
- Ouzounova, M.; Lee, E.; Piranlioglu, R.; El Andaloussi, A.; Kolhe, R.; Demirci, M.F.; Marasco, D.; Asm, I.; Chadli, A.; Hassan, K.A.; et al. Monocytic and Granulocytic Myeloid Derived Suppressor Cells Differentially Regulate Spatiotemporal Tumour Plasticity during Metastatic Cascade. Nat. Commun. 2017, 8, 14979. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 Regulates Endothelial Expression of Vascular Cell Adhesion Molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Carlsson, J.; Christiansen, J.; Davidsson, S.; Giunchi, F.; Fiorentino, M.; Sundqvist, P. The Potential Role of MiR-126, MiR-21 and MiR-10b as Prognostic Biomarkers in Renal Cell Carcinoma. Oncol. Lett. 2019, 17, 4566–4574. [Google Scholar] [CrossRef]
- Snowdon, J.; Boag, S.; Feilotter, H.; Izard, J.; Siemens, D.R. A Pilot Study of Urinary MicroRNA as a Biomarker for Urothelial Cancer. Can. Urol. Assoc. J. 2013, 7, 28–32. [Google Scholar] [CrossRef]
- Khella, H.W.Z.; Scorilas, A.; Mozes, R.; Mirham, L.; Lianidou, E.; Krylov, S.N.; Lee, J.Y.; Ordon, M.; Stewart, R.; Jewett, M.A.S.; et al. Low Expression of MiR-126 Is a Prognostic Marker for Metastatic Clear Cell Renal Cell Carcinoma. Am. J. Pathol. 2015, 185, 693–703. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Gopalan, V.; Smith, R.A.; Lam, A.K.Y. MiR-126 in Human Cancers: Clinical Roles and Current Perspectives. Exp. Mol. Pathol. 2014, 96, 98–107. [Google Scholar] [CrossRef]
- Casciaro, M.; Di Salvo, E.; Brizzi, T.; Rodolico, C.; Gangemi, S. Involvement of MiR-126 in Autoimmune Disorders. Clin. Mol. Allergy 2018, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Chipollini, J.; da Costa, W.H.; Werneck da Cunha, I.; de Almeida e Paula, F.; Guilherme, O.; Salles, P.; Azizi, M.; Spiess, P.E.; Abreu, D.; de Cássio Zequi, S. Prognostic Value of PD-L1 Expression for Surgically Treated Localized Renal Cell Carcinoma: Implications for Risk Stratification and Adjuvant Therapies. Ther. Adv. Urol. 2019, 11, 1756287219882600. [Google Scholar] [CrossRef]
- Walter, B.A.; Valera, V.A.; Pinto, P.A.; Merino, M.J. Comprehensive MicroRNA Profiling of Prostate Cancer. J. Cancer 2013, 4, 350–357. [Google Scholar] [CrossRef]
- Agudo, J.; Ruzo, A.; Tung, N.; Salmon, H.; Leboeuf, M.; Hashimoto, D.; Becker, C.; Garrett-Sinha, L.A.; Baccarini, A.; Merad, M.; et al. The MiR-126-VEGFR2 Axis Controls the Innate Response to Pathogen-Associated Nucleic Acids. Nat. Immunol. 2014, 15, 54–62. [Google Scholar] [CrossRef]
- Huang, T.H.; Chu, T.Y. Repression of MiR-126 and Upregulation of Adrenomedullin in the Stromal Endothelium by Cancer-Stromal Cross Talks Confers Angiogenesis of Cervical Cancer. Oncogene 2014, 33, 3636–3647. [Google Scholar] [CrossRef]
- Khella, H.W.Z.; White, N.M.A.; Faragalla, H.; Gabril, M.; Boazak, M.; Dorian, D.; Khalil, B.; Antonios, H.; Bao, T.T.; Pasic, M.D.; et al. Exploring the Role of MiRNAs in Renal Cell Carcinoma Progression and Metastasis through Bioinformatic and Experimental Analyses. Tumour Biol. 2012, 33, 131–140. [Google Scholar] [CrossRef]
- White, N.M.A.; Khella, H.W.Z.; Grigull, J.; Adzovic, S.; Youssef, Y.M.; Honey, R.J.; Stewart, R.; Pace, K.T.; Bjarnason, G.A.; Jewett, M.A.S.; et al. MiRNA Profiling in Metastatic Renal Cell Carcinoma Reveals a Tumour-Suppressor Effect for MiR-215. Br. J. Cancer 2011, 105, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.L.; Herzog, R.W. One MicroRNA Controls Both Angiogenesis and TLR-Mediated Innate Immunity to Nucleic Acids. Mol. Ther. 2014, 22, 249–250. [Google Scholar] [CrossRef]
- Hartmann, E.; Wollenberg, B.; Rothenfusser, S.; Wagner, M.; Wellisch, D.; Mack, B.; Giese, T.; Gires, O.; Endres, S.; Hartmann, G.; et al. Identification and Functional Analysis of Tumor-Infiltrating Plasmacytoid Dendritic Cells in Head and Neck Cancer. Cancer Res. Am. Assoc. Cancer Res. 2003, 63, 6478–6487. Available online: https://aacrjournals.org/cancerres/article/63/19/6478/510430/Identification-and-Functional-Analysis-of-Tumor (accessed on 15 February 2023).
- Ferretti, C.; La Cava, A. MiR-126, a New Modulator of Innate Immunity. Cell. Mol. Immunol. 2014, 11, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Lechman, E.R.; Gentner, B.; Van Galen, P.; Giustacchini, A.; Saini, M.; Boccalatte, F.E.; Hiramatsu, H.; Restuccia, U.; Bachi, A.; Voisin, V.; et al. Attenuation of MiR-126 Activity Expands HSC in Vivo without Exhaustion. Cell Stem Cell 2012, 11, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Meira, M.; Sievers, C.; Hoffmann, F.; Derfuss, T.; Kuhle, J.; Kappos, L.; Lindberg, R.L.P. MiR-126: A Novel Route for Natalizumab Action? Mult. Scler. 2014, 20, 1363–1370. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | № of Patients | % |
|---|---|---|
| Age, years | 62 | |
| Median | 41–79 | |
| Gender | ||
| Male | 16 | 45.7 |
| Female | 19 | 54.3 |
| Histology | ||
| Clear cell | 35 | 100.0 |
| Non-clear cell | 0 | 0 |
| IMDC risk classification | ||
| Favorable | 2 | 5.71 |
| Intermediate | 25 | 71.43 |
| Poor | 8 | 22.86 |
| Category | Grading, Number of Patients | |
|---|---|---|
| Grades 0–2, n (%) | Grades 3–4, n (%) | |
| Skin-related events | 7 (35.0) | 5 (33.3) |
| Pneumonitis | 1 (5.0) | 5 (33.3) |
| Diarrhea/colitis | 5 (25.0) | 2 (13.3) |
| Endocrine-related events | 12 (60.0) | 3 (20.0) |
| Pancreatitis | 1 (5.0) | 2 (13.3) |
| Colitis | 2 (10.0) | 0 |
| Hepatitis | 1 (5.0) | 1 (6.67) |
| Nephritis | 1 (5.0) | 0 |
| Myalgia | 1 (5.0) | 0 |
| Joint pain | 1 (5.0) | 0 |
| Others | 0 | 2 (13.3) |
| Symbol | Ensembl Gene ID | Chr | Position (Mbp) | Description |
|---|---|---|---|---|
| CASP8 | ENSG00000064012 | 2 | 201.2334 | caspase 8 |
| TNFSF10 | ENSG00000121858 | 3 | 172.5055 | TNF superfamily member 10 |
| TNFRSF10B | ENSG00000120889 | 8 | 23.0201 | TNF receptor superfamily member 10b |
| TOLLIP | ENSG00000078902 | 11 | 1.2744 | Toll-interacting protein |
| EIF4G2 | ENSG00000110321 | 11 | 10.7971 | eukaryotic translation initiation factor 4 gamma 2 |
| FADD | ENSG00000168040 | 11 | 70.2033 | Fas-associated death domain |
| EIF4A1 | ENSG00000161960 | 17 | 7.5728 | eukaryotic translation initiation factor 4A1 |
| IRAK1 | ENSG00000184216 | X | 154.0105 | Interleukin-1 receptor-associated kinase 1 |
| Ontology | Description | Strength | False Discovery Rate |
|---|---|---|---|
| hsa04620 | Toll-like receptor signaling pathway | 1.57 | 0.00052 |
| hsa05130 | Pathogenic Escherichia coli infection | 1.4 | 0.00052 |
| hsa05132 | Salmonella infection | 1.35 | 0.00052 |
| hsa04210 | Apoptosis | 1.45 | 0.0010 |
| hsa04217 | Necroptosis | 1.4 | 0.0013 |
| hsa05164 | Influenza A | 1.35 | 0.0016 |
| hsa05142 | Chagas disease | 1.45 | 0.0082 |
| hsa04668 | TNF signaling pathway | 1.4 | 0.0102 |
| hsa05162 | Measles | 1.31 | 0.0165 |
| hsa04215 | Apoptosis—multiple species | 1.79 | 0.0179 |
| hsa05161 | Hepatitis B | 1.25 | 0.0202 |
| hsa05152 | Tuberculosis | 1.22 | 0.0217 |
| hsa05169 | Epstein–Barr virus infection | 1.16 | 0.0297 |
| hsa05170 | Human immunodeficiency virus 1 infection | 1.14 | 0.0323 |
| hsa05416 | Viral myocarditis | 1.53 | 0.0378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, E.; Asadullina, D.; Gilyazova, G.; Rakhimov, R.; Izmailov, A.; Pavlov, V.; Khusnutdinova, E.; Gilyazova, I. Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma. Biomedicines 2023, 11, 801. https://doi.org/10.3390/biomedicines11030801
Ivanova E, Asadullina D, Gilyazova G, Rakhimov R, Izmailov A, Pavlov V, Khusnutdinova E, Gilyazova I. Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma. Biomedicines. 2023; 11(3):801. https://doi.org/10.3390/biomedicines11030801
Chicago/Turabian StyleIvanova, Elizaveta, Dilara Asadullina, Gulshat Gilyazova, Radmir Rakhimov, Adel Izmailov, Valentin Pavlov, Elza Khusnutdinova, and Irina Gilyazova. 2023. "Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma" Biomedicines 11, no. 3: 801. https://doi.org/10.3390/biomedicines11030801
APA StyleIvanova, E., Asadullina, D., Gilyazova, G., Rakhimov, R., Izmailov, A., Pavlov, V., Khusnutdinova, E., & Gilyazova, I. (2023). Exosomal MicroRNA Levels Associated with Immune Checkpoint Inhibitor Therapy in Clear Cell Renal Cell Carcinoma. Biomedicines, 11(3), 801. https://doi.org/10.3390/biomedicines11030801






