Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohorts
2.2. Diagnostic and Analytical Methods
2.3. Radiological Evaluation of NET Disease
2.4. Histological Diagnosis
2.5. Statistical Analyses
3. Results
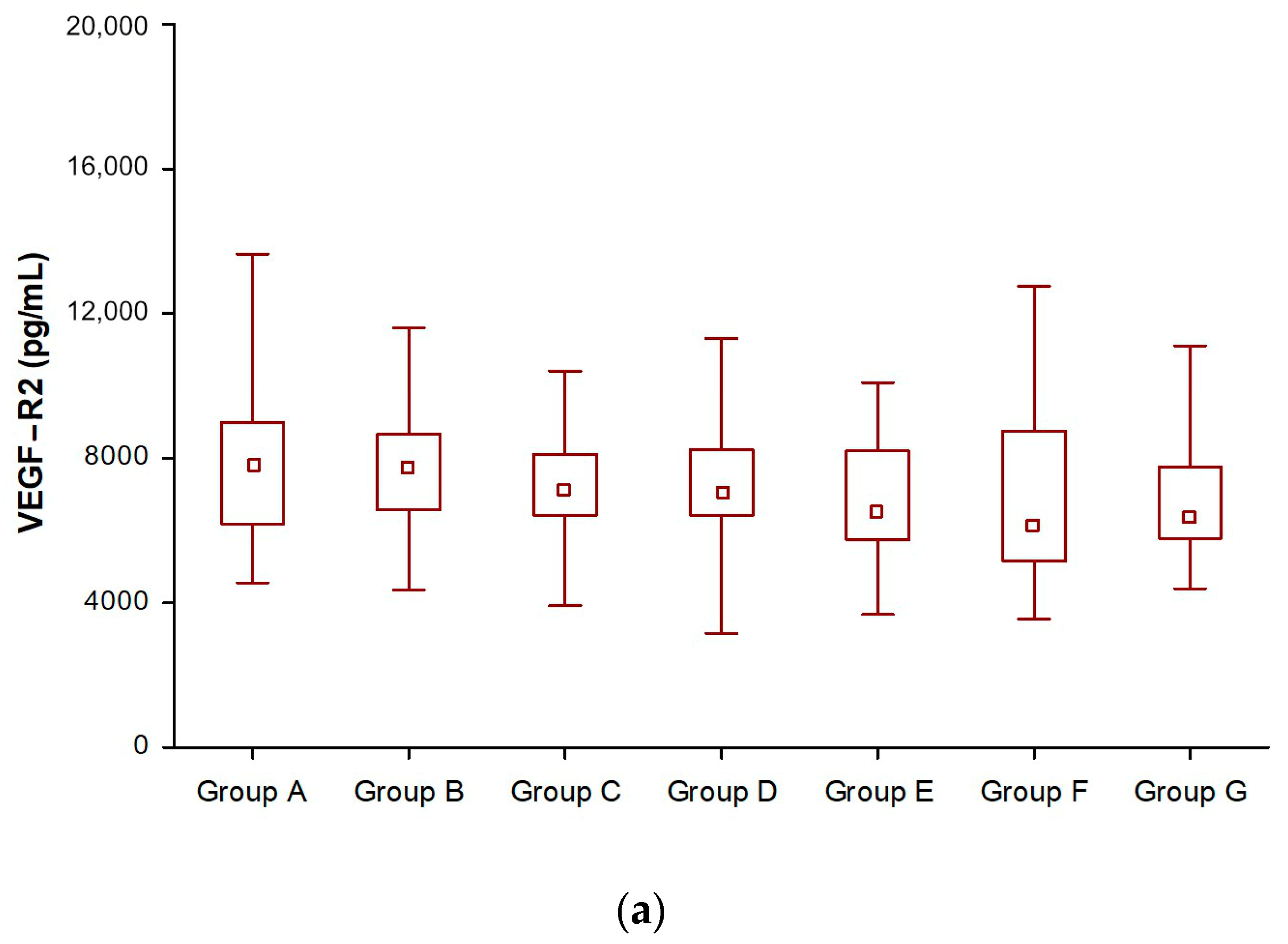
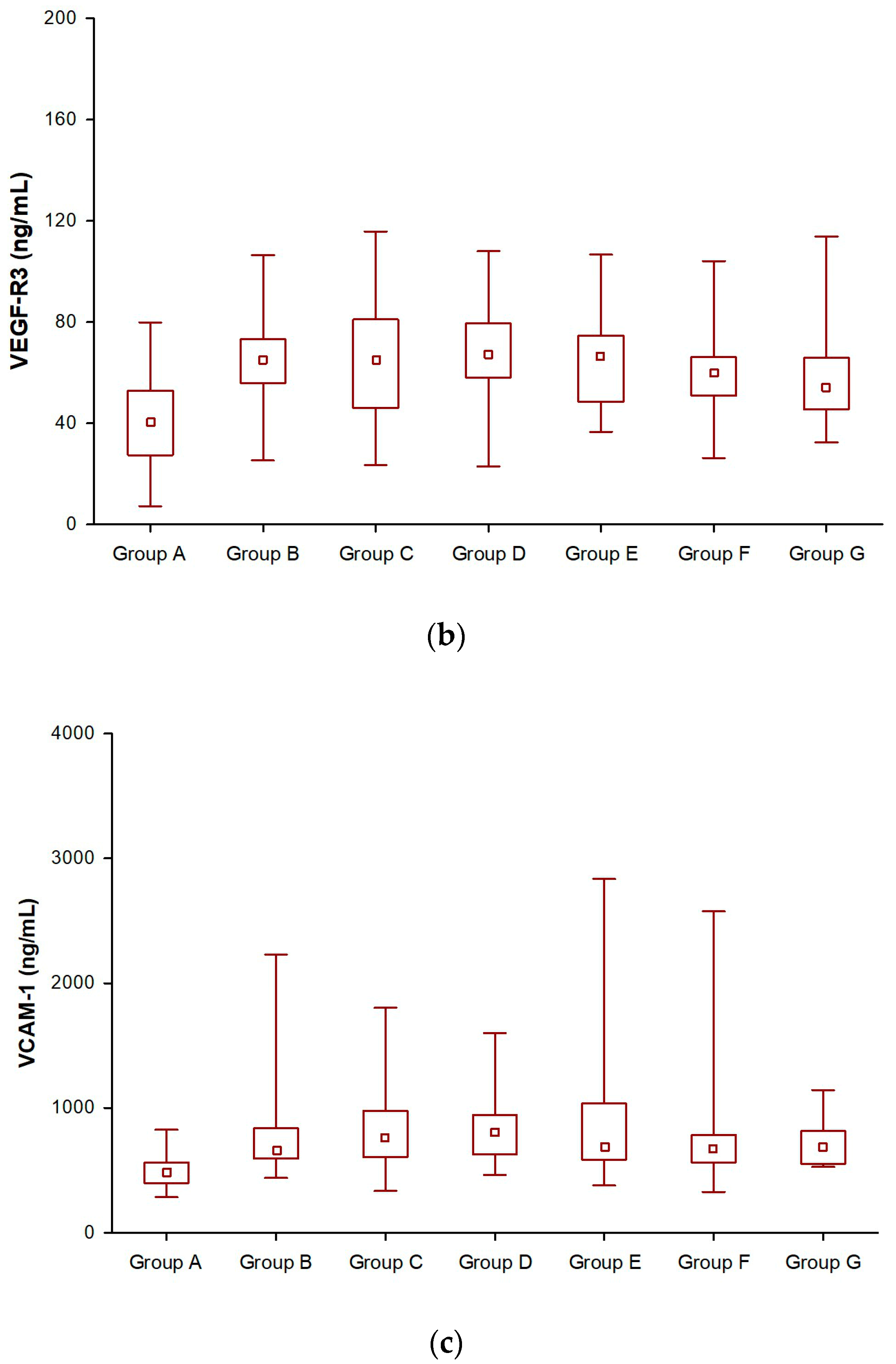
3.1. Angiogenic Factors (VEGF-R2, VEGF-R3, and VCAM-1) in SSA-Treated NET Patients
3.2. Assessment of Angiogenic Factors (VEGF-R2, VEGF-R3, and VCAM-1) Levels According to Tumour Grade
3.3. Assessment of Angiogenic Factors (VEGF-R2, VEGF-R3, and VCAM-1) Levels According to the Disease Stage
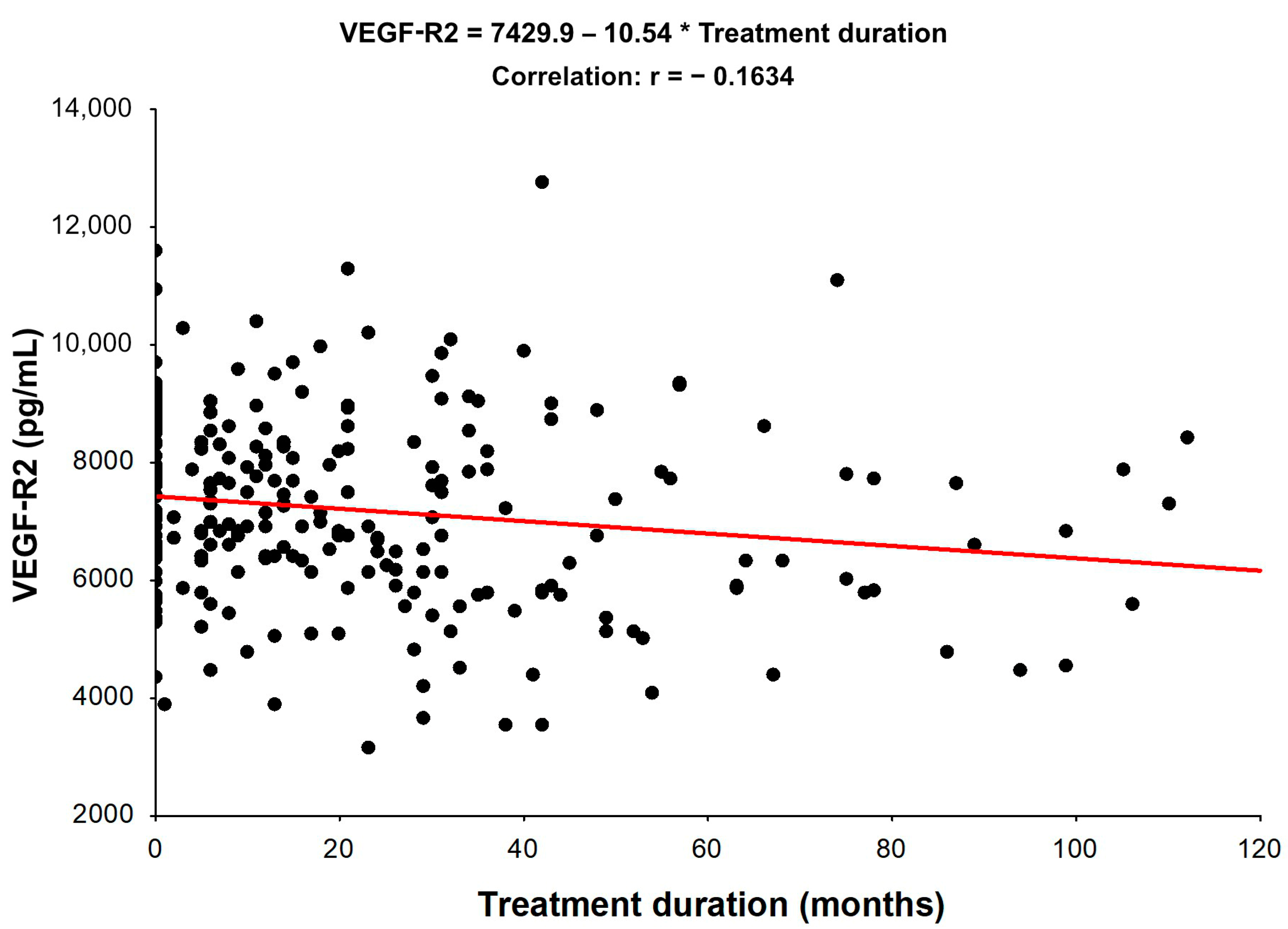
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 387–454. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Blicharz-Dorniak, J.; Strzelczyk, J.; Bałdys-Waligórska, A.; Bednarczuk, T.; Bolanowski, M.; Boratyn-Nowicka, A.; Borowska, M.; Cichocki, A.; Ćwikła, J.B.; et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2017, 68, 79–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarczuk, T.; Zemczak, A.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; Hubalewska-Dydejczyk, A.; et al. Neuroendocrine neoplasms of the small intestine and the appendix–update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 549–583. [Google Scholar] [CrossRef]
- Perez, K.; Chan, J. Medical management of gastrointestinal neuroendocrine tumors. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Valle, J.W.; Eriksson, B.; Rinke, A.; Caplin, M.; Chen, J.; Costa, F.; Falkerby, J.; Fazio, N.; Gorbounova, V.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy–Biotherapy and Novel Targeted Agents. Neuroendocrinology 2017, 105, 266–280. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; Wang, L.; Rashid, A.; Zhu, Z.; Evans, D.B.; Vauthey, J.N.; Xie, K.; Yao, J.C. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007, 109, 1478–1486. [Google Scholar] [CrossRef]
- Besig, S.; Voland, P.; Baur, D.M.; Perren, A.; Prinz, C. Vascular Endothelial Growth Factors, Angiogenesis, and Survival in Human Ileal Enterochromaffin Cell Carcinoids. Neuroendocrinology 2009, 90, 402–415. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef] [Green Version]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF serum values are associated with locoregional spread of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, W.; Jordan, C.T.; Wiegand, G.W.; Pardoll, D.; Lemischka, I.R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 1991, 65, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Pajusola, K.; Aprelikova, O.; Korhonen, J.; Kaipainen, A.; Pertovaara, L.; Alitalo, R.; Alitalo, K. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992, 52, 5738–5743. [Google Scholar] [PubMed]
- Shibuya, M.; Yamaguchi, S.; Yamane, A.; Ikeda, T.; Tojo, A.; Matsushime, H.; Sato, M. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990, 5, 519–524. [Google Scholar]
- Terman, B.I.; Carrion, M.E.; Kovacs, E.; Rasmussen, B.A.; Eddy, R.L.; Shows, T.B. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene 1991, 6, 1677–1683. [Google Scholar] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms, and regulation of endothelial VEGF receptor signaling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couvelard, A.; O’Toole, D.; Turley, H.; Leek, R.; Sauvanet, A.; Degott, C.; Ruszniewski, P.; Belghiti, J.; Harris, A.L.; Gatter, K.; et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and VEGF expression with tumour progression. Br. J. Cancer 2005, 92, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bi, J.; Liang, Q.; Wang, S.; Zhang, L.; Han, F.; Li, S.; Qiu, B.; Fan, X.; Chen, W.; et al. VCAM1 Promotes Tumor Cell Invasion and Metastasis by Inducing EMT and Transendothelial Migration in Colorectal Cancer. Front. Oncol. 2020, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jang, H.J. Deep learning-based prediction of molecular cancer biomarkers from tissue slides: A new tool for precision oncology. Clin. Mol. Hepatol. 2022, 28, 754–772. [Google Scholar] [CrossRef]
- Huang, S.C.; Liang, J.D.; Hsu, S.J.; Hong, T.C.; Yang, H.C.; Kao, J.H. Direct comparison of biopsy techniques for hepatic malignancies. Clin. Mol. Hepatol. 2021, 27, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, H.E.; Harris, A.L.; Melmed, S.; Wass, J.A. Angiogenesis in endocrine tumors. Endocr. Rev. 2003, 24, 600–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [Green Version]
- Dam, G.; Grønbæk, H.; Sorbye, H.; Thiis Evensen, E.; Paulsson, B.; Sundin, A.; Jensen, C.; Ebbesen, D.; Knigge, U.; Tiensuu Janson, E. Prospective Study of Chromogranin A as a Predictor of Progression in Patients with Pancreatic, Small-Intestinal, and Unknown Primary Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 217–224. [Google Scholar] [CrossRef]
- Puliani, G.; Sesti, F.; Anastasi, E.; Verrico, M.; Tarsitano, M.G.; Feola, T.; Campolo, F.; Di Gioia, C.R.T.; Venneri, M.A.; Angeloni, A.; et al. Angiogenic factors as prognostic markers in neuroendocrine neoplasms. Endocrine 2022, 76, 208–217. [Google Scholar] [CrossRef]
- Sesti, F.; Puliani, G.; Feola, T.; Campolo, F.; Sciarra, F.; Hasenmajer, V.; Lenzi, A.; Faggiano, A.; Isidori, A.M.; Venneri, M.A.; et al. Characterization of circulating immune cells and correlation with Tie2/Angiopoietins level in well differentiated neuroendocrine gastroenteropancreatic tumors: A cross-sectional analysis. Endocrine 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Rosiek, V.; Janas, K. Assessment of VEGF and VEGF R1 serum levels in patients with neuroendocrine neoplasms before and after treatment with first-generation somatostatin analogues. Endokrynol. Pol. 2022, 73, 612–618. [Google Scholar] [CrossRef]
- Sandra, I.; Cazacu, I.M.; Croitoru, V.M.; Mihaila, M.; Herlea, V.; Diculescu, M.M.; Dima, S.O.; Croitoru, A.E. Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review. Curr. Issues Mol. Biol. 2022, 44, 4001–4014. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hassler, G.; Baum, U.; Hahn, E.G.; Lohmann, T.; Schuppan, D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin. Endocrinol. 2005, 62, 434–443. [Google Scholar] [CrossRef]
- Kajdaniuk, D.; Marek, B.; Foltyn, W.; Kos-Kudła, B. Vascular endothelial growth factor (VEGF)–part 2: In endocrinology and oncology. Endokrynol. Pol. 2011, 62, 456–464. [Google Scholar]
- Zurita, A.J.; Khajavi, M.; Wu, H.K.; Tye, L.; Huang, X.; Kulke, M.H.; Lenz, H.; Meropol, N.; Carley, W.; DePrimo, S.; et al. Circulating cytokines and monocyte subpopulations as biomarkers of outcome and biological activity in sunitinib-treated patients with advanced neuroendocrine tumours. Br. J. Cancer 2015, 112, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevilla, J.; De Vries, E.; et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Capdevila, J.; Castellano, D.; Teulé, A.; Durán, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; Sastre, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.B.; Chen, G.Y.; Xia, J.G.; Zang, X.W.; Yang, H.Y.; Yang, L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J. Gastroenterol. 2003, 9, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.J.; Ghellal, A.; Iddon, J.; Blann, A.D.; Venizelos, V.; Kumar, S.; Howell, A.; Bundred, N.J. Serum soluble vascular cell adhesion molecule-1: Role as a surrogate marker of angiogenesis. J. Natl. Cancer Inst. 2000, 92, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Wolters, T.L.C.; van der Heijden, C.D.C.C.; Pinzariu, O.; Hijmans-Kersten, B.T.P.; Jacobs, C.; Kaffa, C.; Hoischen, A.; Netea, M.G.; Smit, J.W.A.; Thijssen, D.H.J.; et al. The association between treatment and systemic inflammation in acromegaly. Growth Horm. IGF Res. 2021, 57–58, 101391. [Google Scholar] [CrossRef]
- Xiang, Z.; Deng, X.; He, W.; Yang, Q.; Ni, L.; Dehghan Shasaltaneh, M.; Maghsoudloo, M.; Yang, G.; Wu, J.; Imani, S.; et al. Treatment of malignant pleural effusion in non-small cell lung cancer with VEGF-directed therapy. Ann. Med. 2022, 54, 1357–1371. [Google Scholar] [CrossRef]
| NET Patients | Healthy Controls | |
|---|---|---|
| Number of patients (n) | 56 | 49 |
| Gender | ||
| Male | 30 (54%) | 14 (29%) |
| Female | 26 (46%) | 35 (71%) |
| Age (years) | ||
| Mean/Median | 61.57/64.30 | 53.36/54.50 |
| Tumour grade | ||
| G1 | 38 (68%) | N/A |
| G2 | 18 (32%) | |
| Disease stage | ||
| I + II | 22 (39%) | N/A |
| III | 10 (18%) | |
| IV | 24 (43%) | |
| Disease stage | ||
| SD | 10 (18%) | N/A |
| PD | 24 (43%) | |
| Disease extent-metastases | ||
| yes | 34 (61%) | N/A |
| no | 22 (39%) | |
| NET site: | ||
| Gastrointestinal | 21 (38%) | |
| Pancreatic | 23 (42%) | N/A |
| Unknown | 6 (11%) | |
| Lung/Thymus | 4/1 (9%) | |
| Functionality status: | ||
| NF-NET | 45 (80%) | N/A |
| F-NET: CS/Glucagonoma | 11: 10/1 (20%) | |
| Previous surgery | ||
| yes | 29 (52%) | N/A |
| no | 27 (48%) | |
| Kind of SSAs therapy: | ||
| Sandostatin LAR 30 mg | 14 (25%) | N/A |
| Somatuline Autogel 120 mg | 42 (75%) |
| Variable | NET Patients | Healthy Controls | p-Value * |
|---|---|---|---|
| VEGF-R2 (median/IR) | (7717/6567–8662) | (7793/6186–8980) | NS |
| VEGF-R3 (median/IR) | (65/56–73) | (41/27–53) | <0.001 |
| VCAM-1 (median/IR) | (667/595–838) | (479/400–565) | <0.001 |
| Matched Pairs of Variables | z | p |
|---|---|---|
| VEGF-R2 Group B & VEGF-R2 Group C | 6.45 | <0.001 |
| VEGF-R2 Group B & VEGF-R2 Group D | 5.73 | <0.001 |
| VEGF-R2 Group B & VEGF-R2 Group E | 5.16 | <0.001 |
| VEGF-R2 Group B & VEGF-R2 Group F | 4.28 | <0.001 |
| VEGF-R2 Group B & VEGF-R2 Group G | 4.11 | <0.001 |
| VEGF-R3 Group B & VEGF-R3 Group C | 0.23 | NS |
| VEGF-R3 Group B & VEGF-R3 Group D | 1.08 | NS |
| VEGF-R3 Group B & VEGF-R3 Group E | 5.14 | <0.001 |
| VEGF-R3 Group B & VEGF-R3 Group F | 4.46 | <0.001 |
| VEGF-R3 Group B & VEGF-R3 Group G | 3.72 | <0.001 |
| VCAM-1 Group B & VCAM-1 Group C | 5.21 | <0.001 |
| VCAM-1 Group B & VCAM-1 Group D | 2.21 | 0.027 |
| VCAM-1 Group B & VCAM-1 Group E | 2.21 | 0.027 |
| VCAM-1 Group B & VCAM-1 Group F | 3.92 | <0.001 |
| VCAM-1 Group B & VCAM-1 Group G | 4.11 | < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiek, V.; Janas, K.; Kos-Kudła, B. Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues. Biomedicines 2023, 11, 842. https://doi.org/10.3390/biomedicines11030842
Rosiek V, Janas K, Kos-Kudła B. Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues. Biomedicines. 2023; 11(3):842. https://doi.org/10.3390/biomedicines11030842
Chicago/Turabian StyleRosiek, Violetta, Ksenia Janas, and Beata Kos-Kudła. 2023. "Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues" Biomedicines 11, no. 3: 842. https://doi.org/10.3390/biomedicines11030842






