Reinforcement of Calcium Phosphate Cement with Hybrid Silk Fibroin/Kappa-Carrageenan Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CPC Composites
2.2.1. Calcium Phosphate Cement
2.2.2. Electrospinning Silk Fibroin/k-CG
2.2.3. Composite CPC
2.3. Mineralization CPC Composites
2.4. Characterization
2.4.1. Morphological Analysis
2.4.2. X-ray Diffraction (XRD) Analysis
2.4.3. Setting Time
2.4.4. Mechanical Tests
2.4.5. Injectability
2.4.6. Cell Seeding and Morphology
2.4.7. Cell Mineralization
2.4.8. Statistical Analysis
3. Results and Discussion
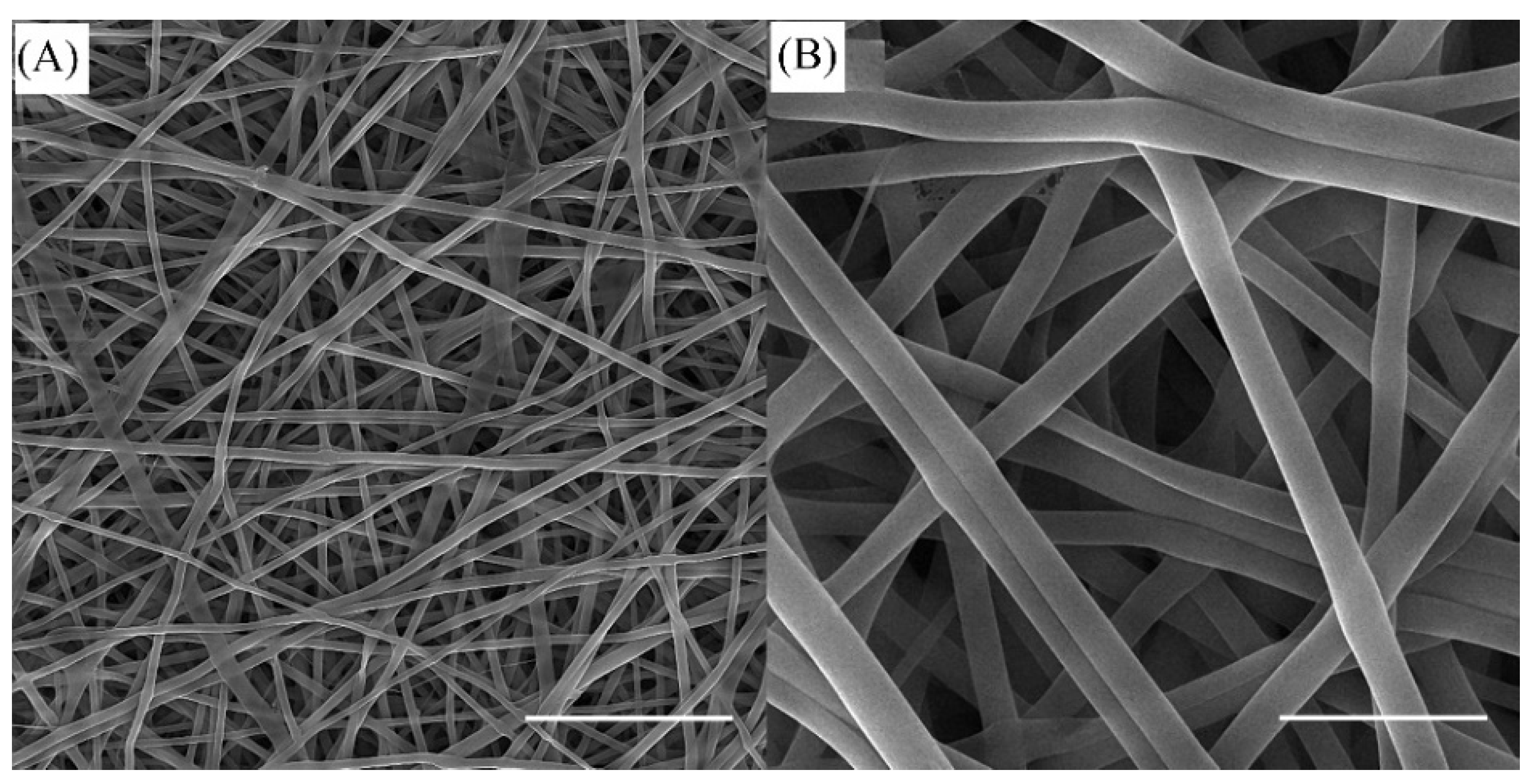
3.1. SEM Observations of SF/k-CG Fibers
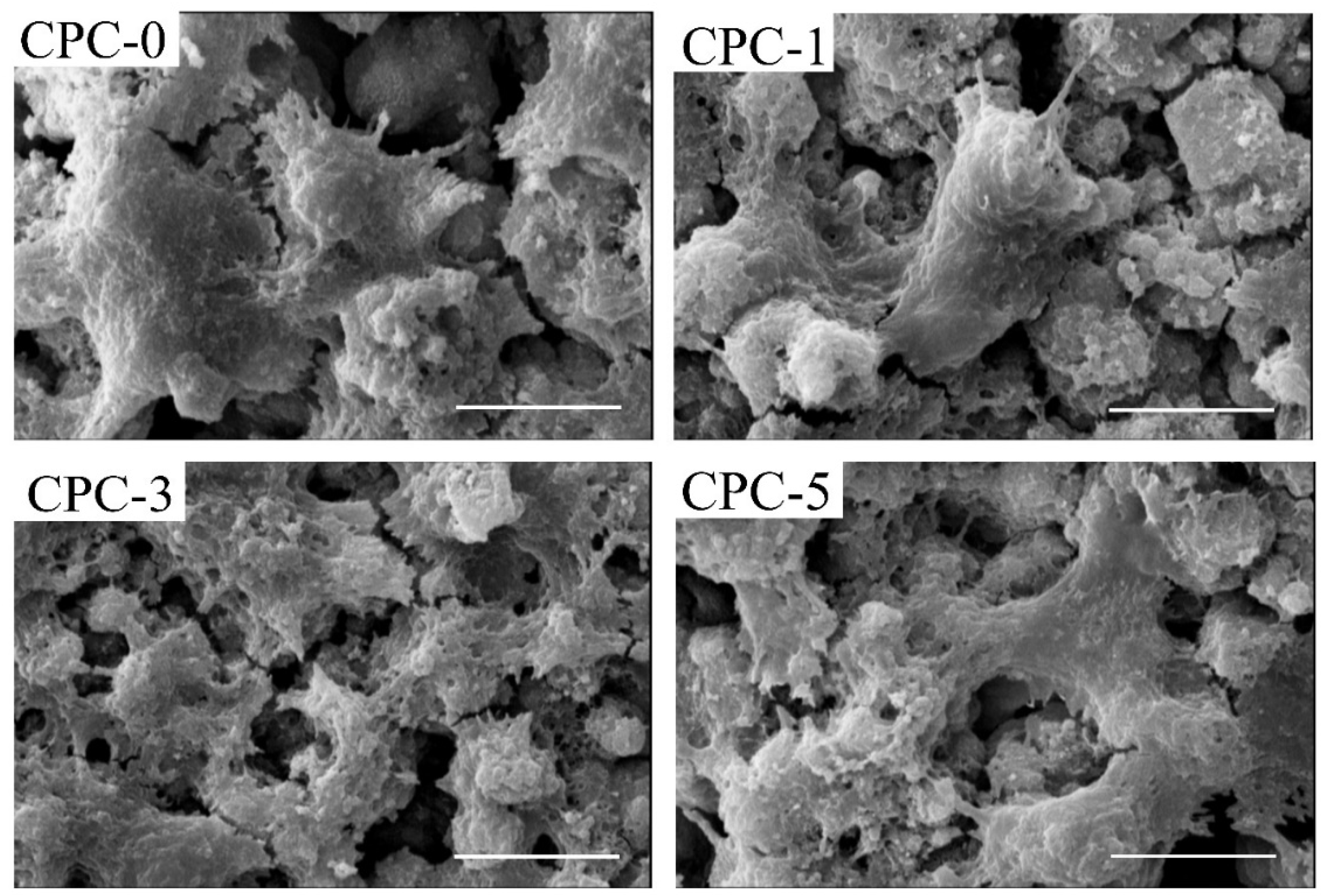
3.2. Morphology and Microstructure of CPC Composites
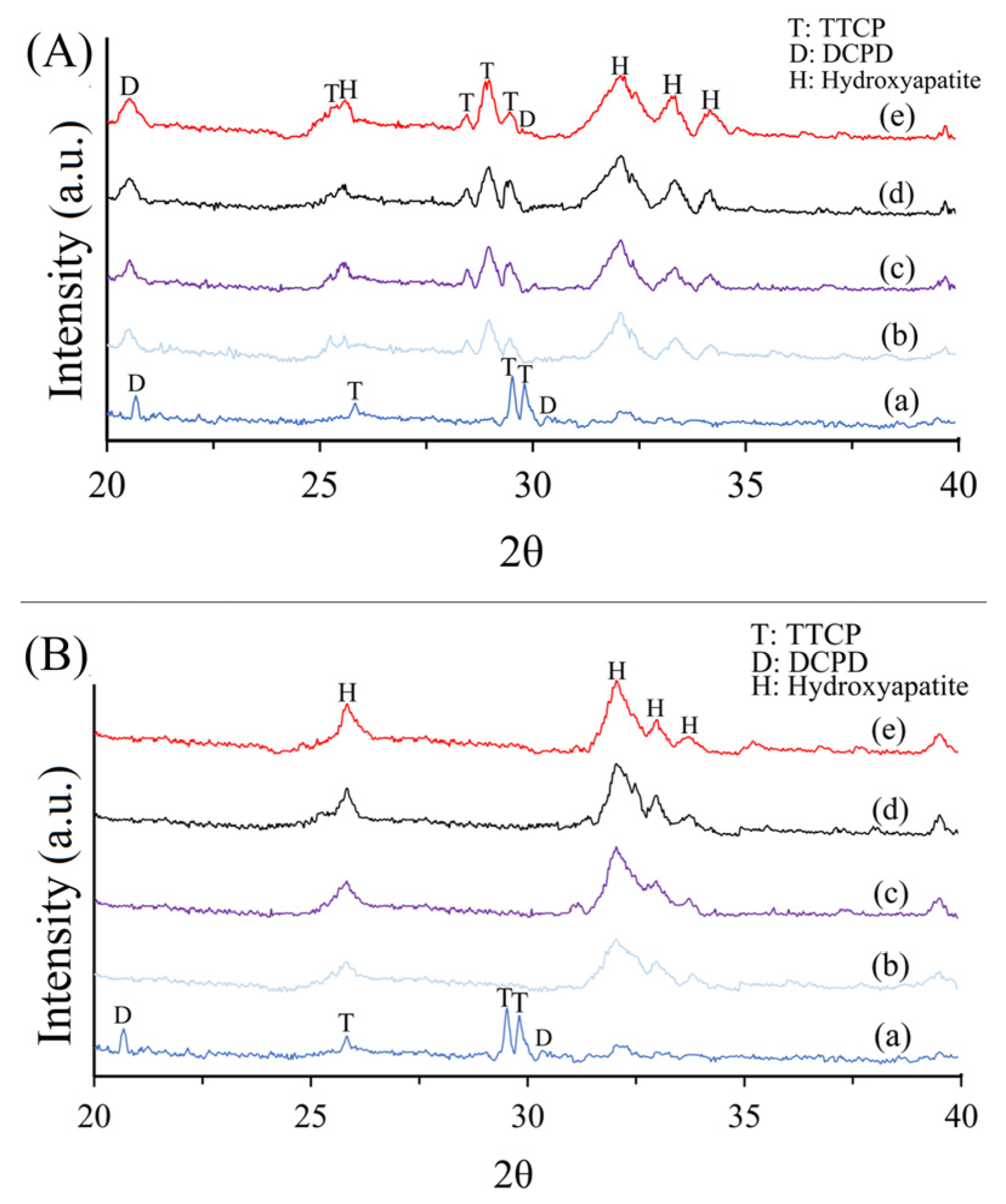
3.3. XRD
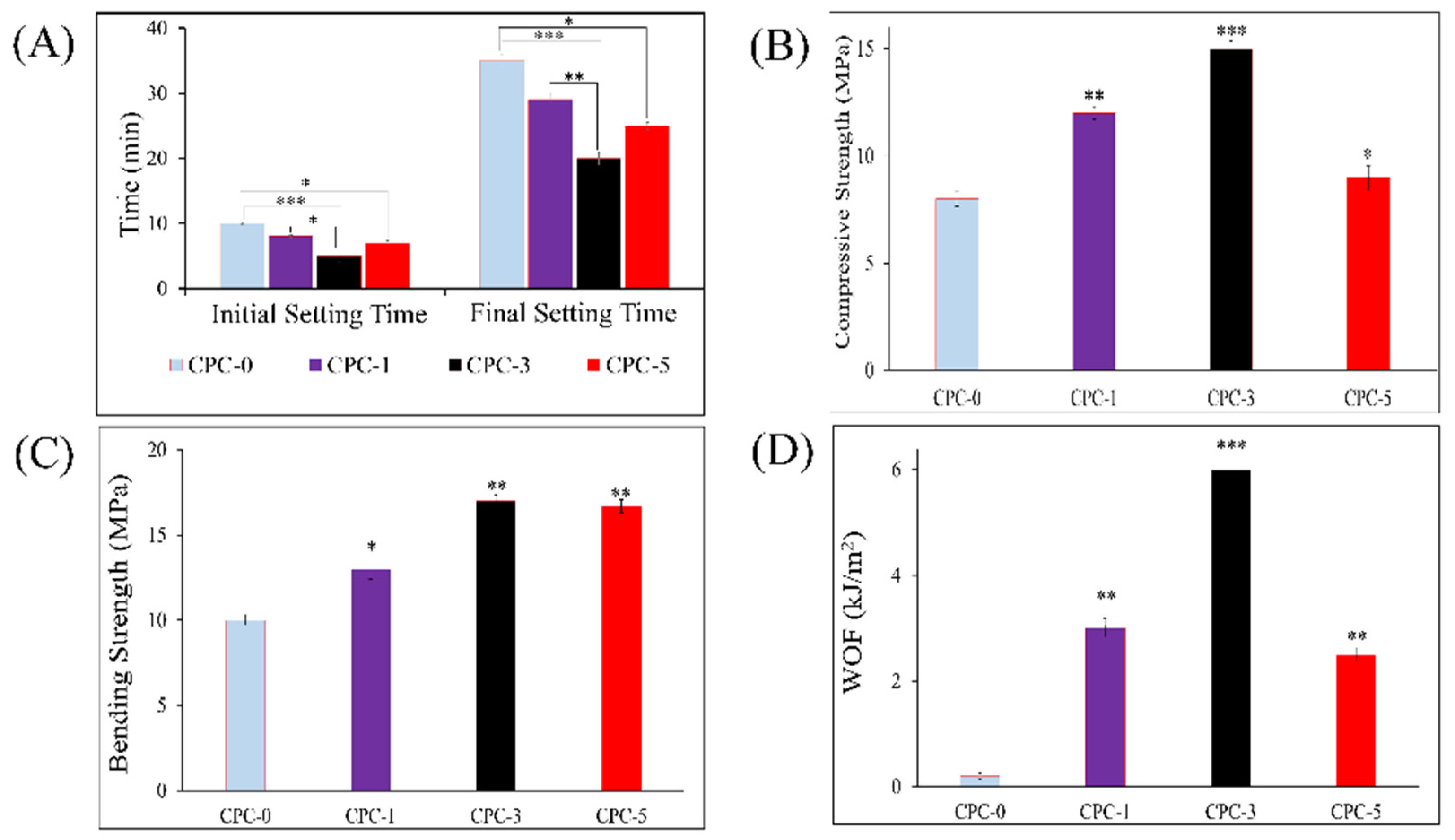
3.4. Setting Time
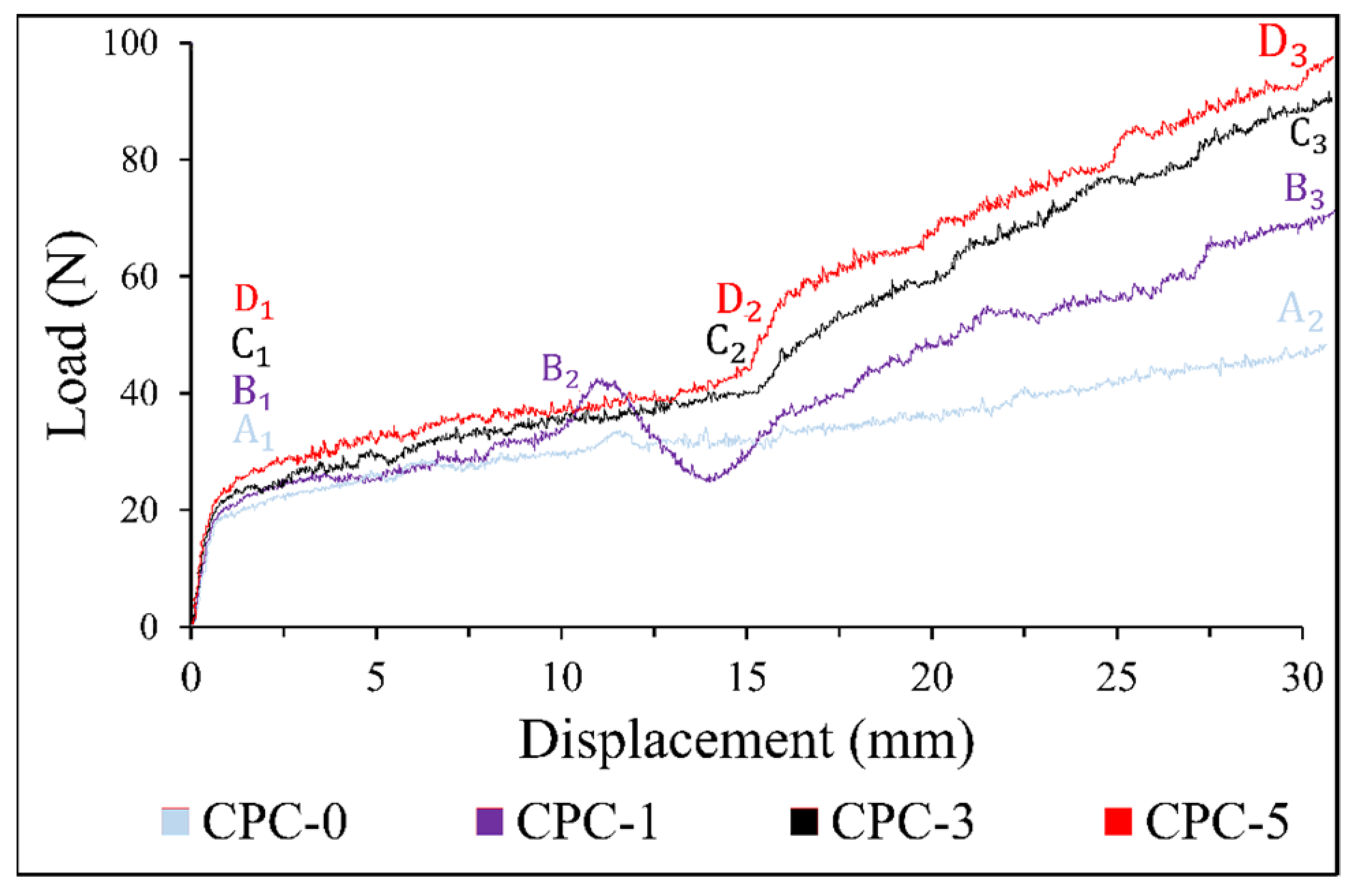
3.5. Mechanical Strength
3.6. Injectability
3.7. Cell Morphology
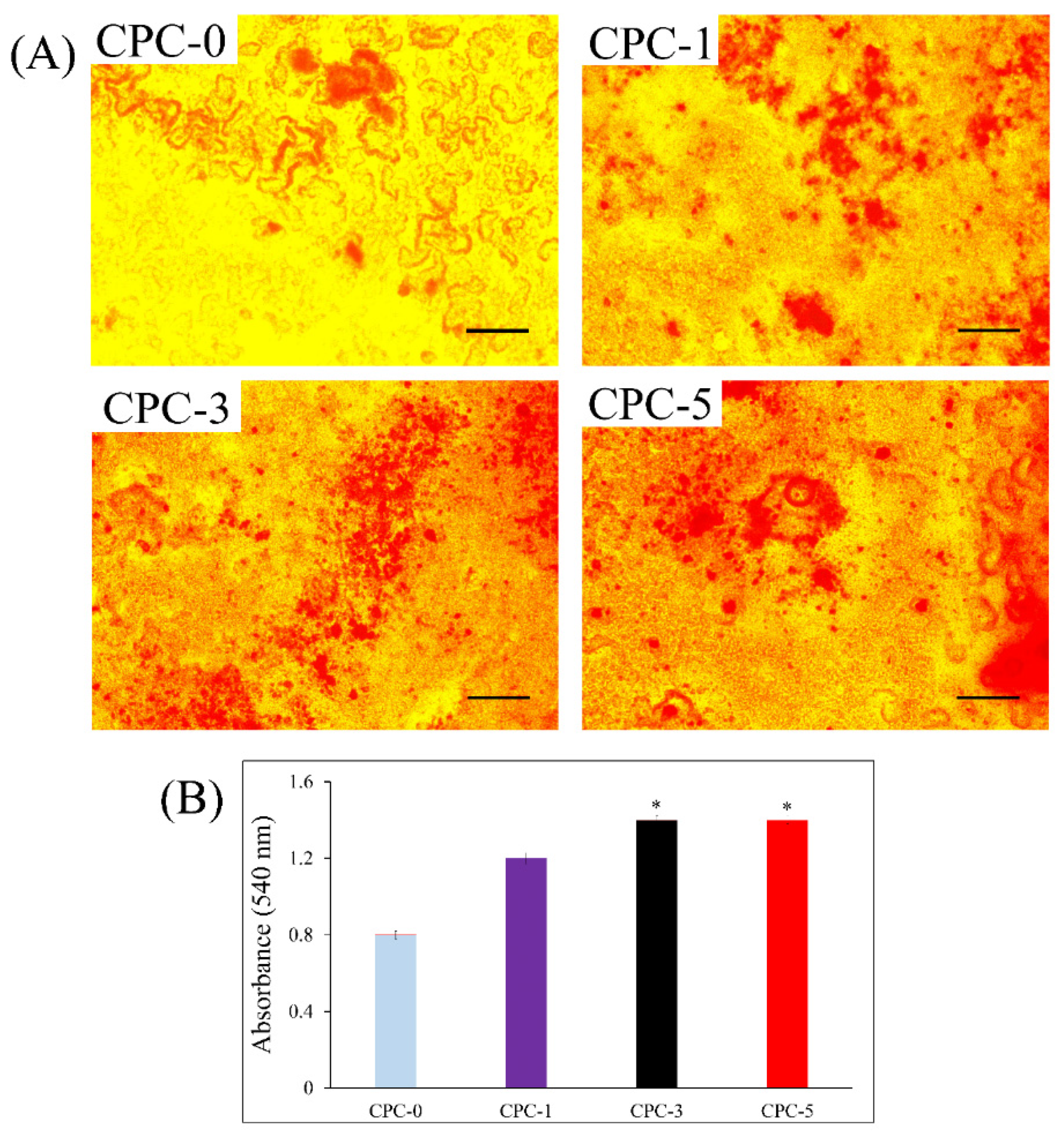
3.8. Cell Mineralization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, P.; Lu, S.; Yu, J.; Xiao, L.; Wang, J.; Liang, H.; Huang, L.; Han, G.; Bian, M.; Zhang, S.; et al. Injectable nanofiber-reinforced bone cement with controlled biodegradability for minimally-invasive bone regeneration. Bioact. Mater. 2023, 21, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Jakobsen, T.; Baas, J.; Nygaard, J.V.; Dolatshahi-Pirouz, A.; Hovgaard, M.B.; Foss, M.; Bünger, C.; Besenbacher, F.; Søballe, K. Hydroxyapatite nanoparticles in poly-D,L-lactic acid coatings on porous titanium implants conducts bone formation. J. Biomed. Mater. Res. Part A 2010, 95, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Dolatshahi-Pirouz, A.; Foss, M.; Baas, J.; Lovmand, J.; Duch, M.; Pedersen, F.S.; Kassem, M.; Bünger, C.; Søballe, K.; et al. Interaction of human mesenchymal stem cells with osteopontin coated hydroxyapatite surfaces. Colloids Surf. B Biointerfaces 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.G.; Polini, A.; Azami, Z.; Leeuwenburgh, S.C.; Jansen, J.A.; Yang, F.; Beucken, J.J.V.D. Incorporation of PLLA micro-fillers for mechanical reinforcement of calcium-phosphate cement. J. Mech. Behav. Biomed. Mater. 2017, 71, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Schnieders, J.; Gbureck, U.; Thull, R.; Kissel, T. Controlled release of gentamicin from calcium phosphate—Poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials 2006, 27, 4239–4249. [Google Scholar] [CrossRef]
- Takechi, M.; Miyamoto, Y.; Ishikawa, K.; Nagayama, M.; Kon, M.; Asaoka, K.; Suzuki, K. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. J. Biomed. Mater. Res. 1998, 39, 308–316. [Google Scholar] [CrossRef]
- Ghosh, S.; Wu, V.; Pernal, S.; Uskoković, V. Self-Setting Calcium Phosphate Cements with Tunable Antibiotic Release Rates for Advanced Antimicrobial Applications. ACS Appl. Mater. Interfaces 2016, 8, 7691–7708. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Zuo, Y.; Yang, F.; Wolke, J.G.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Wang, Y.; Sun, X.; Sun, K. Reduced graphene oxide/carbon nanotubes reinforced calcium phosphate cement. Ceram. Int. 2017, 43, 13083–13088. [Google Scholar] [CrossRef]
- Vezenkova, A.; Locs, J. Sudoku of porous, injectable calcium phosphate cements—Path to osteoinductivity. Bioact. Mater. 2022, 17, 109–124. [Google Scholar] [CrossRef]
- Praveen, K.M.; Murickan, R.T.; Joy, J.; Maria, H.J.; Haponiuk, J.T.; Thomas, S. (Eds.) Electrospun Nanofibers from Bioresources for High-Performance Applications; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Canal, C.; Gallinetti, S.; Ginebra, M.-P. Low-Pressure Plasma Treatment of Polylactide Fibers for Enhanced Mechanical Performance of Fiber-Reinforced Calcium Phosphate Cements. Plasma Process. Polym. 2014, 11, 694–703. [Google Scholar] [CrossRef]
- Canal, C.; Ginebra, M. Fibre-reinforced calcium phosphate cements: A review. J. Mech. Behav. Biomed. Mater. 2011, 4, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.A.; De Oliveira, L.C.; Rigo, E.; Carrodeguas, R.G.; Boschi, A.O.; De Arruda, A.C.F. Fiber Reinforced Calcium Phosphate Cement. Artif. Organs 2000, 24, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Gorst, N.; Perrie, Y.; Gbureck, U.; Hutton, A.; Hofmann, M.; Grover, L.; Barralet, J. Effects of fibre reinforcement on the mechanical properties of brushite cement. Acta Biomater. 2006, 2, 95–102. [Google Scholar] [CrossRef]
- Boehm, A.V.; Meininger, S.; Tesch, A.; Gbureck, U.; Müller, F.A. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials 2018, 11, 192. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Zhou, Z.-B.; He, Z.-W.; Ren, W.-P.; Yu, X.-W.; Huang, Y. Reinforcement of a new calcium phosphate cement with RGD-chitosan-fiber. J. Biomed. Mater. Res. Part A 2013, 102, 68–75. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Wang, Y.; Sun, K.; Bi, J. Properties of reduced graphene/carbon nanotubes reinforced calcium phosphate bone cement in a microwave environment. J. Mater. Sci. Mater. Med. 2019, 30, 1–8. [Google Scholar] [CrossRef]
- Hasan, M.S.; Carpenter, N.; Wei, T.L.; McNally, D.; Ahmed, I.; Boszczyk, B. Effects of Adding Resorbable Phosphate Glass Fibres and PLA to Calcium Phosphate Bone Cements. J. Appl. Biomater. Funct. Mater. 2014, 12, 203–209. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Type 2 Immune Mechanisms in Carbon Nanotube-Induced Lung Fibrosis. Front. Immunol. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Morawska-Chochół, A.; Uszko, P.; Szaraniec, B.; Gryń, K.; Chłopek, J. The influence of bioactive additives on polylactide accelerated degradation. E-Polymers 2016, 16, 475–480. [Google Scholar] [CrossRef]
- Schickert, S.D.L.; Pinto, J.C.; Jansen, J.; Leeuwenburgh, S.C.G.; Beucken, J.J.J.P.V.D. Tough and injectable fiber reinforced calcium phosphate cement as an alternative to polymethylmethacrylate cement for vertebral augmentation: A biomechanical study. Biomater. Sci. 2020, 8, 4239–4250. [Google Scholar] [CrossRef]
- Nourmohammadi, J.; Roshanfar, F.; Farokhi, M.; Nazarpak, M.H. Silk fibroin/kappa-carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications. Mater. Sci. Eng. C 2017, 76, 951–958. [Google Scholar] [CrossRef]
- Roshanfar, F.; Hesaraki, S.; Dolatshahi-Pirouz, A. Electrospun Silk Fibroin/kappa-Carrageenan Hybrid Nanofibers with Enhanced Osteogenic Properties for Bone Regeneration Applications. Biology 2022, 11, 751. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Kim, I.Y.; Iwatsuki, R.; Kikuta, K.; Morita, Y.; Miyazaki, T.; Ohtsuki, C. Thermoreversible behavior of κ-carrageenan and its apatite-forming ability in simulated body fluid. Mater. Sci. Eng. C 2011, 31, 1472–1476. [Google Scholar] [CrossRef]
- Bao, C.; Chen, W.; Weir, M.D.; Thein-Han, W.; Xu, H.H. Effects of electrospun submicron fibers in calcium phosphate cement scaffold on mechanical properties and osteogenic differentiation of umbilical cord stem cells. Acta Biomater. 2011, 7, 4037–4044. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Eichmiller, F.C.; Giuseppetti, A.A. Reinforcement of a self-setting calcium phosphate cement with different fibers. J. Biomed. Mater. Res. 2000, 52, 107–114. [Google Scholar] [CrossRef]
- Shams, M.; Nezafati, N.; Poormoghadam, D.; Zavareh, S.; Zamanian, A.; Salimi, A. Synthesis and characterization of electrospun bioactive glass nanofibers-reinforced calcium sulfate bone cement and its cell biological response. Ceram. Int. 2020, 46, 10029–10039. [Google Scholar] [CrossRef]
- Sugawara, A.; Asaoka, K.; Ding, S.-J. Calcium phosphate-based cements: Clinical needs and recent progress. J. Mater. Chem. B 2013, 1, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Baheiraei, N.; Shahrezaee, M. Biomimetic reduced graphene oxide coated collagen scaffold for in situ bone regeneration. Sci. Rep. 2021, 11, 16783. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshanfar, F.; Hesaraki, S.; Dolatshahi-Pirouz, A.; Saeidi, M.; Leal-Marin, S.; Glasmacher, B.; Orive, G.; Khan Einipour, S. Reinforcement of Calcium Phosphate Cement with Hybrid Silk Fibroin/Kappa-Carrageenan Nanofibers. Biomedicines 2023, 11, 850. https://doi.org/10.3390/biomedicines11030850
Roshanfar F, Hesaraki S, Dolatshahi-Pirouz A, Saeidi M, Leal-Marin S, Glasmacher B, Orive G, Khan Einipour S. Reinforcement of Calcium Phosphate Cement with Hybrid Silk Fibroin/Kappa-Carrageenan Nanofibers. Biomedicines. 2023; 11(3):850. https://doi.org/10.3390/biomedicines11030850
Chicago/Turabian StyleRoshanfar, Fahimeh, Saeed Hesaraki, Alireza Dolatshahi-Pirouz, Mohsen Saeidi, Sara Leal-Marin, Birgit Glasmacher, Gorka Orive, and Sajjad Khan Einipour. 2023. "Reinforcement of Calcium Phosphate Cement with Hybrid Silk Fibroin/Kappa-Carrageenan Nanofibers" Biomedicines 11, no. 3: 850. https://doi.org/10.3390/biomedicines11030850
APA StyleRoshanfar, F., Hesaraki, S., Dolatshahi-Pirouz, A., Saeidi, M., Leal-Marin, S., Glasmacher, B., Orive, G., & Khan Einipour, S. (2023). Reinforcement of Calcium Phosphate Cement with Hybrid Silk Fibroin/Kappa-Carrageenan Nanofibers. Biomedicines, 11(3), 850. https://doi.org/10.3390/biomedicines11030850







