Prevalence of mecA- and mecC-Associated Methicillin-Resistant Staphylococcus aureus in Clinical Specimens, Punjab, Pakistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Transportation, and Preservation
2.2. Isolation and Confirmation of Staphylococcus aureus
2.3. Phenotypic Identification of MRSA and Antimicrobial Susceptibility Testing
2.4. DNA Extraction for PCR
2.5. Molecular Detection of mecA and mecC Genes in MRSA
2.6. Quality Control
2.7. Statistical Analysis
3. Results
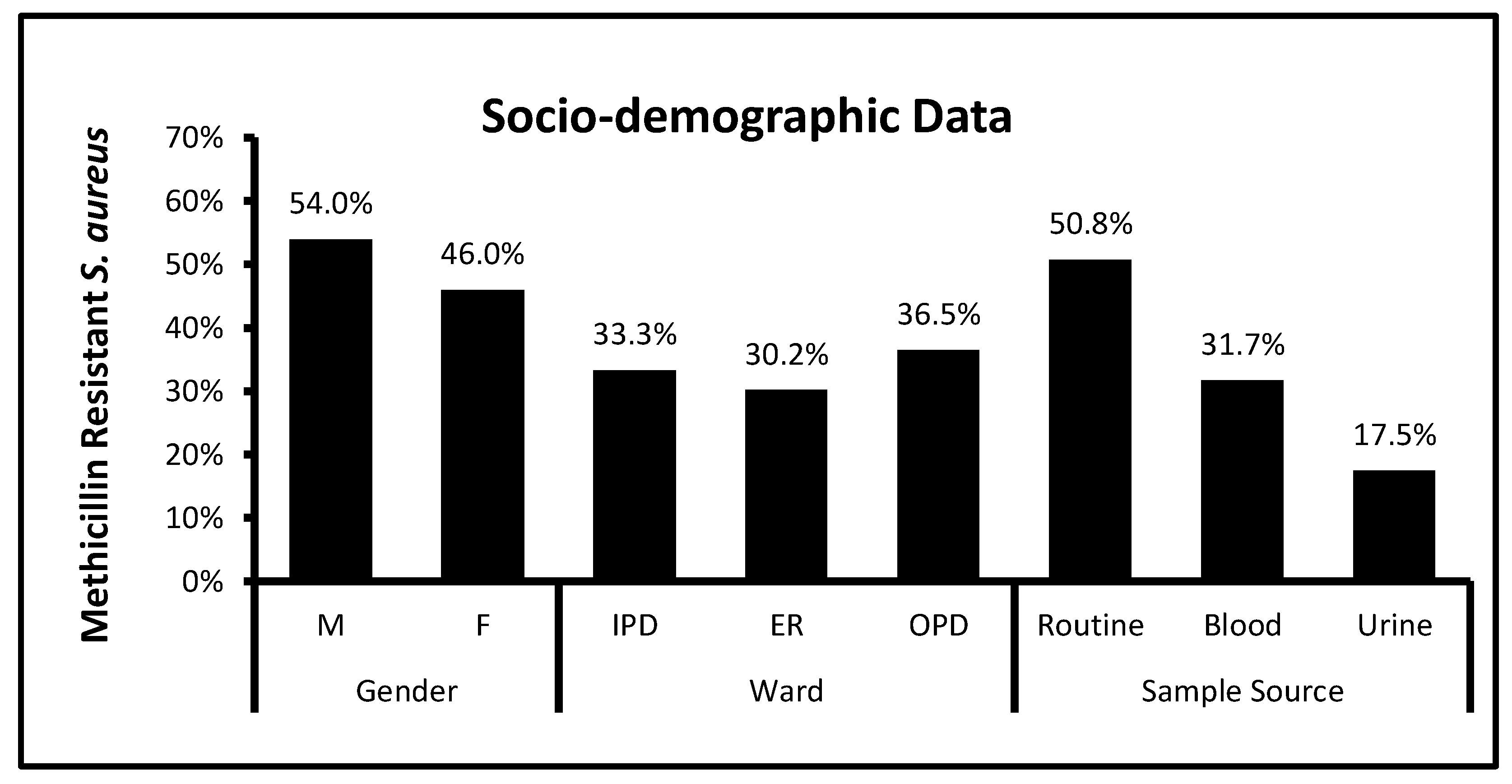
3.1. Socio-Demographic Data and Bacterial Distribution among Clinical Patients
3.2. Antibiotic Susceptibility Test
3.3. Prevalence of mecA and mecC Genes in MRSA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollitt, E.J.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Giamarellou, H. Community-acquired methicillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2006, 27, 87–96. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Huang, Y.-C. New epidemiology of Staphylococcus aureus infection in Asia. Clin. Microbiol. Infect. 2014, 20, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Milheiriço, C.; Gardete, S.; Holmes, M.A.; Holden, M.T.; de Lencastre, H.; Tomasz, A. Properties of a novel PBP2A protein homolog from Staphylococcus aureus strain LGA251 and its contribution to the β-lactam-resistant phenotype. J. Biol. Chem. 2012, 287, 36854–36863. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.M.; Saeed, A. Genetic and molecular mechanisms of multidrug-resistance in uropathogens and novel therapeutic combat. In Biochemistry of Drug Resistance; Springer: Berlin, Germany, 2021; pp. 505–538. [Google Scholar]
- Vandendriessche, S.; Vanderhaeghen, W.; Soares, F.V.; Hallin, M.; Catry, B.; Hermans, K.; Butaye, P.; Haesebrouck, F.; Struelens, M.J.; Denis, O. Prevalence, risk factors and genetic diversity of methicillin-resistant Staphylococcus aureus carried by humans and animals across livestock production sectors. J. Antimicrob. Chemother. 2013, 68, 1510–1516. [Google Scholar] [CrossRef]
- Long, S.W.; Olsen, R.J.; Mehta, S.C.; Palzkill, T.; Cernoch, P.L.; Perez, K.K.; Musick, W.L.; Rosato, A.E.; Musser, J.M. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2014, 58, 6668–6674. [Google Scholar] [CrossRef]
- Abdeen, E.E.; Mousa, W.S.; Abdel-Tawab, A.A.; El-Faramawy, R.; Abo-Shama, U.H. Phenotypic, genotypic and antibiogram among Staphylococcus aureus isolated from bovine subclinical mastitis. Pak. Vet. J. 2021, 41, 289–293. [Google Scholar]
- Aqib, A.I.; Ijaz, M.; Anjum, A.A.; Malik, M.A.R.; Mehmood, K.; Farooqi, S.H.; Hussain, K. Antibiotic susceptibilities and prevalence of Methicillin resistant Staphylococcus aureus (MRSA) isolated from bovine milk in Pakistan. Acta Trop. 2017, 176, 168–172. [Google Scholar] [CrossRef]
- Stefani, S.; Varaldo, P. Epidemiology of methicillin-resistant staphylococci in Europe. Clin. Microbiol. Infect. 2003, 9, 1179–1186. [Google Scholar] [CrossRef]
- Rohner, P.; Pepey, B.; Auckenthaler, R. Advantage of combining resin with lytic BACTEC blood culture media. J. Clin. Microbiol. 1997, 35, 2634–2638. [Google Scholar] [CrossRef]
- Sánchez-Romero, M.I.; Moya, J.M.G.-L.; López, J.J.G.; Mira, N.O. Collection, transport and general processing of clinical specimens in Microbiology laboratory. Enferm. Infecc. Microbiol. Clin. 2019, 37, 127–134. [Google Scholar] [CrossRef]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries, Part 2; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Sharp, S.E.; Searcy, C. Comparison of mannitol salt agar and blood agar plates for identification and susceptibility testing of Staphylococcus aureus in specimens from cystic fibrosis patients. J. Clin. Microbiol. 2006, 44, 4545–4546. [Google Scholar] [CrossRef]
- Kateete, D.P.; Kimani, C.N.; Katabazi, F.A.; Okeng, A.; Okee, M.S.; Nanteza, A.; Joloba, M.L.; Najjuka, F.C. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 23. [Google Scholar] [CrossRef]
- Yamasato, K.; Okuno, D.; Ohtomo, T. Preservation of bacteria by freezing at moderately low temperatures. Cryobiology 1973, 10, 453–463. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef]
- Lee, J.H. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 2003, 69, 6489–6494. [Google Scholar] [CrossRef]
- Stegger, Á.; Andersen, P.; Kearns, A.; Pichon, B.; Holmes, M.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef]
- Cauwelier, B.; Gordts, B.; Descheemaecker, P.; Van Landuyt, H. Evaluation of a disk diffusion method with cefoxitin (30 μg) for detection of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 389–392. [Google Scholar] [PubMed]
- Pai, V.; Rao, V.I.; Rao, S.P. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus [MRSA] isolates at a tertiary care hospital in Mangalore, South India. J. Lab. Physicians 2010, 2, 82–84. [Google Scholar] [CrossRef] [PubMed]
- William, S.; Feil, H.; Copeland, A. Bacterial Genomic DNA Isolation Using CTAB. Available online: https://jgi.doe.gov/wp-content/uploads/2014/02/JGI-Bacterial-DNA-isolation-CTAB-Protocol-2012.pdf (accessed on 3 January 2023).
- Cartwright, E.J.; Paterson, G.K.; Raven, K.E.; Harrison, E.M.; Gouliouris, T.; Kearns, A.; Pichon, B.; Edwards, G.; Skov, R.L.; Larsen, A.R. Use of Vitek 2 antimicrobial susceptibility profile to identify mecC in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2013, 51, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Agrawal, P.; Kumar, S.; Kapila, K. Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR for mecA gene for detection of MRSA. Indian J. Med. Microbiol. 2009, 27, 27–29. [Google Scholar] [CrossRef]
- Akhter, R.; Khan, K.M.A.; Hasan, F. Isolation and antimicrobial susceptibility pattern of methicillin-resistant and methicillin sensitive Staphylococcus aureus. J. Surg. Pak. 2009, 14, 161–164. [Google Scholar]
- Idrees, M.M.; Rasool, M.F.; Imran, I.; Khalid, A.; Saeed, A.; Ahmad, T.; Alqahtani, F. A Cross-Sectional Study to Evaluate Antimicrobial Susceptibility of Uropathogens from South Punjab, Pakistan. Infect. Drug Resist. 2022, 15, 1845–1855. [Google Scholar] [CrossRef]
- Kaleem, F.; Usman, J.; Hassan, A.; Omair, M.; Khalid, A.; Uddin, R. Sensitivity pattern of methicillin resistant Staphylococcus aureus isolated from patients admitted in a tertiary care hospital of Pakistan. Iran. J. Microbiol. 2010, 2, 143. [Google Scholar]
- Shabir, S.; Hardy, K.J.; Abbasi, W.S.; McMurray, C.L.; Malik, S.A.; Wattal, C.; Hawkey, P.M. Epidemiological typing of meticillin-resistant Staphylococcus aureus isolates from Pakistan and India. J. Med. Microbiol. 2010, 59, 330–337. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Sierra, J.M.; Camoez, M.; Tubau, F.; Gasch, O.; Pujol, M.; Martin, R.; Domínguez, M.A. Low Prevalence of C fr-Mediated Linezolid Resistance among Methicillin-Resistant Staphylococcus aureus in a Spanish Hospital: Case Report on Linezolid Resistance Acquired during Linezolid Therapy. PLoS ONE 2013, 8, e59215. [Google Scholar] [CrossRef]
- Arfat, Y.; Johnson, M.; Malik, S.; Morrissey, J.; Bayliss, C. Epidemiology of methicillin resistant Staphylococcus aureus (MRSA) isolates from Pakistan. Afr. J. Microbiol. Res. 2013, 7, 568–576. [Google Scholar]
- Arfat, Y. Genotyping of Methicillin Resistant Staphylococcus aureus (MRSA) from Local Hospital of Rawalpindi/Islamabad, Pakistan. Ph.D. Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2013. [Google Scholar]
- Shebl, H.R.; Zaki, W.K.; Saleh, A.N.; Salam, S.A.A. Prevalence of MecC Gene Among Methicillin Resistant Staphylococcus aureus isolated from Patients in Ain-Shams University Hospital. J. Pure Appl. Microbiol. 2020, 14, 2807–2813. [Google Scholar] [CrossRef]
- Paterson, G.; Morgan, F.; Harrison, E.; Cartwright, E.; Török, M.; Zadoks, R.; Parkhill, J.; Peacock, S.; Holmes, M. Prevalence and characterization of human mecC methicillin-resistant Staphylococcus aureus isolates in England. J. Antimicrob. Chemother. 2014, 69, 907–910. [Google Scholar] [CrossRef]
- Khan, A.A.; Ali, A.; Tharmalingam, N.; Mylonakis, E.; Zahra, R. First report of mecC gene in clinical methicillin resistant S. aureus (MRSA) from tertiary care hospital Islamabad, Pakistan. J. Infect. Public Health 2020, 13, 1501–1507. [Google Scholar] [CrossRef]
- Ullah, N.; Dar, H.A.; Naz, K.; Andleeb, S.; Rahman, A.; Saeed, M.T.; Hanan, F.; Bae, T.; Ali, A. Genomic Investigation of Methicillin-Resistant Staphylococcus aureus ST113 Strains Isolated from Tertiary Care Hospitals in Pakistan. Antibiotics 2021, 10, 1121. [Google Scholar] [CrossRef]
- Shore, A.C.; Coleman, D.C. Staphylococcal cassette chromosome mec: Recent advances and new insights. Int. J. Med. Microbiol. 2013, 303, 350–359. [Google Scholar] [CrossRef]
- Paterson, G.K.; Harrison, E.M.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014, 22, 42–47. [Google Scholar] [CrossRef]
- Zafar, A.; Stone, M.; Ibrahim, S.; Parveen, Z.; Hasan, Z.; Khan, E.; Hasan, R.; Wain, J.; Bamford, K. Prevalent genotypes of meticillin-resistant Staphylococcus aureus: Report from Pakistan. J. Med. Microbiol. 2011, 60, 56–62. [Google Scholar] [CrossRef]
- Kırmusaoğlu, S. MRSA and MSSA: The mechanism of methicillin resistance and the influence of methicillin resistance on biofilm phenotype of Staphylococcus aureus. In The Rise of Virulence and Antibiotic Resistance in Staphylococcus aureus; Enany, S., Ed.; IntechOpen: London, UK, 2017; pp. 25–41. [Google Scholar]
- Abd El-Hamid, M.; Bendary, M.; Merwad, A.; Elsohaby, I.; Mohammad Ghaith, D.; Alshareef, W. What is behind phylogenetic analysis of hospital-, community-and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar]



| Target Gene | Primer Sequence (5′ to 3′) | Fragment Size (bp) | Reference |
|---|---|---|---|
| nuc * | F -GCGATTGATGGTGATACGGTI- R -AGCCAAGCCTTGACGAACTAAAGC- | 279 bp | [20] |
| mecA | F -AAAATCGATGGTAAAGGTTGGC- R -AGTTCTGGAGTACCGGATTTGC- | 533 bp | [21] |
| mecC | F -TCACCAGGTTCAAC[Y]CAAAA- R -CCTGAATC[W]GCTAATAATATTTC- | 356 bp | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, M.M.; Saeed, K.; Shahid, M.A.; Akhtar, M.; Qammar, K.; Hassan, J.; Khaliq, T.; Saeed, A. Prevalence of mecA- and mecC-Associated Methicillin-Resistant Staphylococcus aureus in Clinical Specimens, Punjab, Pakistan. Biomedicines 2023, 11, 878. https://doi.org/10.3390/biomedicines11030878
Idrees MM, Saeed K, Shahid MA, Akhtar M, Qammar K, Hassan J, Khaliq T, Saeed A. Prevalence of mecA- and mecC-Associated Methicillin-Resistant Staphylococcus aureus in Clinical Specimens, Punjab, Pakistan. Biomedicines. 2023; 11(3):878. https://doi.org/10.3390/biomedicines11030878
Chicago/Turabian StyleIdrees, Muhammad Mubashar, Khadija Saeed, Muhammad Akbar Shahid, Muhammad Akhtar, Khadija Qammar, Javariya Hassan, Tayyaba Khaliq, and Ali Saeed. 2023. "Prevalence of mecA- and mecC-Associated Methicillin-Resistant Staphylococcus aureus in Clinical Specimens, Punjab, Pakistan" Biomedicines 11, no. 3: 878. https://doi.org/10.3390/biomedicines11030878
APA StyleIdrees, M. M., Saeed, K., Shahid, M. A., Akhtar, M., Qammar, K., Hassan, J., Khaliq, T., & Saeed, A. (2023). Prevalence of mecA- and mecC-Associated Methicillin-Resistant Staphylococcus aureus in Clinical Specimens, Punjab, Pakistan. Biomedicines, 11(3), 878. https://doi.org/10.3390/biomedicines11030878







