Designing Effective Multi-Target Drugs and Identifying Biomarkers in Recurrent Pregnancy Loss (RPL) Using In Vivo, In Vitro, and In Silico Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Mining and Identification of Genes Expression
2.2. Recurrent Pregnancy Loss Regulatory Network Construction and Module Finding
2.3. Functional Enrichment Analyses
2.4. Samples Collection
2.5. RNA-Seq Validation
2.6. Immunohistochemistry
2.7. Western Blotting
2.8. Cell Culture
2.9. 5-Ethynyl-2′-deoxyuridine (EdU) and Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) Assay
2.10. Flow Cytometry
2.11. Wound-Healing Assay
2.12. Transwell Assay
2.13. Plasmid Transfection
2.14. Lentivirus Infection
2.15. Animal Preparation and RPL Model
2.16. Immunofluorescence Staining of Embryos
2.17. Mitochondrial Superoxide Measuring
2.18. Potential of Mitochondrial Membrane Determination
2.19. Statistical Analysis
3. Results
3.1. RPL Regulatory Network Construction and Hub High Traffic Genes Detection
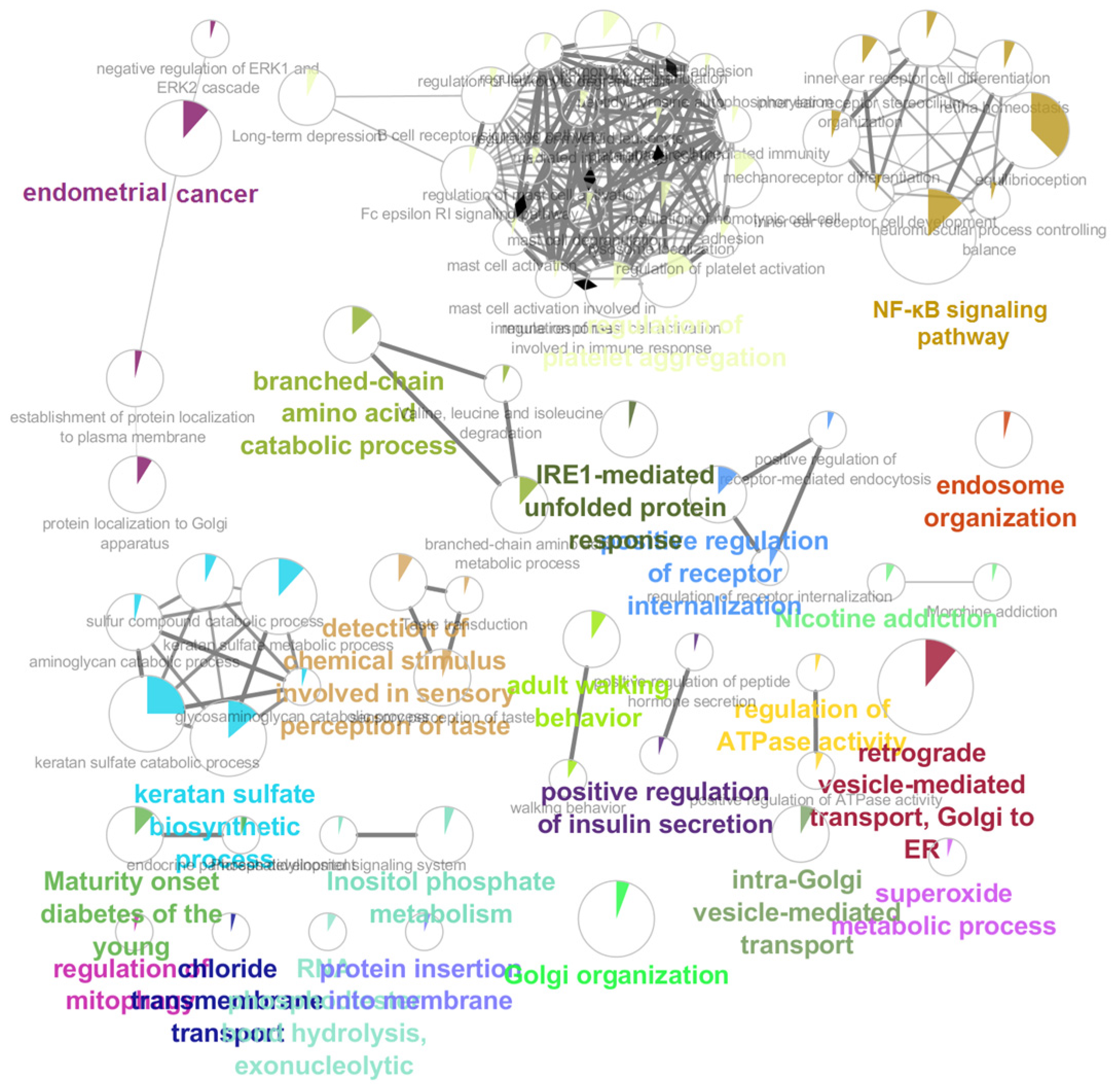
3.2. Pathway Enrichment Analysis
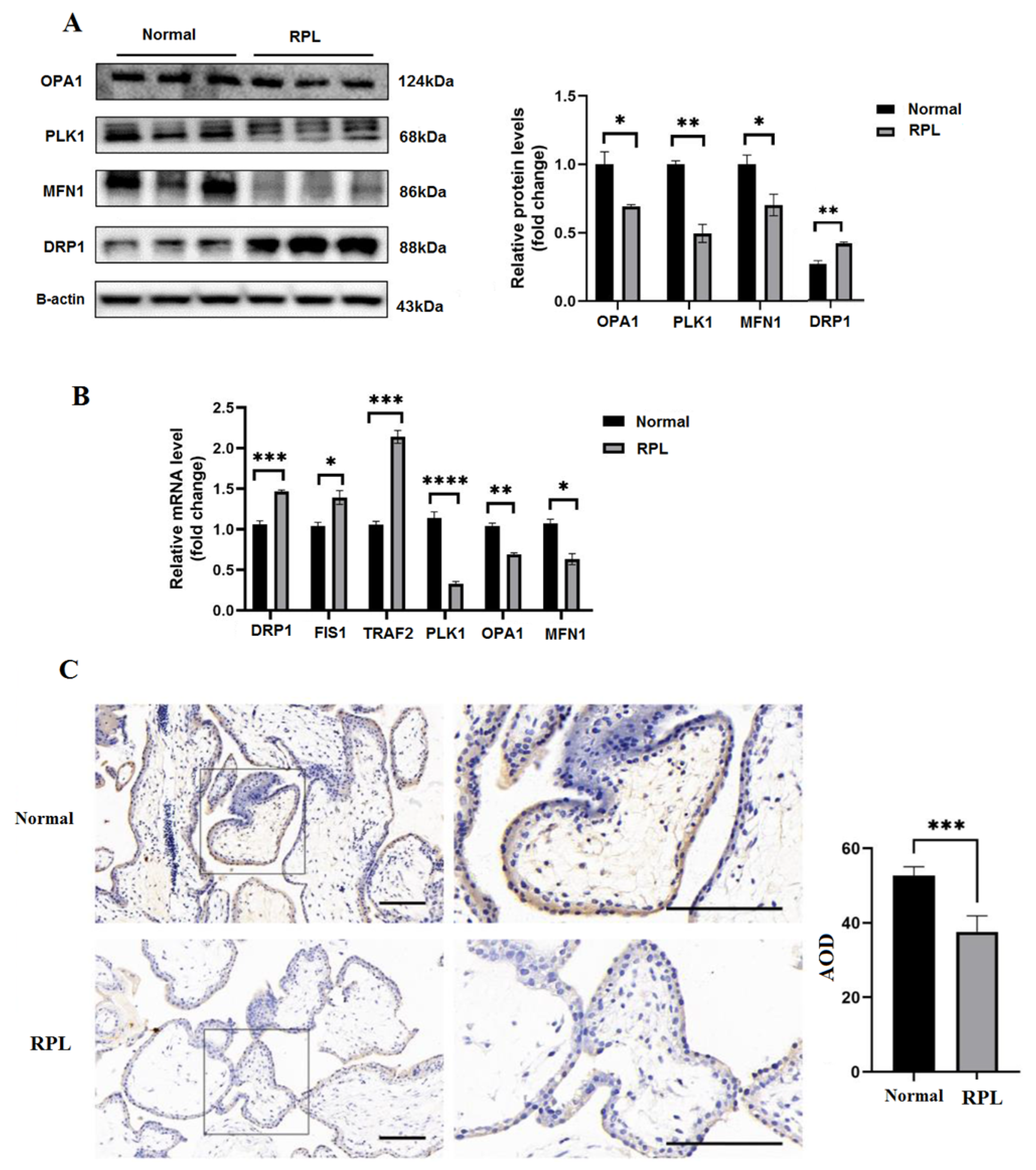
3.3. Biological Processes and Protein Expression
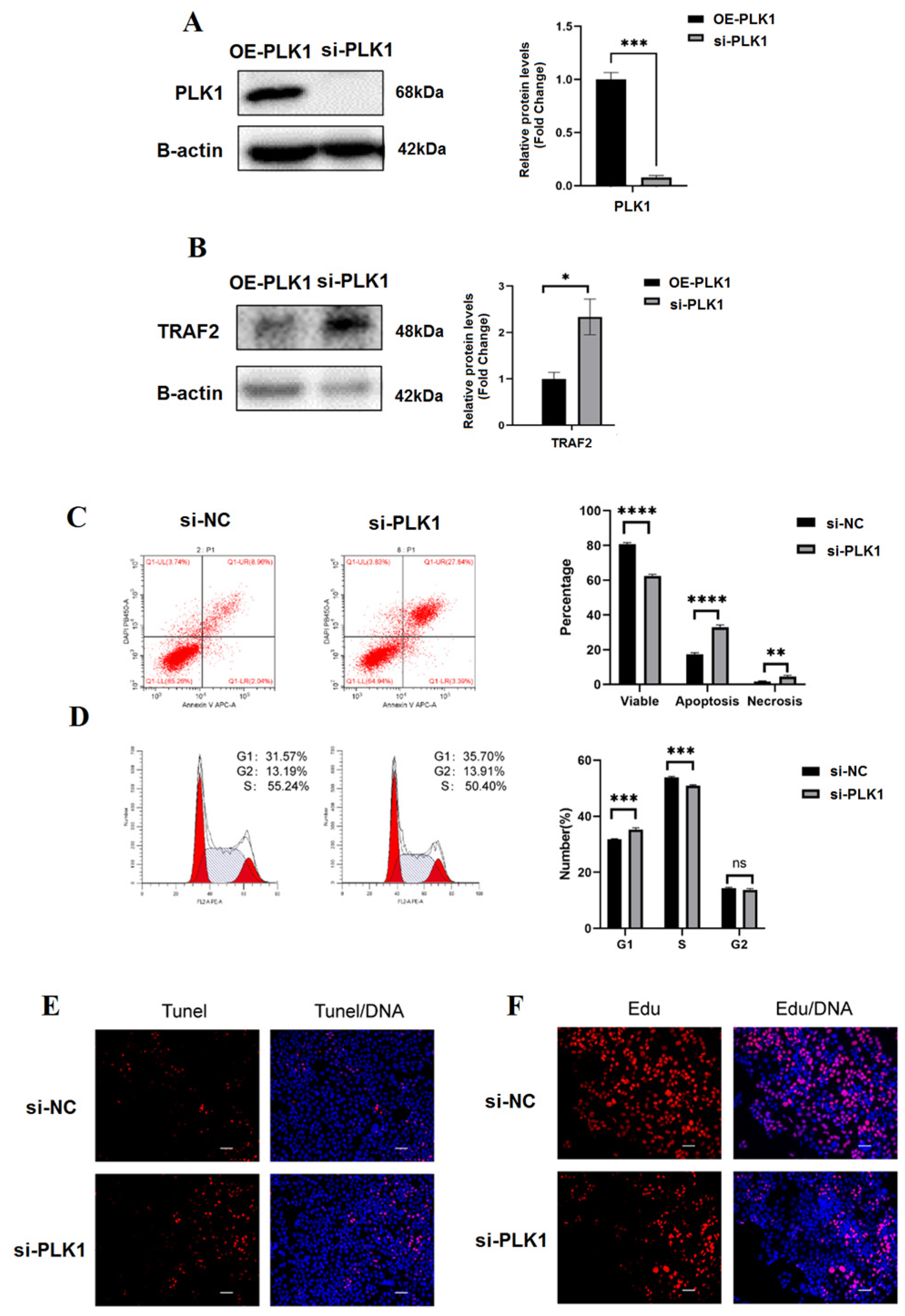
3.4. PLK1 as Hub High Traffic Gene Exerts Positive Effect on the Cell Differentiation
3.5. In Trophoblasts, PLK1 Knockdown Decreases Proliferation and Increases Apoptosis
3.6. PLK1 Promotes Trophoblasts’ G1-S Transition
3.7. Plk1 Stimulates Trophoblast Migration and Invasion
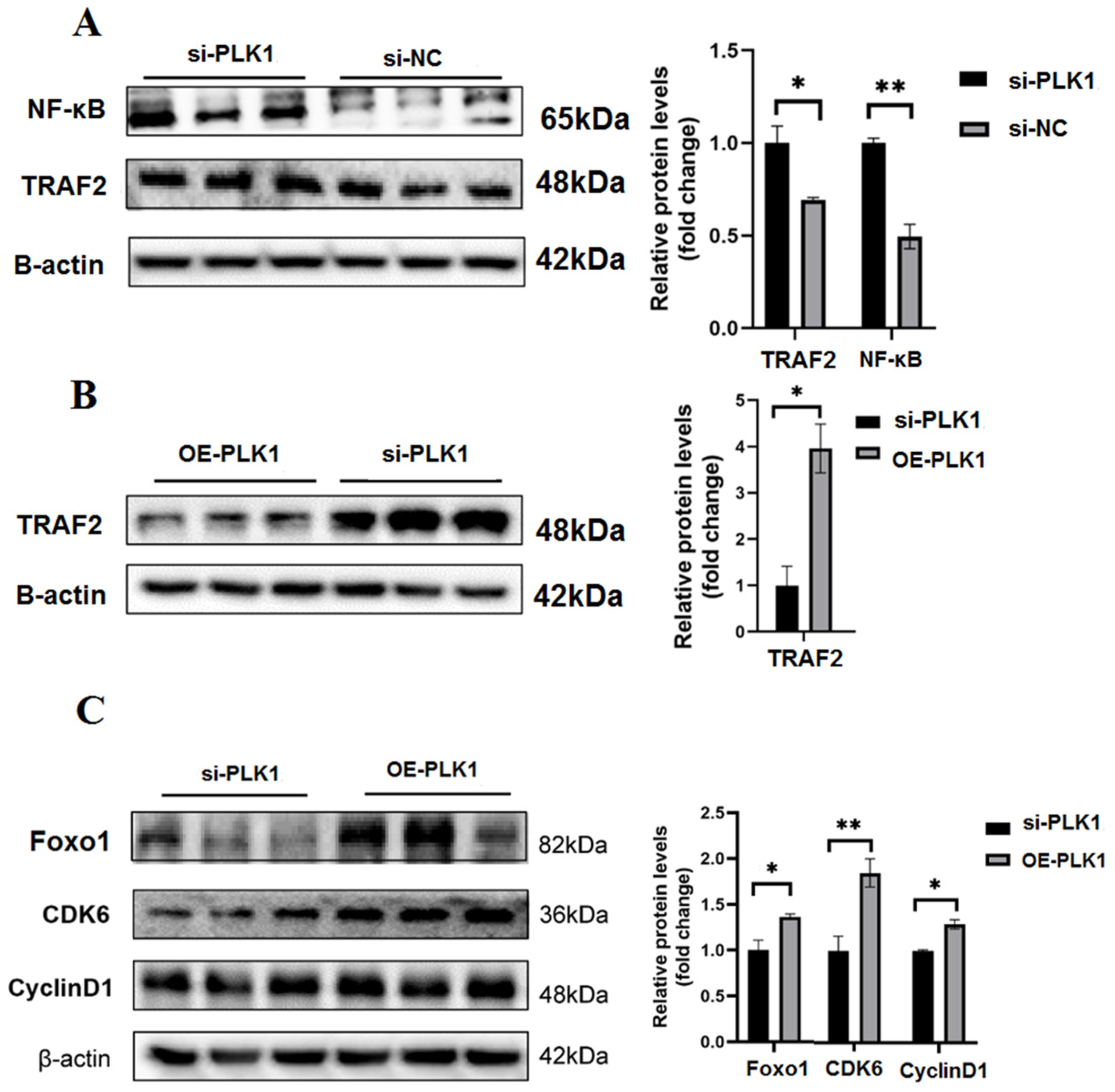
3.8. PLK1 Stimulates the Trophoblast Cell Cycle and Proliferation
3.9. Mice Blastocyst Development and Trophectoderm Differentiation Are Both Negatively Regulated by the PLK1 Inhibitor
3.10. Expression of PLK1-Induced ROS and Dysfunction of the Mitochondria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammon Avalos, L.; Galindo, C.; Li, D.K. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res. 2012, 94, 417–423. [Google Scholar]
- Li, T.C.; Makris, M.; Tomsu, M.; Tuckerman, E.; Laird, S. Recurrent Miscarriage: Aetiology, Management and Prognosis. Hum. Reprod. 2002, 8, 463–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, J.L.; Schust, D.J. Recurrent First Trimester Pregnancy Loss: Revised Definitions and Novel Causes. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 446–450. [Google Scholar] [PubMed]
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers. 2020, 6, 1–19. [Google Scholar]
- Hocher, B.; Hocher, C.F. Epigenetics of Recurrent Pregnancy Loss. EBiomedicine 2018, 35, 18–19. [Google Scholar] [CrossRef] [Green Version]
- Magnus, M.C.; Wilcox, A.J.; Morken, N.-H.; Weinberg, C.R.; Håberg, S.E. Role of maternal age and pregnancy history in risk of miscarriage: Prospective register based study. BMJ 2019, 364, l869. [Google Scholar]
- Mulley, J.F. Greater loss of female embryos during human pregnancy: A novel mechanism. BioEssays 2019, 41, e1900063. [Google Scholar]
- Huang, W.; Man, Y.; Gao, C.; Zhou, L.; Gu, J.; Xu, H.; Wan, Q.; Long, Y.; Chai, L.; Xu, Y.; et al. Short-Chain Fatty Acids Ameliorate Diabetic Nephropathy via GPR43-Mediated Inhibition of Oxidative Stress and NF-kappaB Signaling. Oxid. Med. Cell Longev. 2020, 2020, 4074832. [Google Scholar]
- Gutteridge, R.E.; Ndiaye, M.A.; Liu, X.; Ahmad, N. Plk1 Inhibitors in Cancer Therapy: From Laboratory to Clinics. Mol. Cancer Ther. 2016, 15, 1427–1435. [Google Scholar]
- Ciardo, D.; Haccard, O.; Narassimprakash, H.; Chiodelli, V.; Goldar, A.; Marheineke, K. Polo-like kinase 1 (Plk1) is a positive regulator of DNA replication in the Xenopus in vitro system. Cell Cycle 2020, 19, 1817–1832. [Google Scholar]
- Shakeel, I.; Basheer, N.; Hasan, G.M.; Afzal, M.; Hassan, M.I. Polo-like Kinase 1 as an emerging drug target: Structure, function and therapeutic implications. J. Drug Target 2021, 29, 168–184. [Google Scholar]
- Zhang, W.; Wang, J.; Zhang, Y.; Yuan, Y.; Guan, W.; Jin, C.; Chen, H.; Wang, X.; Yang, X.; He, F. The scaffold protein TANK/I-TRAF inhibits NF-kappaB activation by recruiting polo-like kinase 1. Mol. Biol. Cell 2010, 21, 2500–2513. [Google Scholar] [CrossRef] [Green Version]
- Lipecki, J.; Mitchell, A.E.; Muter, J.; Lucas, E.S.; Makwana, K.; Fishwick, K.; Odendaal, J.; Hawkes, A.; Vrljicak, P.; Brosens, J.; et al. EndoTime: Non-categorical timing estimates for luteal endometrium. Hum. Reprod. 2022, 37, 747–761. [Google Scholar]
- Karahalil, B. Overview of Systems Biology and Omics Technologies. Curr. Med. Chem. 2016, 23, 4221–4230. [Google Scholar] [PubMed]
- Rull, K.; Nagirnaja, L.; Laan, M. Genetics of Recurrent Miscarriage: Challenges, Current Knowledge, Future Directions. Front. Genet. 2012, 3, 34. [Google Scholar]
- Hong, L.; Zhu, Y.C.; Liu, S.; Wu, T.; Li, Y.; Ye, L.; Diao, L.; Zeng, Y. Multi-Omics Reveals a Relationship Between Endometrial Amino Acid Metabolism and Autophagy in Women With Recurrent Miscarriagedagger. Biol. Reprod. 2021, 105, 393–402. [Google Scholar] [PubMed]
- Du, L.; Deng, W.; Zeng, S.; Xu, P.; Huang, L.; Liang, Y.; Wang, Y.; Xu, H.; Tang, J.; Bi, S.; et al. Single-Cell Transcriptome Analysis Reveals Defective Decidua Stromal Niche Attributes to Recurrent Spontaneous Abortion. Cell Prolif. 2021, 54, e13125. [Google Scholar] [PubMed]
- Li, Y.; Wang, R.; Wang, M.; Huang, W.; Liu, C.; Fang, Z.; Liao, S.; Jin, L. RNA Sequencing of Decidua Reveals Differentially Expressed Genes in Recurrent Pregnancy Loss. Reprod. Sci. 2021, 28, 2261–2269. [Google Scholar] [PubMed]
- Mann, O.N.; Kong, C.S.; Lucas, E.S.; Brosens, J.J.; Hanyaloglu, A.C.; Brighton, P.J. Expression and function of the luteinizing hormone choriogonadotropin receptor in human endometrial stromal cells. Sci. Rep. 2022, 12, 8624. [Google Scholar] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Chatr-Aryamontri, A.; Breitkreutz, B.J.; Heinicke, S.; Boucher, L.; Winter, A.; Stark, C.; Nixon, J.; Ramage, L.; Kolas, N.; O’Donnell, L.; et al. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2012, 41, 816–823. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.; Skardal, P.S.; Sun, J. Introduction to Focus Issue: Symmetry and optimization in the synchronization and collective behavior of complex systems. Chaos 2020, 30, 060401. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [Green Version]
- Sherman, B.T.; Huang, D.W.; Tan, Q.; Guo, Y.; Bour, S.; Liu, D.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. DAVID Knowledgebase: A gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinform. 2007, 8, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Schlitt, T.; Brazma, A. Modelling gene networks at different organisational levels. FEBS Lett. 2005, 579, 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlebach, G.; Shamir, R. Modelling and analysis of gene regulatory networks. Nat. Rev. Mol. Cell Biol. 2008, 9, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Affeldt, S.; Verny, L.; Isambert, H. A network reconstruction algorithm based on 2-point and 3-point information statistics. BMC Bioinform. 2016, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaremek, A.; Jeyarajah, M.J.; Jaju Bhattad, G.; Renaud, S.J. Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast. Front. Cell Dev. Biol. 2021, 9, 674162. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Anderson, K.V. The Primary Cilium: A Signalling Centre during Vertebrate Development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and Functional Biology of Arachidonic Acid 15-Lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef]
- Sober, S.; Rull, K.; Reiman, M.; Ilisson, P.; Mattila, P.; Laan, M. RNA Sequencing of Chorionic Villi From Recurrent Pregnancy Loss Patients Reveals Impaired Function of Basic Nuclear and Cellular Machinery. Sci. Rep. 2016, 6, 38439. [Google Scholar] [CrossRef]
- Tian, F.J.; Cheng, Y.X.; Li, X.C.; Wang, F.; Qin, C.M.; Ma, X.L.; Yang, J.; Lin, Y. The YY1/MMP2 Axis Promotes Trophoblast Invasion at the Maternal-Fetal Interface. J. Pathol. 2016, 239, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tang, H.; Xiong, Y.; Tang, L. Differential Expression Profile of Long Noncoding RNAs in Human Chorionic Villi of Early Recurrent Miscarriage. Clin. Chim. Acta 2017, 464, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gao, C.; Gao, L.; Cui, Y.; Liu, J. Expression Profile of Micro-RNAs and Functional Annotation Analysis of Their Targets in Human Chorionic Villi From Early Recurrent Miscarriage. Gene 2016, 576, 366–371. [Google Scholar] [CrossRef]
- Ando, K.; Ozaki, T.; Yamamoto, H.; Furuya, K.; Hosoda, M.; Hayashi, S.; Fukuzawa, M.; Nakagawara, A. Polo-Like Kinase 1 (Plk1) Inhibits P53 Function by Physical Interaction and Phosphorylation. J. Biol. Chem. 2004, 279, 25549–25561. [Google Scholar] [CrossRef] [Green Version]
- Demetriou, C.; Chanudet, E.; GOSgene; JosephJoseph, A.; Topf, M.; Thomas, A.C.; Bitner-Glindzicz, M.; Regan, L.; Stanier, P.; E Moore, G. Exome Sequencing Identifies Variants in Fkbp4 that Are Associated with Recurrent Fetal Loss in Humans. Hum. Mol. Genet. 2019, 28, 3466–3474. [Google Scholar] [CrossRef]
- Maddirevula, S.; Awartani, K.; Coskun, S.; Alnaim, L.F.; Ibrahim, N.; Abdulwahab, F. A Genomics Approach to Females with Infertility and Recurrent Pregnancy Loss. Hum. Genet. 2020, 139, 605–613. [Google Scholar] [CrossRef]
- Qian, J.; Nguyen, N.M.P.; Rezaei, M.; Huang, B.; Tao, Y.; Zhang, X.; Cheng, Q.; Yang, H.; Asangla, A.; Majewski, J.; et al. Biallelic Padi6 Variants Linking Infertility, Miscarriages, and Hydatidiform Moles. Eur. J. Hum. Genet. 2018, 26, 1007–1013. [Google Scholar]
- Quintero-Ronderos, P.; Jiménez, K.M.; Esteban-Pérez, C.; Ojeda, D.A.; Bello, S.; Fonseca, D.J.; Coronel, M.A.; Moreno-Ortiz, H.; Sierra-Díaz, D.C.; Lucena, E.; et al. Foxd1 Mutations Are Related to Repeated Implantation Failure, Intra-uterine Growth Restriction and Preeclampsia. Mol. Med. 2019, 25, 37. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Ronderos, P.; Mercier, E.; Fukuda, M.; González, R.; Suárez, C.F.; Patarroyo, M.A.; Vaiman, D.; Gris, J.-C.; Laissue, P. Novel Genes and Mutations in Patients Affected by Recurrent Pregnancy Loss. PLoS ONE 2017, 12, e0186149. [Google Scholar] [CrossRef]
- Ruf, S.; Heberle, A.M.; Langelaar-Makkinje, M.; Gelino, S.; Wilkinson, D.; Gerbeth, C.; Schwarz, J.J.; Holzwarth, B.; Warscheid, B.; Meisinger, C.; et al. PLK1 (Polo Like Kinase 1) Inhibits MTOR Complex 1 and Promotes Autophagy. Autophagy 2017, 13, 486–505. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Feng, Y.F.; Liu, X.T.; Li, Y.C.; Zhu, H.M.; Sun, M.R.; Li, P.; Liu, B.; Yang, H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021, 38, 101771. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, T.; Song, K.; Du, S.; He, S.; Hu, X.; Li, X.; Li, H.; Dong, S.; Zhang, Y.; et al. Dexmedetomidine ameliorates endotoxin-induced acute lung injury in vivo and in vitro by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway. Redox Biol. 2021, 41, 101954. [Google Scholar] [CrossRef] [PubMed]
- Whitley, B.N.; Engelhart, E.A.; Hoppins, S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion 2019, 49, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Hoppins, S.; Lackner, L.; Nunnari, J. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 2007, 76, 751–780. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Bazylianska, V.; Recanati, M.A.; Fite, A.; Liu, J.; Wan, J.; Mantena, N.; Malek, M.; Podgorski, I.; Heath, E.; et al. Tissue specific regulation of cytochrome c by post-translational modifications: Respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 2019, 33, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V. Mitochondria as decision-makers in cell death. Environ. Mol. Mutagen. 2010, 51, 406–416. [Google Scholar] [CrossRef]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Zhan, L.; Cao, H.; Li, J.; Lyu, Y.; Guo, X.; Zhang, J.; Ji, L.; Ren, T.; An, J.; et al. Increased mitochondrial fission promotes autophagy and hepatocellular carcinoma cell survival through the ROS-modulated coordinated regulation of the NFKB and TP53 pathways. Autophagy 2016, 12, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, K.; Kawagoe, T.; Kondo, T.; Akira, S.; Takeuchi, O. TRAF family member-associated NF kappaB activator (TANK) is a negative regulator of osteoclastogenesis and bone formation. J. Biol. Chem. 2012, 287, 29114–29124. [Google Scholar] [CrossRef] [Green Version]
- Houghton, F.D.; Thompson, J.G.; Kennedy, C.J.; Leese, H.J. Oxygen consumption and energy metabolism of the early mouse embryo. Mol. Reprod. Dev. 1996, 44, 476–485. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Wilding, M.; Dale, B.; Marino, M.; di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef]
- Hamatani, T.; Falco, G.; Carter, M.G.; Akutsu, H.; Stagg, C.A.; Sharov, A.A.; Dudekula, D.B.; VanBuren, V.; Ko, M.S. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004, 13, 2263–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenlaub-Ritter, U.; Wieczorek, M.; Lüke, S.; Seidel, T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 2011, 11, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Heizenrieder, T.; Di Emidio, G.; Treffon, P.; Amicarelli, F.; Seidel, T.; Eichenlaub-Ritter, U. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum. Reprod. 2011, 26, 1843–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Coronel, A.A.; Rostami, A.; Younus, L.A.; Arias Gonzáles, J.L.; Lafta, M.H.; Amin, A.H.; Saadoon, M.A.; Salman, H.M.; Bahrami, A.; Feilei, R.; et al. Designing Effective Multi-Target Drugs and Identifying Biomarkers in Recurrent Pregnancy Loss (RPL) Using In Vivo, In Vitro, and In Silico Approaches. Biomedicines 2023, 11, 879. https://doi.org/10.3390/biomedicines11030879
Ramírez-Coronel AA, Rostami A, Younus LA, Arias Gonzáles JL, Lafta MH, Amin AH, Saadoon MA, Salman HM, Bahrami A, Feilei R, et al. Designing Effective Multi-Target Drugs and Identifying Biomarkers in Recurrent Pregnancy Loss (RPL) Using In Vivo, In Vitro, and In Silico Approaches. Biomedicines. 2023; 11(3):879. https://doi.org/10.3390/biomedicines11030879
Chicago/Turabian StyleRamírez-Coronel, Andrés Alexis, Amirabbas Rostami, Laith A. Younus, José Luis Arias Gonzáles, Methaq Hadi Lafta, Ali H. Amin, Mohammed Abdulkadhim Saadoon, Hayder Mahmood Salman, Abolfazl Bahrami, Rossa Feilei, and et al. 2023. "Designing Effective Multi-Target Drugs and Identifying Biomarkers in Recurrent Pregnancy Loss (RPL) Using In Vivo, In Vitro, and In Silico Approaches" Biomedicines 11, no. 3: 879. https://doi.org/10.3390/biomedicines11030879




