From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications
Abstract
:1. Introduction: From NAFLD to MAFLD
2. Definition of MAFLD
- Waist circumference ≥ 102/88 cm in Caucasian men/women or ≥ 90/80 cm in Asian men/women;
- Blood pressure ≥ 130/85 mmHg or antihypertensive medication;
- Plasma Triglycerides ≥ 150 mg/dl or triglycerides lowering medication;
- Plasma high-density lipoprotein cholesterol (HDL-C) < 40 mg/dl for men and < 50 mg/dl for women or lipid lowering medication;
- Prediabetes (fasting plasma glucose levels between 100–125 mg/dl or 2 h post load glucose levels between 140–199 mg/dl or glycosylated haemoglobin (HbA1c) between 5.7–6.4%;
- Homeostasis model assessment (HOMA) with insulin resistance score ≥ 2.5;
- High-sensitivity C-reactive protein levels > 2 mg/L.

3. MAFLD: Molecular and Pathophysiological Considerations
4. What Link between MAFLD and Cardiovascular Events?
5. MAFLD and Complication of CVD
5.1. Ischemic Stroke
5.2. Structural Cardiac Abnormalities
5.3. Cardiac Arrhythmias
5.4. Coronary Artery Disease (CAD)
5.5. High Blood Pressure
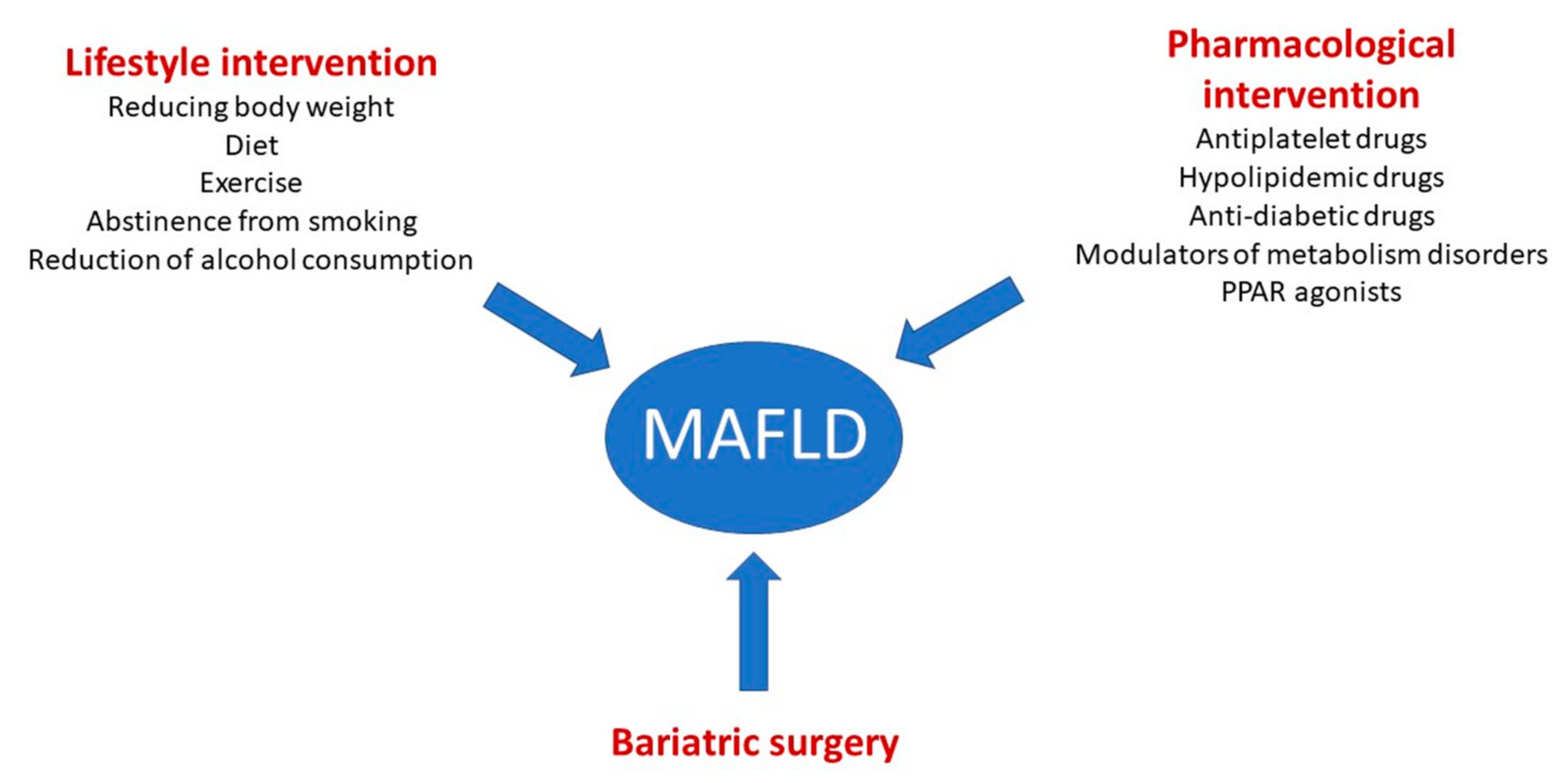
6. Therapeutic Strategies
6.1. Lifestyle Intervention
6.2. Pharmacological Intervention
6.2.1. Aspirin and Hypolipidemic Drugs
6.2.2. Anti-Diabetic Drugs
6.2.3. Modulators of Metabolism Disorders
6.2.4. PPAR Agonists
6.3. Bariatric Surgery
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, E.S.; Hur, W.; Bae, S.H.; Choi, J.Y.; Song, M.J.; Kim, C.W.; Jo, S.H.; Lee, C.D.; Lee, Y.S.; et al. Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2013, 19, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2020, 19, 580–589.e5. [Google Scholar] [CrossRef] [PubMed]
- Loria, P.; Lonardo, A.; Carulli, N. Should nonalcoholic fatty liver disease be renamed? Dig. Dis. 2005, 23, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Eslam, M.; George, J. Refining the role of epicardial adipose tissue in non-alcoholic fatty liver disease. Hepatol. Int. 2019, 13, 662–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, S.E.; Kumar, R.B.; Shukla, A.P. Nonalcoholic steatohepatitis, obesity, and cardiac dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 315–320. [Google Scholar] [CrossRef]
- Liu, S.-S.; Ma, X.-F.; Zhao, J.; Du, S.-X.; Zhang, J.; Dong, M.-Z.; Xin, Y.-N. Association between nonalcoholic fatty liver disease and extrahepatic cancers: A systematic review and meta-analysis. Lipids Health Dis. 2020, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, A.A.; Ahmed, A.; Kim, D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver 2020, 14, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Colognesi, M.; Gabbia, D.; De Martin, S. Depression and Cognitive Impairment—Extrahepatic Manifestations of NAFLD and NASH. Biomedicines 2020, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Santos, R.D.; Valenti, L.; Romeo, S. Does nonalcoholic fatty liver disease cause cardiovascular disease? Current knowledge and gaps. Atherosclerosis 2019, 282, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Boeckmans, J.; Natale, A.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, R.M. Human-based systems: Mechanistic NASH modelling just around the corner? Pharmacol. Res. 2018, 134, 257–267. [Google Scholar] [CrossRef]
- Caligiuri, A.; Gentilini, A.; Marra, F. Molecular Pathogenesis of NASH. Int. J. Mol. Sci. 2016, 17, 1575. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Sanyal, A.; Charles, E.D.; A Neuschwander-Tetri, B.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2018, 392, 2705–2717. [Google Scholar] [CrossRef]
- Ballestri, S.; Lonardo, A.; Bonapace, S.; Byrne, C.D.; Loria, P.; Targher, G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1724–1745. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal communication in liver fibrosis and regeneration. J. Hepatol. 2016, 65, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Gracia-Sancho, J.; Caparrós, E.; Fernández-Iglesias, A.; Francés, R. Role of liver sinusoidal endothelial cells in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Wentworth, B.J.; Zimmet, A.; Rinella, M.E.; Loomba, R.; Caldwell, S.H.; Argo, C. Systematic review with meta-analysis: Risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol. Ther. 2018, 48, 696–703. [Google Scholar] [CrossRef]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The changing scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2021, 41, 585–597. [Google Scholar] [CrossRef]
- Vitale, A.; Svegliati-Baroni, G.; Ortolani, A.; Cucco, M.; Riva, G.V.D.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.; Marco, M.D.; Caturelli, E.; et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: The ITA.LI.CA database. Gut 2023, 72, 141–152. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease-related risk of cardiovascular disease and other cardiac complications. Diabetes Obes. Metab. 2021, 24, 28–43. [Google Scholar] [CrossRef]
- Meyersohn, N.M.; Mayrhofer, T.; Corey, K.E.; Bittner, D.O.; Staziaki, P.V.; Szilveszter, B.; Hallett, T.; Lu, M.T.; Puchner, S.B.; Simon, T.G.; et al. Association of Hepatic Steatosis With Major Adverse Cardiovascular Events, Independent of Coronary Artery Disease. Clin. Gastroenterol. Hepatol. 2020, 19, 1480–1488.e14. [Google Scholar] [CrossRef]
- D’Ardes, D.; Boccatonda, A.; Cocco, G.; Fabiani, S.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Schiavone, C.; Cipollone, F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J. Gastroenterol. 2022, 28, 1102–1112. [Google Scholar] [CrossRef]
- Brouwers, M.C.G.J.; Simons, N.; Stehouwer, C.D.A.; Isaacs, A. Non-alcoholic fatty liver disease and cardiovascular disease: Assessing the evidence for causality. Diabetologia 2019, 63, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Adiels, M.; Taskinen, M.R.; Borén, J. Fatty liver, insulin resistance, and dyslipidemia. Curr. Diabetes Rep. 2008, 8, 60–64. [Google Scholar] [CrossRef]
- Altun, Ö.; Dikker, O.; Arman, Y.; Ugurlukisi, B.; Kutlu, O.; Cil, E.O.; Yoldemir, S.A.; Akarsu, M.; Ozcan, M.; Kalyon, S.; et al. Serum Angiopoietin-like peptide 4 levels in patients with hepatic steatosis. Cytokine 2018, 111, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.; D’Erasmo, L.; Di Costanzo, A.; Minicocci, I.; Pecce, V.; Arca, M. The Interplay between Angiopoietin-Like Proteins and Adipose Tissue: Another Piece of the Relationship between Adiposopathy and Cardiometabolic Diseases? Int. J. Mol. Sci. 2021, 22, 742. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, J.; Zhao, J.; Endo, M.; Kadomatsu, T.; Miyata, K.; Sugizaki, T.; Okadome, Y.; Tian, Z.; Horiguchi, H.; Miyashita, K.; et al. Association of circulating ANGPTL 3, 4, and 8 levels with medical status in a population undergoing routine medical checkups: A cross-sectional study. PLoS ONE 2018, 13, e0193731. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Sninsky, J.J.; Baca, A.M.; Superko, H.R.; Portillo Sanchez, P.; Biernacki, D.; Maximos, M.; Lomonaco, R.; Orsak, B.; Suman, A.; et al. Hepatic Steatosis and Insulin Resistance, But Not Steatohepatitis, Promote Atherogenic Dyslipidemia in NAFLD. J. Clin. Endocrinol. Metab. 2016, 101, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.-M.; Ho, S.-L.; Jeng, Y.-M.; Lai, Y.-S.; Chen, Y.-H.; Lu, S.-C.; Chen, H.-L.; Chang, P.-Y.; Hu, R.-H.; Lee, P.-H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. 2019, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Matikainen, N.; Mänttäri, S.; Westerbacka, J.; Vehkavaara, S.; Lundbom, N.; Yki-Järvinen, H.; Taskinen, M.-R. Postprandial Lipemia Associates with Liver Fat Content. J. Clin. Endocrinol. Metab. 2007, 92, 3052–3059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 2014, 30, 1489–1503. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Rametta, R.; Meroni, M.; Valenti, L. The role of insulin resistance in nonalcoholic steatohepatitis and liver disease development—A potential therapeutic target? Expert Rev. Gastroenterol. Hepatol. 2016, 10, 229–242. [Google Scholar] [CrossRef]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.-H.; Dutcher, A.K.; Towle, H.C. Glucose and Insulin Function through Two Distinct Transcription Factors to Stimulate Expression of Lipogenic Enzyme Genes in Liver. J. Biol. Chem. 2001, 276, 9437–9445. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lim, T.S.; Kim, S.U.; Kim, H.C. Long-term cardiovascular outcomes differ across metabolic dysfunction-associated fatty liver disease subtypes among middle-aged population. Hepatol. Int. 2022, 16, 1308–1317. [Google Scholar] [CrossRef]
- Alkagiet, S.; Papagiannis, A.; Tziomalos, K. Associations between nonalcoholic fatty liver disease and ischemic stroke. World J. Hepatol. 2018, 10, 474–478. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Jung, M.-H.; Jeong, S.-H.; Hong, Y.-P.; Kim, Y.I.; An, S.J. The Association between Nonalcoholic Fatty Liver Disease and Stroke: Results from the Korean Genome and Epidemiology Study (KoGES). Int. J. Environ. Res. Public Health 2020, 17, 9568. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Panjawatanan, P.; Kroner, P.T.; Cheungpasitporn, W.; Ungprasert, P. Association between cardiac conduction defect and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef]
- Chiu, L.S.; Pedley, A.; Massaro, J.M.; Benjamin, E.J.; Mitchell, G.F.; McManus, D.D.; Aragam, J.; Vasan, R.S.; Cheng, S.; Long, M.T. The association of non-alcoholic fatty liver disease and cardiac structure and function–Framingham Heart Study. Liver Int. 2020, 40, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Ballestri, S.; Ricci, F.; Di Vincenzo, A.; Ticinesi, A.; Gallina, S.; Giamberardino, M.A.; Cipollone, F.; Sutton, R.; Vettor, R.; et al. Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease: Mechanisms and Therapeutic Implications. Int. J. Environ. Res. Public Health 2019, 16, 3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismaiel, A.; Spinu, M.; Socaciu, C.; Budisan, L.; Leucuta, D.-C.; Popa, S.-L.; Chis, B.A.; Berindan-Neagoe, I.; Olinic, D.M.; Dumitrascu, D.L. Metabolic biomarkers related to cardiac dysfunction in metabolic-dysfunction-associated fatty liver disease: A cross-sectional analysis. Nutr. Diabetes 2022, 12, 4. [Google Scholar] [CrossRef]
- Thanassoulis, G.; Massaro, J.M.; O’Donnell, C.J.; Hoffmann, U.; Levy, D.; Ellinor, P.T.; Wang, T.J.; Schnabel, R.B.; Vasan, R.S.; Fox, C.S.; et al. Pericardial fat is associated with prevalent atrial fibrillation: The Framingham Heart Study. Circ. Arrhythmia Electrophysiol. 2010, 3, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Sone, H.; Mizuno, S.; Fujii, H.; Yoshimura, Y.; Yamasaki, Y.; Ishibashi, S.; Katayama, S.; Saito, Y.; Ito, H.; Ohashi, Y.; et al. Is the diagnosis of metabolic syndrome useful for predicting cardiovascular disease in asian diabetic patients? Analysis from the Japan Diabetes Complications Study. Diabetes Care 2005, 28, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Muthiah, M.; Ng, C.H.; Chan, K.E.; Fu, C.E.; Lim, W.H.; Tan, D.J.H.; Nah, B.; Kong, G.; Xiao, J.; Yong, J.N.; et al. Type 2 diabetes mellitus in metabolic-associated fatty liver disease vs. type 2 diabetes mellitus non-alcoholic fatty liver disease: A longitudinal cohort analysis. Ann. Hepatol. 2023, 28, 100762. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Núñez-Córdoba, J.M.; Ezponda, A.; Mendoza, F.J.; Ampuero, J.; Bastarrika, G.; Frühbeck, G.; Escalada, G. Cardiometabolic characterization in metabolic dysfunction-associated fatty liver disease. Front. Med. 2022, 9, 1023583. [Google Scholar] [CrossRef]
- Bessho, R.; Kashiwagi, K.; Ikura, A.; Yamataka, K.; Inaishi, J.; Takaishi, H.; Kanai, T. A significant risk of metabolic dysfunction-associated fatty liver disease plus diabetes on subclinical atherosclerosis. PLoS ONE 2022, 17, e0269265. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.J.; Ahn, S.H.; Lee, K.S.; Lee, B.K.; Baik, S.J.; Kim, S.U.; Lee, J.I. MAFLD Predicts the Risk of Cardiovascular Disease Better than NAFLD in Asymptomatic Subjects with Health Check-Ups. Dig. Dis. Sci. 2022, 67, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Yamashita, M.; Uchida, S.; Maekawa, E.; Terada, T.; Reed, J.L.; et al. The Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease and Its Association with Physical Function and Prognosis in Patients with Acute Coronary Syndrome. J. Clin. Med. 2022, 11, 1847. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Cao, Y.-X.; Jin, J.-L.; Guo, Y.-L.; Zhu, C.-G.; Wu, N.-Q.; Gao, Y.; Xu, R.-X.; Dong, Q.; Zheng, M.-H.; et al. Metabolic-associated fatty liver disease and major adverse cardiac events in patients with chronic coronary syndrome: A matched case–control study. Hepatol. Int. 2021, 15, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Staels, B. Hepatic sexual dimorphism—Implications for non-alcoholic fatty liver disease. Nat. Rev. Endocrinol. 2021, 17, 662–670. [Google Scholar] [CrossRef]
- Mori, K.; Tanaka, M.; Hosaka, I.; Mikami, T.; Endo, K.; Hanawa, N.; Ohnishi, H.; Furuhashi, M. Metabolic dysfunction-associated fatty liver disease is associated with an increase in systolic blood pressure over time: Linear mixed-effects model analyses. Hypertens Res. 2023. [Google Scholar] [CrossRef]
- Theofilis, P.; Vordoni, A.; Tsimihodimos, V.; Kalaitzidis, R.G. Metabolic Dysfunction-Associated Fatty Liver Disease in Newly Diagnosed, Treatment-Naive Hypertensive Patients and Its Association with Cardiorenal Risk Markers. High Blood Press Cardiovasc. Prev. 2023, 30, 63–72. [Google Scholar] [CrossRef]
- Liu, J.; Lv, H.; Wang, J.; Zhu, Q.; Chen, G.; Jiang, Y.; Zhao, K.; Shao, L.; Shi, J.; Pan, X. Blood pressure stratification for predicting liver fibrosis risk in metabolic dysfunction associated fatty liver disease. Ann. Hepatol. 2022, 28, 100892. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- EASL-EASD-EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.-M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [Green Version]
- Lessiani, G.; Santilli, F.; Boccatonda, A.; Iodice, P.; Liani, R.; Tripaldi, R.; Saggini, R.; Davì, G. Arterial stiffness and sedentary lifestyle: Role of oxidative stress. Vasc. Pharmacol. 2016, 79, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Tripaldi, R.; Davì, G.; Santilli, F. Oxidative Stress Modulation Through Habitual Physical Activity. Curr. Pharm. Des. 2016, 22, 3648–3680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goh, G.B.; Chan, W.; Wong, G.L.; Fan, J.; Seto, W.; Huang, Y.; Lin, H.; Lee, I.; Lee, H.W.; et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non-alcoholic fatty liver disease. Liver Int. 2020, 40, 2719–2731. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Kawaguchi, T.; Bekki, M.; Omoto, M.; Matsuse, H.; Nago, T.; Takano, Y.; Ueno, T.; Koga, H.; George, J.; et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J. Hepatol. 2016, 66, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Niederseer, D.; Wernly, B.; Aigner, E.; Stickel, F.; Datz, C. NAFLD and Cardiovascular Diseases: Epidemiological, Mechanistic and Therapeutic Considerations. J. Clin. Med. 2021, 10, 467. [Google Scholar] [CrossRef]
- Simeone, P.; Boccatonda, A.; Liani, R.; Santilli, F. Significance of urinary 11-dehydro-thromboxane B2 in age-related diseases: Focus on atherothrombosis. Ageing Res. Rev. 2018, 48, 51–78. [Google Scholar] [CrossRef]
- Perla, F.M.; Prelati, M.; Lavorato, M.; Visicchio, D.; Anania, C. The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Katzmann, J.L.; Laufs, U. New Insights in the Control of Low-Density Lipoprotein Cholesterol to Prevent Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 69. [Google Scholar] [CrossRef]
- Vadini, F.; Simeone, P.G.; Boccatonda, A.; Guagnano, M.T.; Liani, R.; Tripaldi, R.; Di Castelnuovo, A.; Cipollone, F.; Consoli, A.; Santilli, F. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: A randomized, controlled study. Int. J. Obes. 2020, 44, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.A.; Mells, J.; Dunham, R.M.; Grakoui, A.; Handy, J.; Saxena, N.K.; Anania, F.A. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yao, B.; Kuang, H.; Yang, X.; Huang, Q.; Hong, T.; Li, Y.; Dou, J.; Yang, W.; Qin, G.; et al. Liraglutide, Sitagliptin, and Insulin Glargine Added to Metformin: The Effect on Body Weight and Intrahepatic Lipid in Patients With Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 2414–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, J.-M.; Cercueil, J.-P.; Loffroy, R.; Denimal, D.; Bouillet, B.; Fourmont, C.; Chevallier, O.; Duvillard, L.; Vergès, B. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes. The Lira-NAFLD study. J. Clin. Endocrinol. Metab. 2016, 102, jc20162775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Lai, L.L.; Vethakkan, S.R.; Nik Mustapha, N.R.; Mahadeva, S.; Chan, W.K. Empagliflozin for the Treatment of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes Mellitus. Dig. Dis. Sci. 2020, 65, 623–631. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.R.; Haeusler, R.A. Bile acids in glucose metabolism and insulin signalling—Mechanisms and research needs. Nat. Rev. Endocrinol. 2019, 15, 701–712. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.-S.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Degirolamo, C.; Sabbà, C.; Moschetta, A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat. Rev. Drug Discov. 2016, 15, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A. Is it necessary to target lipid metabolism in different organs for effective treatment of NASH?—The results of the Pan-PPAR Lanifibranor trial. HepatoBiliary Surg. Nutr. 2022, 11, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Pierre, B.; Elisabetta, B.; Vlad, R.; Philippe, H.M.; Bruno, S.; Jean-Louis, J.; Philippe, H.M.; Bruno, S.; Jean-Louis, J.; Pierre, B.; et al. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp. Clin. Trials 2020, 98, 106170. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Idowu, M.O.; Parmar, D.; Borg, B.B.; Denham, D.; Loo, N.M.; Lazas, D.; Younes, Z.; Sanyal, A.J. A Phase 2 Double Blinded, Randomized Controlled Trial of Saroglitazar in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 19, 2670–2672. [Google Scholar] [CrossRef]
- Malik, A.; Nadeem, M.; Malik, M.I. Efficacy of elafibranor in patients with liver abnormalities especially non-alcoholic steatohepatitis: A systematic review and meta-analysis. Clin. J. Gastroenterol. 2021, 14, 1579–1586. [Google Scholar] [CrossRef]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Brar, K.; Banfield, L.; Gmora, S.; Anvari, M.; Hong, D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1040–1060.e11. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.; Narwaria, M.; Mahawar, K.K. A Systematic Review of Bariatric Surgery in Patients with Liver Cirrhosis. Obes. Surg. 2015, 25, 1518–1526. [Google Scholar] [CrossRef]
- Are, V.S.; Knapp, S.M.; Banerjee, A.; Shamseddeen, H.; Ghabril, M.; Orman, E.; Patidar, K.R.; Chalasani, N.; Desai, A.P. Improving Outcomes of Bariatric Surgery in Patients With Cirrhosis in the United States: A Nationwide Assessment. Am. J. Gastroenterol. 2020, 115, 1849–1856. [Google Scholar] [CrossRef]
- Ramai, D.; Singh, J.; Lester, J.; Khan, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; D’Erasmo, L.; Bini, S.; Pastori, D.; Angelico, F.; Del Ben, M.; Arca, M.; Di Costanzo, A. Heterogeneity of non-alcoholic fatty liver disease (NAFLD): Implication for cardiovascular risk stratification. Atherosclerosis 2022, 357, 51–59. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Class | Trial | Phase | Trial ID |
|---|---|---|---|---|
| Obeticholic acid | FXR agonist 1st gen. | FLINT study | IIb | NCT01265498 |
| REGENERATE study (NASH with significant fibrosis) | III | NCT02548351 | ||
| REVERSE study (NASH with significant cirrhosis) | III | NCT03439254 | ||
| Cilofexor | FXR agonist 2nd gen. | Cilofexor in patients with noncirrhotic NASH | II | NCT02854605 |
| Elafibranor | PPAR α/δ agonist | GOLDEN- 505 study | IIb | NCT01694849 |
| RESOLVE- IT study | III | NCT02704403 | ||
| Lanifibranor | Pan- PPAR agonist | NATIVE study | IIb | NCT03008070 |
| Saroglitazar | Dual PPAR- α/γ agonist | EVIDENCE IV study | II | NCT03061721 |
| Liraglutide | GLP-1 receptor agonist | LEAN study | II | NCT01237119 |
| Semaglutide | GLP-1 receptor agonist | Subcutaneous semaglutide in NASH | II | NCT02970942 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. https://doi.org/10.3390/biomedicines11030883
Boccatonda A, Andreetto L, D’Ardes D, Cocco G, Rossi I, Vicari S, Schiavone C, Cipollone F, Guagnano MT. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines. 2023; 11(3):883. https://doi.org/10.3390/biomedicines11030883
Chicago/Turabian StyleBoccatonda, Andrea, Lorenzo Andreetto, Damiano D’Ardes, Giulio Cocco, Ilaria Rossi, Susanna Vicari, Cosima Schiavone, Francesco Cipollone, and Maria Teresa Guagnano. 2023. "From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications" Biomedicines 11, no. 3: 883. https://doi.org/10.3390/biomedicines11030883






