Hepatoprotective and Neuroprotective Effects of Naringenin against Lead-Induced Oxidative Stress, Inflammation, and Apoptosis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
2.2. Experimental Planning and Animals
2.3. Collection and Processing of Samples
2.4. Biochemical Tests
2.4.1. Evaluation of Liver Markers
2.4.2. Evaluation of Neural Monoamines
2.4.3. Evaluation of Hepatic and Neural Oxidant/Antioxidant Parameters
2.5. Analysis of Gene Expression
2.5.1. Extraction of Total RNA and Reverse Transcription
2.5.2. Quantitative Real-Time PCR
2.6. Histopathological Evaluation
2.7. Analysis of Statistics
3. Results
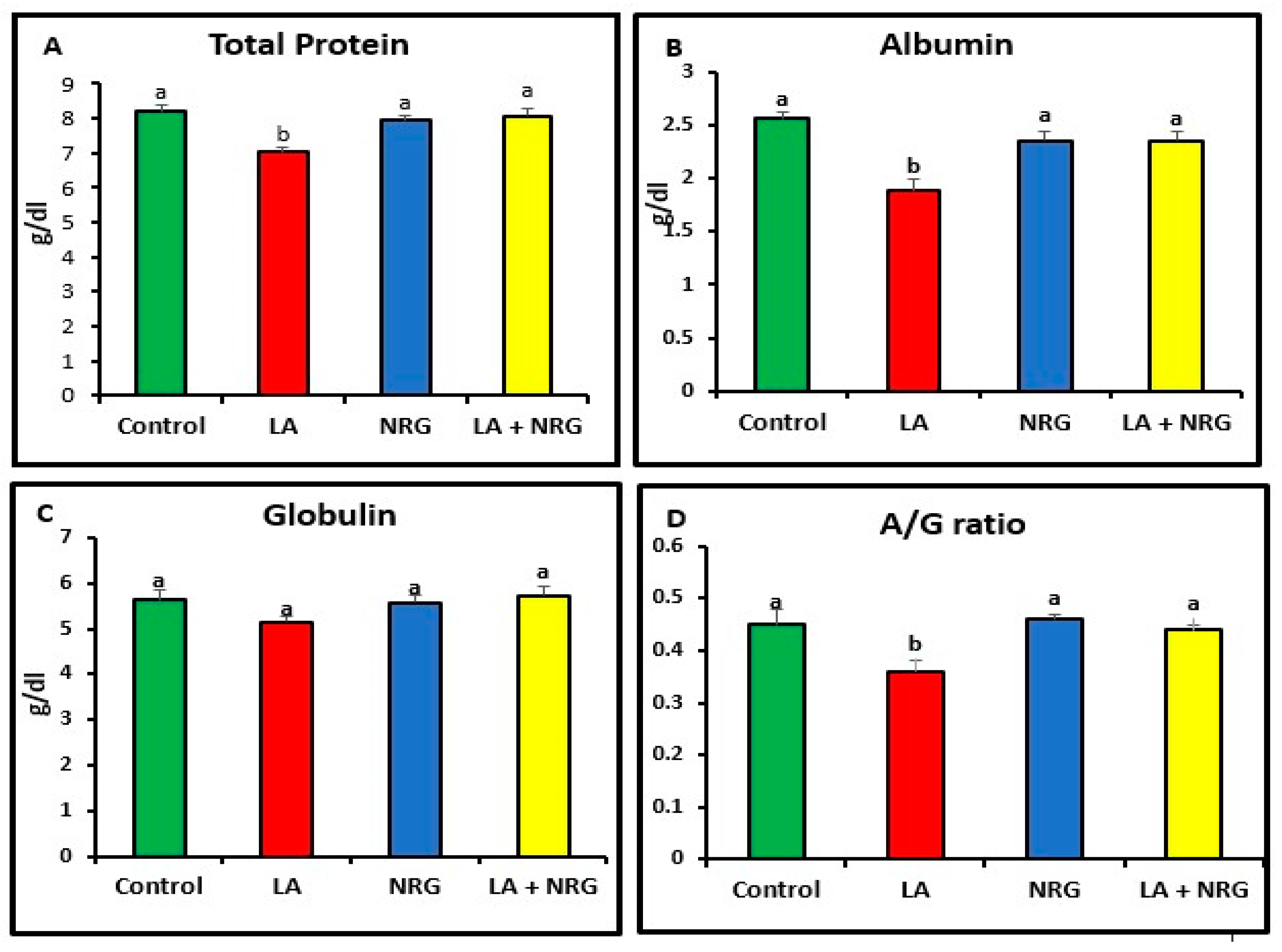
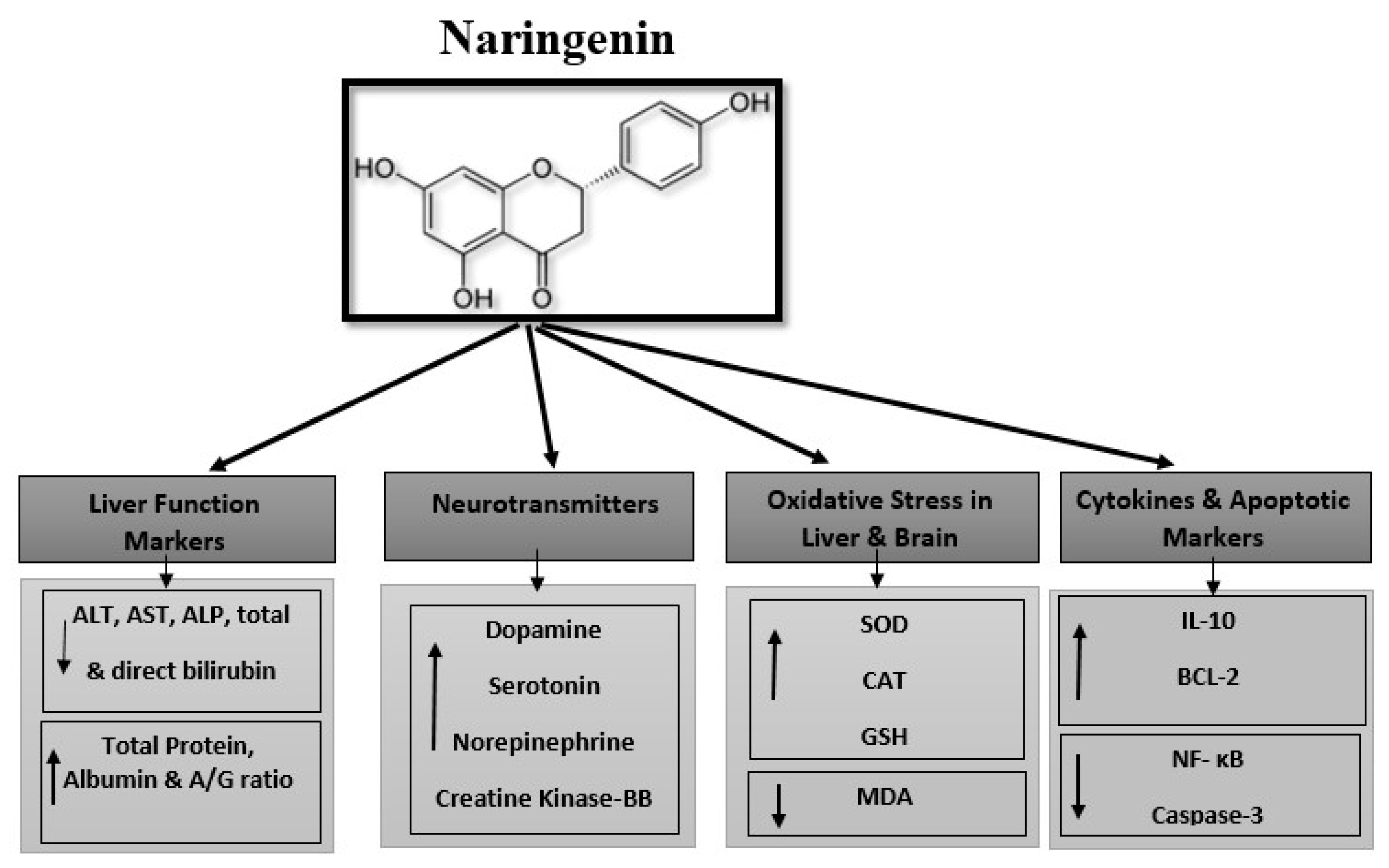
3.1. Protective Effect of Naringenin (NRG) on Liver Function Markers in Lead-Treated Rats
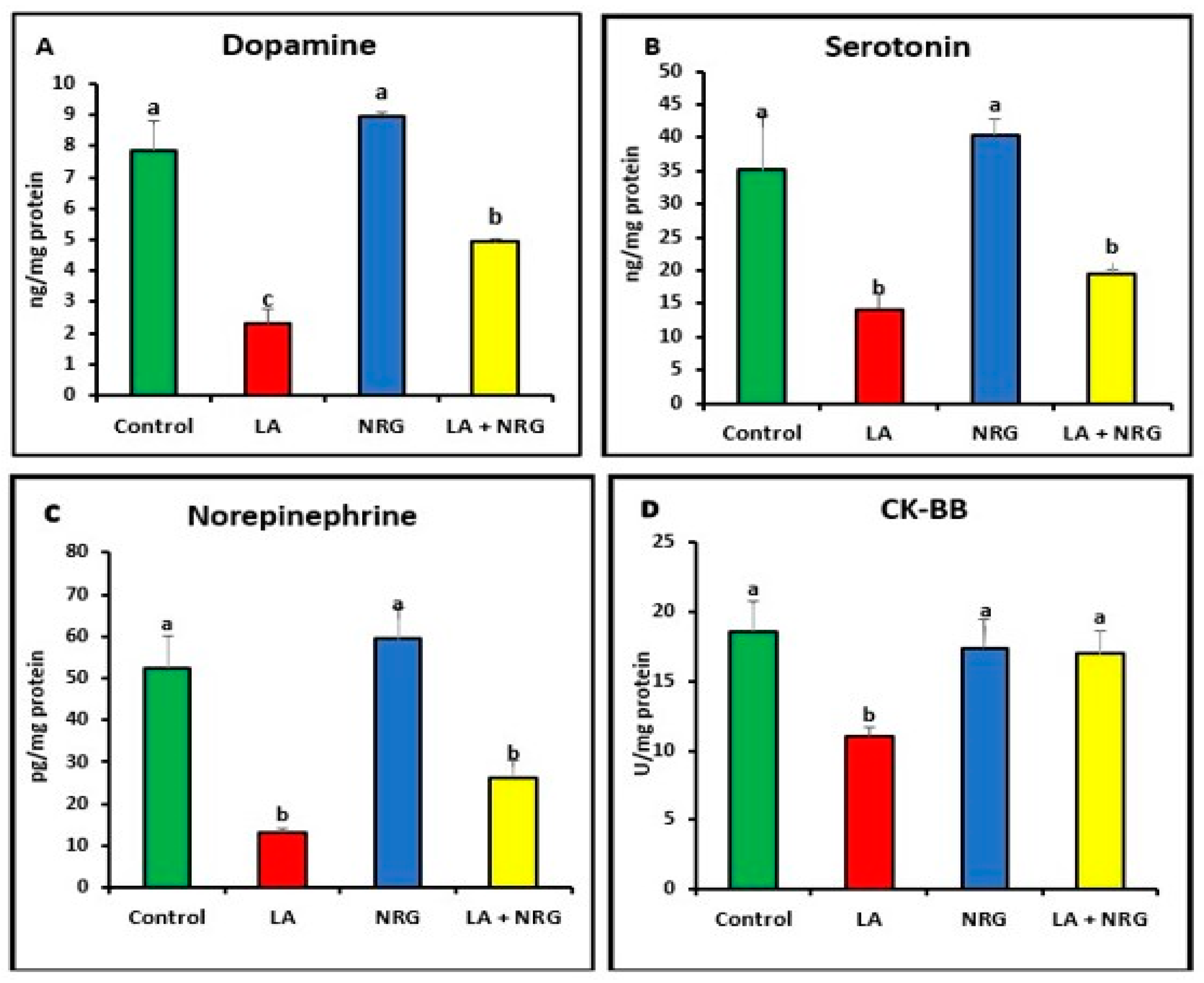
3.2. Protective Effect of Naringenin (NRG) on the Levels of Neurotransmitters in the Brains of Lead-Intoxicated Rats
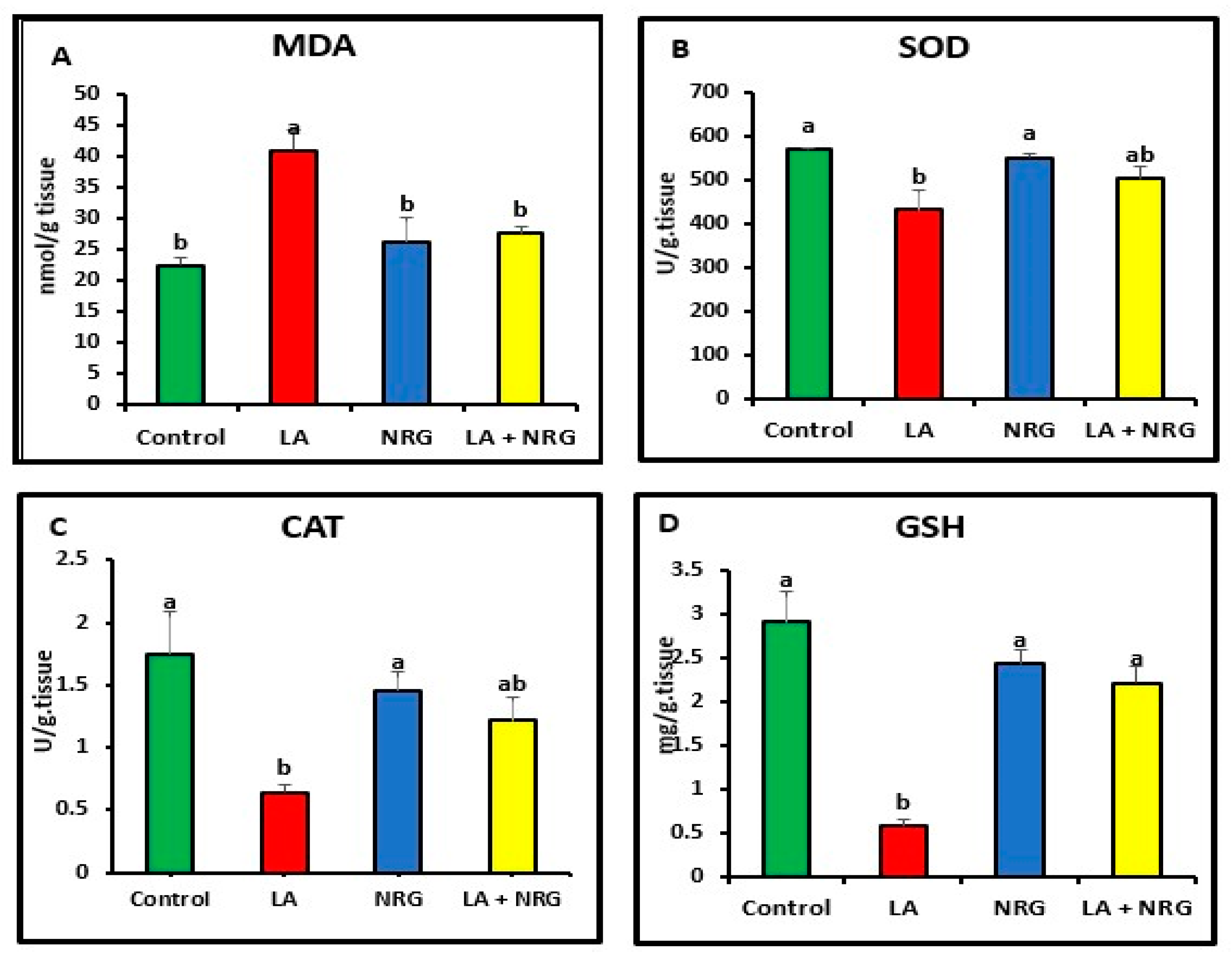
3.3. Protective Effect of Naringenin (NRG) on Hepatic and Neural Lipid Peroxidation and Antioxidant System in Lead-Intoxicated Rats
3.4. Protective Effect of Naringenin (NRG) on Hepatic and Neural Expression of Cytokines and Apoptotic Markers in Lead-Intoxicated Rats
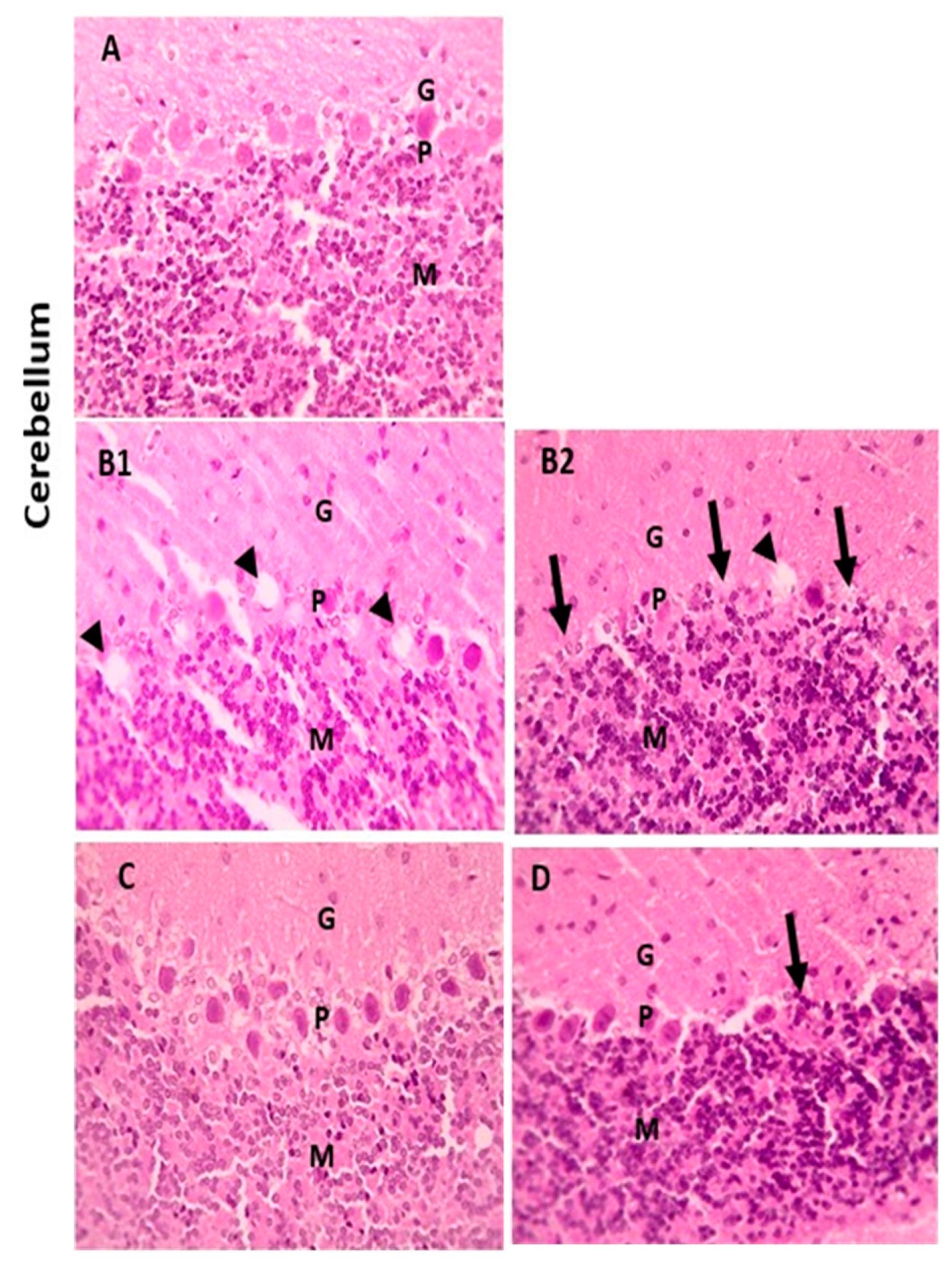
3.5. Protective Effect of Naringenin (NRG) on Histopathological Alterations in Hepatic and Brain Tissues of Lead-Intoxicated Rats
3.5.1. Hepatic Tissue
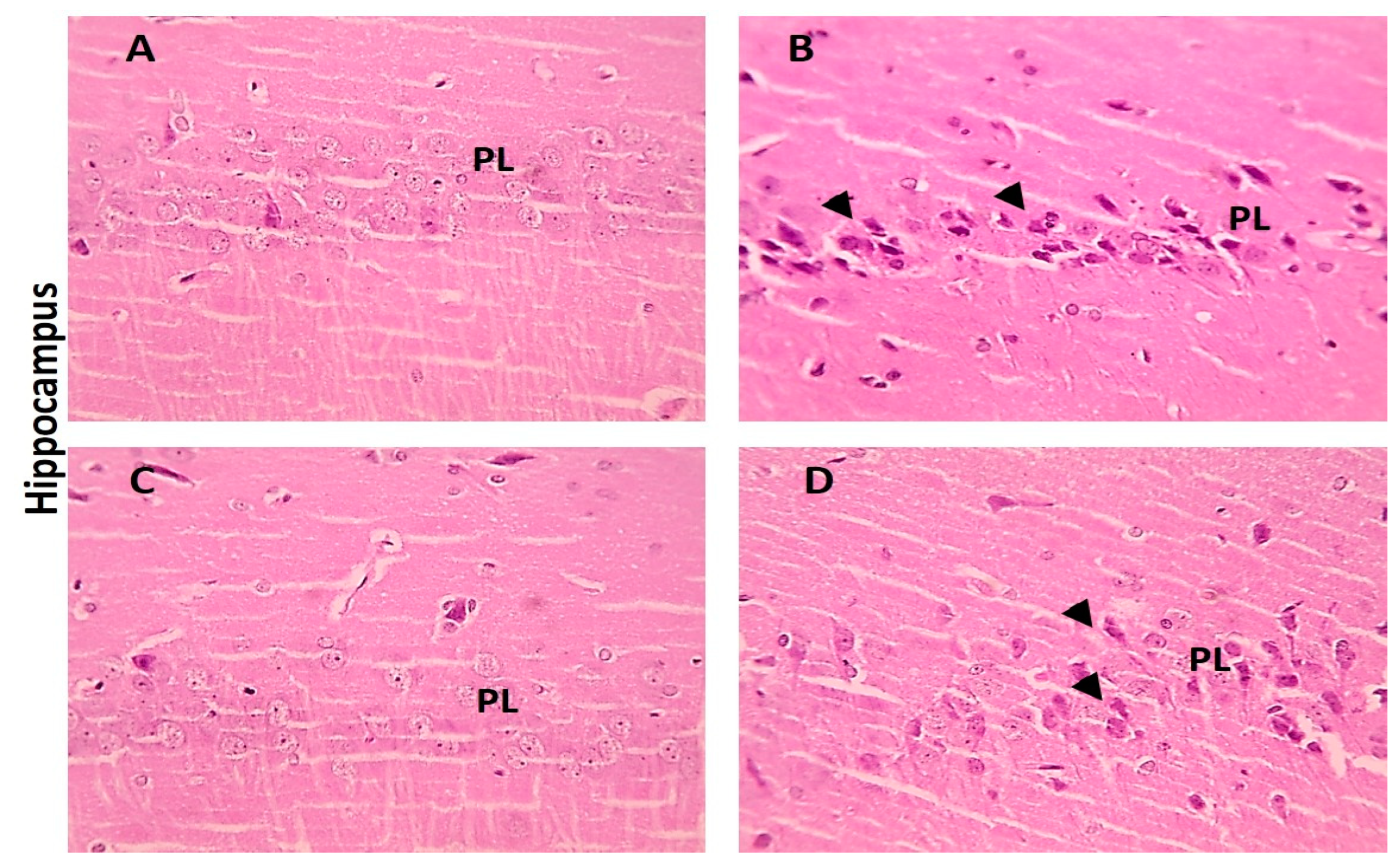
3.5.2. Brain Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousef, A.O.S.; Fahad, A.A.; Abdel Moneim, A.E.; Metwally, D.M.; El-Khadragy, M.F.; Kassab, R.B. The neuroprotective role of coenzyme Q10 against lead acetate-induced neurotoxicity is mediated by antioxidant, anti-inflammatory and anti-apoptotic activities. Int. J. Environ. Res. Public Health 2019, 16, 2895. [Google Scholar] [CrossRef] [PubMed]
- Al-khafaf, A.; Ismail, H.K.; Al-Saidya, A.M. Histopathological effects of experimental exposure to lead on nervous system in albino female rats. Iraqi J. Vet. Sci. 2021, 35, 45–48. [Google Scholar] [CrossRef]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Shinohara, A.; Matsushita, K.; Watanabe, H.; Inaba, Y. Indices of lead-exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. Tohoku J. Exp. Med. 1996, 178, 49–62. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Buha Djordjevic, A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Otong, E.S.; Musa, S.A.; Danborno, B.; Sambo, S.J.; Dibal, N.I. Adansonia digitata ameliorates lead-induced memory impairments in rats by reducing glutamate concentration and oxidative stress. Egypt. J. Basic Appl. Sci. 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Machoń-Grecka, A.; Dobrakowski, M.; Boroń, M.; Lisowska, G.; Kasperczyk, A.; Kasperczyk, S. The influence of occupational chronic lead exposure on the levels of selected pro-inflammatory cytokines and angiogenic factors. Hum. Exp. Toxicol. 2017, 36, 467–473. [Google Scholar] [CrossRef]
- Baty, R.S.; Hassan, K.E.; Alsharif, K.F.; El-Hennamy, R.E.; Elmahallawy, E.K.; Hafez, M.M.; Moneim, A.E.A.; Kassab, R.B. Neuroprotective role of luteolin against lead acetate-induced cortical damage in rats. Hum. Exp. Toxicol. 2020, 39, 1200–1212. [Google Scholar] [CrossRef]
- Kucukler, S.; Benzer, F.; Yildirim, S.; Gur, C.; Kandemir, F.M.; Bengu, A.S.; Ayna, A.; Caglayan, C.; Dortbudak, M.B. Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: A biochemical and histopathological approach. Biol. Trace Elem. Res. 2021, 199, 1501–1514. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Alkahtani, S.; Almeer, R.; Albasher, G. Alleviation of lead acetate-induced nephrotoxicity by Moringa oleifera extract in rats: Highlighting the antioxidant, anti-inflammatory, and anti-apoptotic activities. Environ. Sci. Pollut. Res. 2020, 27, 33723–33731. [Google Scholar] [CrossRef]
- Kahalerras, L.; Otmani, I.; Abdennour, C. The Allium triquetrum L. Leaves Mitigated Hepatotoxicity and Nephrotoxicity Induced by Lead Acetate in Wistar Rats. Biol. Trace Elem. Res. 2022, 200, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.R. Association of Insulin Resistance, β-Cell Function Impairment and Calcium, Magnesium, and Fetuin-A Concentrations in Women with Type 2 Diabetes Mellitus. Acta Fac. Med. Naissensis 2016, 33, 187–197. [Google Scholar] [CrossRef]
- Zobeiri, M.; Belwal, T.; Parvizi, F.; Naseri, R.; Farzaei, M.H.; Nabavi, S.F.; Sureda, A.; Nabavi, S.M. Naringenin and its nano-formulations for fatty liver: Cellular modes of action and clinical perspective. Curr. Pharm. Biotechnol. 2018, 19, 196–205. [Google Scholar] [CrossRef]
- Jaradat, N.; Shawarb, N.; Hussein, F.; Al-Masri, M.; Warad, I.; Khasati, A.; Shehadeh, M.; Qneibi, M.; Hussein, A.M.A.; Makhamreh, S. Antibacterial and antioxidant screening of semi-synthetic naringin based hydrazone and oxime derivatives. Jundishapur J. Microbiol. 2018, 11, e65496. [Google Scholar] [CrossRef]
- Rivoira, M.A.; Rodriguez, V.; Talamoni, G.; de Talamoni, N.T. New perspectives in the pharmacological potential of naringin in medicine. Curr. Med. Chem. 2021, 28, 1987–2007. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, N.A.M.; Virk, P.; Hendi, A.; Awad, M.; Elobeid, M. Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Green Process. Synth. 2021, 10, 392–402. [Google Scholar] [CrossRef]
- Wang, H.; Su, G.; Chen, G.; Bai, J.; Pei, Y. 1H NMR-based metabonomics of the protective effect of Curcuma longa and curcumin on cinnabar-induced hepatotoxicity and nephrotoxicity in rats. J. Funct. Foods 2015, 17, 459–467. [Google Scholar] [CrossRef]
- El-Wafaey, D.I.; Nafea, O.E.; Faruk, E.M. Naringenin alleviates hepatic injury in zinc oxide nanoparticles exposed rats: Impact on oxido-inflammatory stress and apoptotic cell death. Toxicol. Mech. Methods 2022, 32, 58–66. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Heidarian, E.; Rafieian-Kopaei, M. Protective effect of artichoke (Cynara scolymus) leaf extract against lead toxicity in rat. Pharm. Biol. 2013, 51, 1104–1109. [Google Scholar] [CrossRef]
- Uckun, Z.; Guzel, S.; Canacankatan, N.; Yalaza, C.; Kibar, D.; Coskun Yilmaz, B. Potential protective effects of naringenin against vancomycin-induced nephrotoxicity via reduction on apoptotic and oxidative stress markers in rats. Drug Chem. Toxicol. 2020, 43, 104–111. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Messaoudi, I.; Said, L.; El Heni, J.; Kerkeni, A.; Said, K. Metallothionein Gene Expression in Liver of Rats Exposed to Cadmium and Supplemented with Zinc and Selenium. Arch. Environ. Contam. Toxicol. 2010, 59, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Dorogin, A.; Li, Y.; Lye, S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J. Cell. Mol. Med. 2014, 18, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Kermanian, F.; Soleimani, M.; Ebrahimzadeh, A.; Haghir, H.; Mehdizadeh, M. Effects of adenosine A2a receptor agonist and antagonist on hippocampal nuclear factor-kB expression preceded by MDMA toxicity. Metab. Brain Dis. 2012, 28, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, S. Changes in apoptosis-related genes (Bcl-2, Bax) in the urethras of old female rats following estrogen replacement. Yonago Acta Med. 2003, 46, 109–115. [Google Scholar]
- Shi, Y.; Song, Y.; Wang, Y.; Liang, X.; Hu, Y.; Guan, X.; Cheng, J.; Yang, K. p, p′-DDE induces apoptosis of rat Sertoli cells via a FasL-dependent pathway. J. Biomed. Biotechnol. 2009, 2009, 181282. [Google Scholar]
- Bancroft, J.D.; Gamble, M. General acknowledgments. In Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008; p. xiii. [Google Scholar] [CrossRef]
- El-Tantawy, W.H. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J. Tradit. Complement. Med. 2016, 6, 327–331. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Refaat, B.; Qasem, A.H.; Khan, A.; Ghaith, M.; Almasmoum, H.; Mahbub, A.; Almaimani, R.A. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. 2019, 26, 22736–22746. [Google Scholar] [CrossRef]
- Flora, S.; Nayan, J. An in vitro investigation of ameliorative effect of synthetic and herbal antioxidants on lead induced neurotoxicity. World J. Pharm. Res. 2016, 5, 1535–1553. [Google Scholar]
- Bukola, D.; Zaid, A.; Olalekan, E.I.; Falilu, A. Consequences of anthropogenic activities on fish and the aquatic environment. Poult. Fish. Wildl. Sci. 2015, 3, 1000138. [Google Scholar] [CrossRef]
- Wilson, T.D.; Lindsey, S.; Schooler, T.Y. A model of dual attitudes. Psychol. Rev. 2000, 107, 101–126. [Google Scholar] [CrossRef]
- Leret, M.L.; Garcia-Uceda, F.; Antonio, M.T. Effects of maternal lead administration on monoaminergic, GABAergic and glutamatergic systems. Brain Res. Bull. 2002, 58, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.B.; Reddy, G.H.; Prasanthi, R.P.J.; Chetty, C.S.; Reddy, G.R. Developmental lead exposure alters mitochondrial monoamine oxidase and synaptosomal catecholamine levels in rat brain. Int. J. Dev. Neurosci. 2005, 23, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Farida, T.; Salawu, O.A.; Tijani, A.Y.; Ejiofor, J.I. Pharmacological evaluation of Ipomoea asarifolia (Desr.) against carbon tetrachloride-induced hepatotoxicity in rats. J. Ethnopharmacol. 2012, 142, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Laamech, J.; El-Hilaly, J.; Fetoui, H.; Chtourou, Y.; Gouitaa, H.; Tahraoui, A.; Lyoussi, B. Berberis vulgaris L. effects on oxidative stress and liver injury in lead-intoxicated mice. J. Complement. Integr. Med. 2017, 14, 20150079. [Google Scholar] [CrossRef]
- Okeke, N.; Emeka, A.; Johntel, C. Biochemical taurine alleviated modification in male Wistar rats co-exposed to chlorpyrifos and lead. Int. J. Environ. Sci. Toxicol. 2014, 2, 104–115. [Google Scholar]
- Jarrar, B.M.; Taib, N.T. Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J. Biol. Sci. 2012, 19, 203–210. [Google Scholar] [CrossRef]
- Dick, C.F.; Dos-Santos, A.L.A.; Meyer-Fernandes, J.R. Inorganic phosphate as an important regulator of phosphatases. Enzym. Res. 2011, 2011, 103980. [Google Scholar] [CrossRef]
- Burtis, C.A.; Bruns, D.E. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics-e-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Shalan, M.G.; Mostafa, M.S.; Hassouna, M.M.; El-Nabi, S.E.H.; El-Refaie, A. Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 2005, 206, 1–15. [Google Scholar] [CrossRef]
- Moussa, S.A.; Bashandy, S.A. Biophysical and biochemical changes in the blood of rats exposed to lead toxicity. Rom. J. Biophys. 2008, 18, 123–133. [Google Scholar]
- BaSalamah, M.A.; Abdelghany, A.H.; El-Boshy, M.; Ahmad, J.; Idris, S.; Refaat, B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A. Bilirubin: A potential biomarker and therapeutic target for diabetic nephropathy. Diabetes 2014, 63, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Offor, S.J.; Mbagwu, H.O.C.; Orisakwe, O.E. Lead Induced Hepato-renal Damage in Male Albino Rats and Effects of Activated Charcoal. Front. Pharmacol. 2017, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Harinarayan, C.V. Vitamin D and diabetes mellitus. Hormones 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Imam, T.S.; Khalifa, H.A.; Hussein, M.M.; Ali, H.A. Aluminum–induced oxidative stress and hepato-renal impairment in male albino rats: Possible protective trial with naringenin. Life Sci. J. 2016, 13, 93–104. [Google Scholar]
- Mershiba, S.D.; Dassprakash, M.V.; Saraswathy, S.D. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol. Biol. Rep. 2013, 40, 3681–3691. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, B.; Xing, G.; Wang, F.; Hu, Z. Protective effect of naringenin against acetaminophen-induced acute liver injury in metallothionein (MT)-null mice. Food Funct. 2013, 4, 297–302. [Google Scholar] [CrossRef]
- Linthorst, A.C.E. Stress, corticotropin-releasing factor and serotonergic neurotransmission. In Techniques in the Behavioral and Neural Sciences; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 503–524. [Google Scholar]
- Abeer, M. Grape seed extract (Vitisvinifera) alleviate neurotoxicity and hepatotoxicity induced by lead acetate in male albino rats. J. Behav. Brain Sci. 2012, 2, 19559. [Google Scholar]
- Mani, S.; Sekar, S.; Barathidasan, R.; Manivasagam, T.; Thenmozhi, A.J.; Sevanan, M.; Chidambaram, S.B.; Essa, M.M.; Guillemin, G.J.; Sakharkar, M.K. Naringenin decreases α-synuclein expression and neuroinflammation in MPTP-induced Parkinson’s disease model in mice. Neurotox. Res. 2018, 33, 656–670. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.; El-Toweissy, M.Y.; Ali, A.M.; Awad Allah, A.A.M.; Darwish, H.S.; Sadek, I.A. Curcumin ameliorates lead (Pb2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol. Trace Elem. Res. 2015, 168, 206–220. [Google Scholar] [CrossRef]
- Manoj Kumar, V.; Henley, A.K.; Nelson, C.J.; Indumati, O.; Prabhakara Rao, Y.; Rajanna, S.; Rajanna, B. Protective effect of Allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ. Sci. Pollut. Res. 2017, 24, 1544–1552. [Google Scholar] [CrossRef]
- Jangid, J.; Bera, A.K.; Joseph, M.; Singh, V.; Singh, T.P.; Pradhan, B.K.; Das, S. Potential zones identification for harvesting wind energy resources in desert region of India–a multi criteria evaluation approach using remote sensing and GIS. Renew. Sustain. Energy Rev. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Okediran, B.S.; Biobaku, K.T.; Olaifa, F.H.; Atata, A.J. Haematological and antioxidant enzyme response to Lead toxicity in male Wistar rats. Ceylon J. Sci. 2017, 46, 31. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free. Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Horváthová, E.; Srančíková, A.; Regendová-Sedláčková, E.; Melušová, M.; Meluš, V.; Netriová, J.; Krajčovičová, Z.; Slameňová, D.; Pastorek, M.; Kozics, K. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis 2016, 31, 51–59. [Google Scholar] [PubMed]
- El-Newary, S.A.; Shaffie, N.M.; Omer, E.A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac. J. Trop. Med. 2017, 10, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Wali, B.; Khattak, A.J.; Karnowski, T. The relationship between driving volatility in time to collision and crash-injury severity in a naturalistic driving environment. Anal. Methods Accid. Res. 2020, 28, 100136. [Google Scholar] [CrossRef]
- Zeng, H.; Zhou, H.; Srivastava, A.; Kannan, R.; Prasanna, V. Graphsaint: Graph sampling based inductive learning method. arXiv 2019, arXiv:1907.04931. [Google Scholar]
- Sharifi, M.; Fathy, M.; Mahmoudi, M.T. A classified and comparative study of edge detection algorithms. In Proceedings of the International Conference on Information Technology: Coding and Computing, Las Vegas, NV, USA, 8–10 April 2002; pp. 117–120. [Google Scholar]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Tang, Q.; Li, Y.; Hu, B.R.; Ming, Z.Y.; Fu, Q.; Qian, J.Q.; Xiang, J.Z. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid 1. Acta Pharmacol. Sin. 2006, 27, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Yang, H.-X.; Ma, J.-Q.; Yang, W.; Feng, Z.-J.; Sun, J.-M.; Cheng, C.; Li, J.; Jiang, H. Role of AMPK pathway in lead-induced endoplasmic reticulum stress in kidney and in paeonol-induced protection in mice. Food Chem. Toxicol. 2018, 122, 87–94. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, A.A.; Fahim, H.I.; Zaky, M.Y. Quercetin and naringenin abate diethylnitrosamine/acetylaminofluorene-induced hepatocarcinogenesis in Wistar rats: The roles of oxidative stress, inflammation and cell apoptosis. Drug Chem. Toxicol. 2022, 45, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Shedid, S.M.; Abdel-Magied, N.; Saada, H.N. Role of betaine in liver injury induced by the exposure to ionizing radiation. Environ. Toxicol. 2019, 34, 123–130. [Google Scholar] [CrossRef] [PubMed]






| Gene | Primer Sequence [5′-3′] | Reference |
|---|---|---|
| Rat β-actin | F:3′TCCTCCTGAGCGCAAGTACTCT5′ R: 5′GCTCAGTAACAGTCCGCCTAGAA3′ | [23] |
| IL10 | F:3′GCGGCTGAGGCGCTGTCAT5′ R: 5′CGCCTTGTAGACACCTTGGTCTTGG3′ | [24] |
| NF-KB | F:3′GCAAACCTGGGAATACTTCATGTGACTAAG5′ R: 5′ATAGGCAAGGTCAGAATGCACCAGAAGTCC3′ | [25] |
| BCL-2 | F:3′CACCCCTGGCATCTTCTCCTT5′ R: 5′AGCGTCTTCAGAGACAGCCAG3′ | [26] |
| Caspase-3 | F:3′AGTTGGACCCACCTTGTGAG5′ R: 5′AGTCTGCAGCTCCTCCACAT3′ | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, L.A.H.; Elshopakey, G.E.; Abdelhamid, F.M.; Albukhari, T.A.; Almehmadi, S.J.; Refaat, B.; El-Boshy, M.; Risha, E.F. Hepatoprotective and Neuroprotective Effects of Naringenin against Lead-Induced Oxidative Stress, Inflammation, and Apoptosis in Rats. Biomedicines 2023, 11, 1080. https://doi.org/10.3390/biomedicines11041080
Mansour LAH, Elshopakey GE, Abdelhamid FM, Albukhari TA, Almehmadi SJ, Refaat B, El-Boshy M, Risha EF. Hepatoprotective and Neuroprotective Effects of Naringenin against Lead-Induced Oxidative Stress, Inflammation, and Apoptosis in Rats. Biomedicines. 2023; 11(4):1080. https://doi.org/10.3390/biomedicines11041080
Chicago/Turabian StyleMansour, Lubna A. H., Gehad E. Elshopakey, Fatma M. Abdelhamid, Talat A. Albukhari, Samah J. Almehmadi, Bassem Refaat, Mohamed El-Boshy, and Engy F. Risha. 2023. "Hepatoprotective and Neuroprotective Effects of Naringenin against Lead-Induced Oxidative Stress, Inflammation, and Apoptosis in Rats" Biomedicines 11, no. 4: 1080. https://doi.org/10.3390/biomedicines11041080







