Cubosomes in Drug Delivery—A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications
Abstract
1. Introduction
| Cubosomes | Liposomes |
|---|---|
| Distinct, submicron, nanostructured particles of bicontinuous cubic liquid-crystalline phase enclosing two separate regions of water divided by surfactant-controlled bilayers. | Spherical vesicles have an aqueous core enclosed by one or more phospholipid bilayers. The main components are cholesterol and phospholipids. |
| Retain their stability even at high dilution, which is not possible with other liquid-crystalline systems. Higher ratio of particle volume and bilayer area in comparison with the liposomes. | They contain biocompatible and biodegradable lipids and are inert and non-immunogenic. |
| Encapsulate all three types of hydrophilic, hydrophobic and amphiphilic substances. | Can be loaded with hydrophilic and hydrophobic molecules. |
| Better stability than liposomes and, due to their liquid-crystalline membrane architecture, possess a greater ability to envelop and encapsulate hydrophobic chemotherapeutic agents. Extremely high encapsulation efficiency and enhanced apoptotic efficacy. | Sometimes phospholipid undergoes oxidation and hydrolysis-like reaction. |
| High-energy methods such as ultrasonication, homogenisation and micro-fluidisation are used to prepare cubosomes. | Provide selective passive targeting to tumour tissues. Flexibility to couple with site-specific ligands to achieve active targeting. Prepared by physical dispersion, solvent dispersion and detergent solubilisation technique. |
| Challenges faced in optimising various parameters to enhance the loading capacities and subsequent improvement in their release are a few limitations of these novel delivery systems. | Chances for leakage and fusion of encapsulated drugs. High production cost, low solubility with a short half-life. |
2. Cubosomes and Their Types
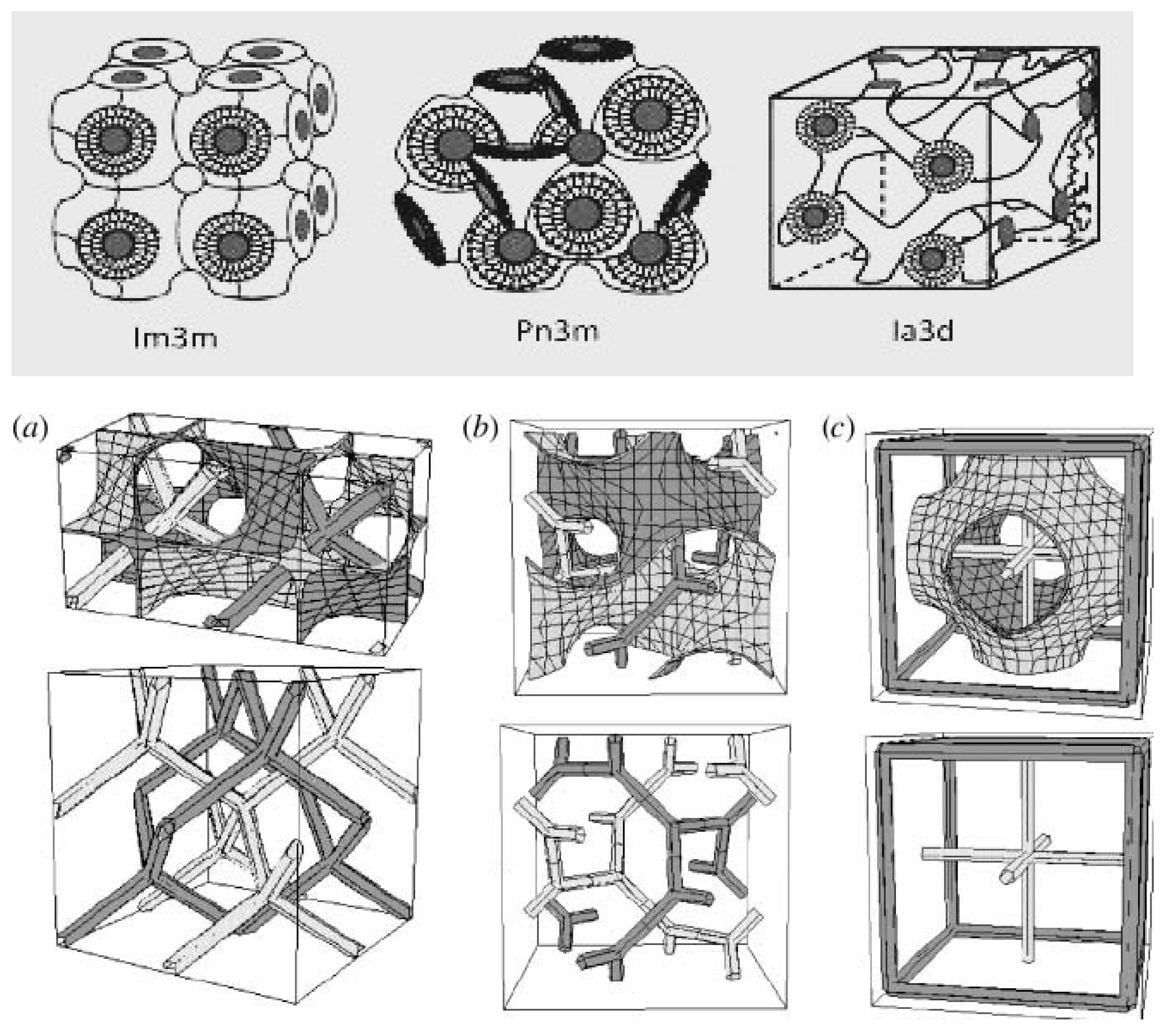
3. Theories on Cubic Phase Structure
3.1. Fontell & Drew Theory
3.2. Gustafson et al. Theory
3.3. Schwarz, Jacob & Anderson Theory
3.4. System Forming Theory
4. Mechanism of Drug Release from Cubosomes
5. Advantages and Disadvantages of Cubosomes
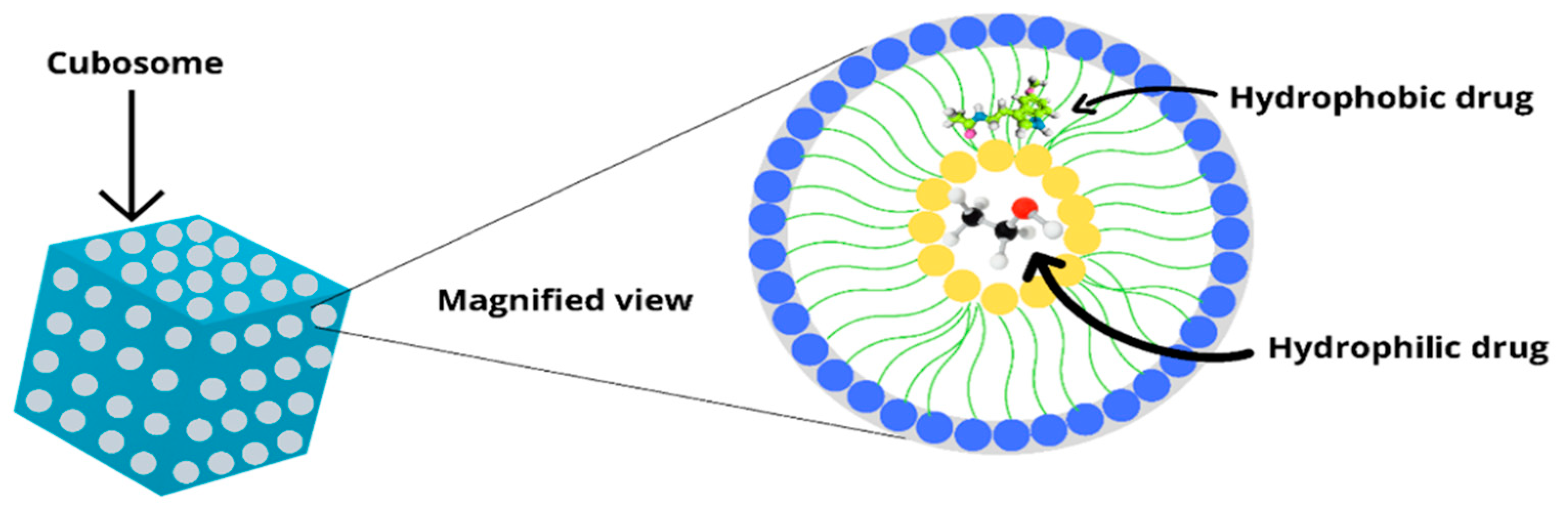
6. Structure and Components of Cubosomes
6.1. Amphiphilic Lipids
6.1.1. Glycerol Monooleate (GMO)
6.1.2. Phytantriol (PHYT)
6.2. Stabilizers
7. Drug Loading in Cubosomes
8. Methods of Preparation
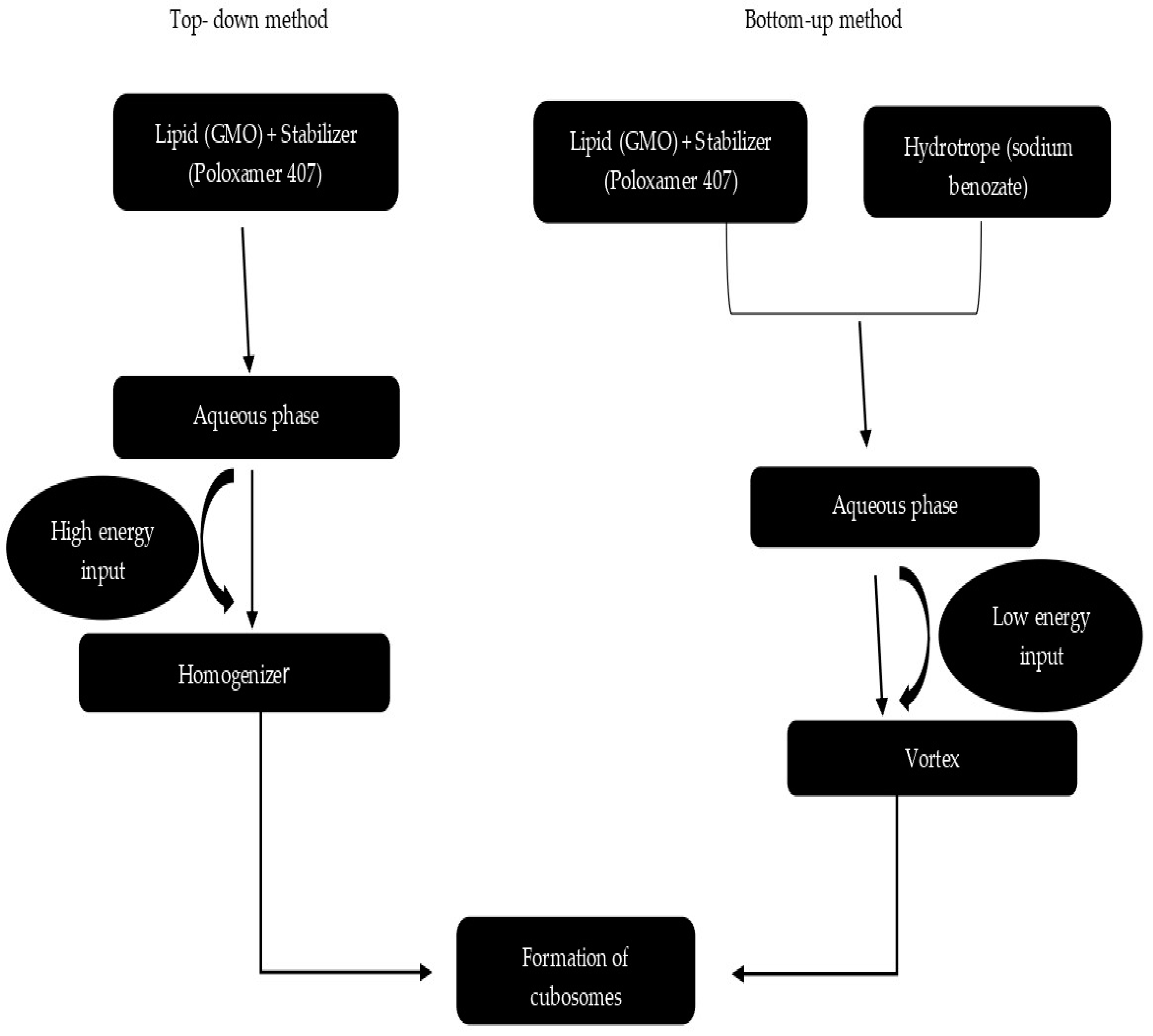
8.1. Top-Down Approach
8.2. Bottom-Up Approach
8.3. Heat Treatment
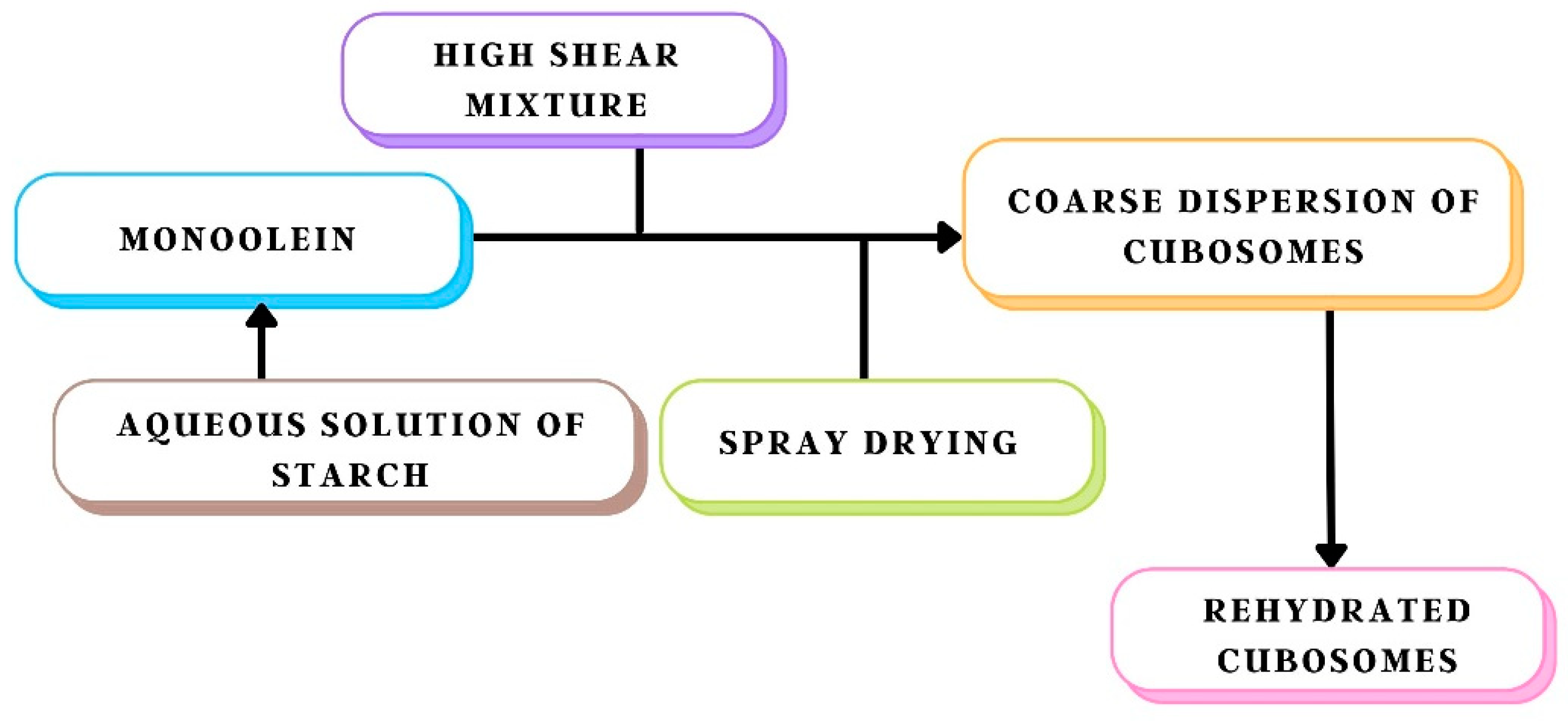
8.4. Spray Drying
| Techniques | Benefits | Drawbacks | References |
|---|---|---|---|
| Bottom-up approach | Requires low energy input; hence it can be used safely for drugs that are temperature sensitive | This method can be preferred only for thermosensitive drugs, formulations are stable for a short period | [47] |
| Top-down approach | Reduces aggregation and improves the stability of formulations for up to one year | High-energy input is required to disperse the aggregates into cubosomes | [47] |
| Solvent evaporation method | Produce cubosomes of smaller particle size with higher physical stability | Due to the large-scale mixing of water and ethanol high polydispersity index is reported | [48] |
| Spray-drying method | A highly versatile, cheap and scalable method. The best method for drying labile products, such as proteins and vaccines | Difficulty in spray drying of the formulation as a cubic phase is formed immediately upon hydration of monoolein | [48] |
9. Characterisation of Cubosomes
9.1. Characterization of Non-Lamellar Liquid Crystalline
9.1.1. Electron Microscopy
9.1.2. X-ray Scattering
9.2. Particle Size Distribution
9.3. Entrapment Efficiency
9.4. Measurement of Drug Release
9.5. Stability Studies
10. Applications of Cubosomes
| Products | Drugs | Target Diseases | Status | References |
|---|---|---|---|---|
| SPI-077 (Alza) | Cisplatin | Solid tumours | Phase II (Development terminated) | [69] |
| CPX-351 (Celator) | Cytarabine:daunorubicin | Acute myeloid leukaemia | Phase II | [69] |
| CPX-1 (Celator) | Irinotecan HCI:floxuridine | Colorectal cancer | Phase II | [70] |
| Brakiva (Talon) | Topotecan | Relapsed solid tumours | Phase I | [71] |
| Lipoplatin (Reglon) | Cisplatin | Non-small cell lung cancer | Phase III | [71] |
| ThermoDo x(Cesion) | Thermosensitive doxorubicin | Primary hepatocellular carcinoma | Phase III | [72] |
| Exparel (Pacira) | Bupivacaine | Nerve block | Phase II | [73] |
| Stimuvax (Oncothyreon/Merck) | Anti-MUC1 cancer vaccine | Non-small cell lung cancer | Phase III | [73] |
| Active Ingredients | Polymers Used | Applications | References |
|---|---|---|---|
| 20 (S)-protopanaxadiol, Piperine | Monoolein (MO), Poloxamer 407 (PF127) | Drug Delivery | [74] |
| 3-bromopyruvate | Monoolein (MO), Poloxamer 407 (PF127), Folic acid | Tumour Targeted Delivery | [75] |
| Camptothecin | Squarain-based NIR-emitting fluorescent probe, Pluronic F108 (PF108), Monoolein | Theranostic and Bioimaging | [75] |
| Curcumin | Polyethylene glycol 400 (PEG-400), RH40, and Monoolein (MO) | Anticancer activity | [76] |
| Doxorubicin (DOX) | Monolinolein, Pyridinylmethyl linoleate | Tumour Targeted Delivery | [76] |
| Gambogenic acid | Monoolein (MO) | Drug delivery in cancer therapy | [77] |
| Meso-Tetraphenylporphine-Mn (III) chloride | Monoolein (MO), Polyethylene glycol (PEG), Phospholipids | Bioimaging | [77] |
| Metformin | Monoolein (MO), Poloxamer (Pol.) 407 (PF127) | Drug Delivery | [78] |
| Paclitaxel (PTX) | Monoolein (MO), Poloxamer 407 (PF127), Polyethylene glycol (PEG) | Drug Delivery | [78] |
| Pemetrexed and Resveratrol | Monoolein (MO) | Drug Delivery in lung cancer | [79] |
| Dacarbazine | GMO, Pol. 407 | First-line chemotherapy medication against melanoma | [79] |
| 5-fluorouracil (5-FU) | GMO, Pol. 407 | For the treatment of advanced gastrointestinal cancers, including hepatocellular carcinoma | [80] |
| 20 (S) protopanaxadiol (PPD) | GMO, Pol. 407 | Anticancer drug | [80] |
| Folic-acid-modified etoposide cubosomes | Polyethylene glycol 400 (PEG-400), RH40, and Monoolein | Breast cancer | [81] |
| Cisplatin- and paclitaxel-loaded cubosomes | Monoolein (MO), Poloxamer 407 (PF127), Polyethylene glycol (PEG) | Liver cancer | [81] |
| Icariin cubosomes | Monoolein (MO), Poloxamer (Pol.) 407 (PF127) | Ovarian Cancer | [81] |
| Cisplatin and metformin nanocubosomes | Monoolein (MO), Poloxamer (Pol.) 407 (PF127) | Colorectal cancer | [82] |
| Loaded Drug | Lipids & Stabilisers | Therapeutic Uses | References |
|---|---|---|---|
| Dexamethasone (DEX) | GMO, Pol. 407 | Treatment of anterior ocular inflammation | [83] |
| Flurbiprofen (FB) | GMO, Pol. 407 | For treatment of ocular inflammation | [83] |
| Ketorolac | GMO, Pol. 407 | For treatment of ocular symptoms due to allergies | [84] |
| Timolol (TM) | GMO, Pol. 407 | Non-selective beta-blocker drug for the treatment of glaucoma | [84] |
| Cyclosporine A | GMO, Pol. 407 | Immunosuppressive agent for treating inflammatory and immune-related ocular diseases | [85] |
| Pilocarpine | GMO, Pol. 407 | To treat open-angle glaucoma and acute angle-closure glaucoma | [85] |
| Loaded Drug | Oil Stabiliser | Therapeutic Use | References |
|---|---|---|---|
| Capsaicin | GMO, PYT, Pol. 407 | Used in the treatment of psoriasis, pruritus, and contact allergy | [86] |
| Silver sulfadiazine | GMO, Pol. 407 | Used for the treatment of infected burns | [86] |
| Indomethacin | GMO, Pol. 407 | Anti-inflammatory drug | [87] |
| Hydroxypropyl β cyclodextrin/minoxidil complex | GMO, Pol. 407 | Minoxidil for hair growth | [87] |
| Antimicrobial peptide (AMP) LL-37 | GMO, Pol. 407 | Used for treatment of skin infection caused by Staphylococcus aureus | [88] |
| Erythromycin | GMO, Pol. 407 | Treatment and prevention of several types of acne as a result of its bacteriostatic activity against Propionibacterium acnes | [89] |
| Dapsone | GMO, Pol. 407 | For treatment of acne, leprosy & systemic lupus erythematosus | [89] |
| Vaccination through transcutaneous immunization (TCI) | Microneedle enhances the permeation of the peptide mixture in water through the skin layers, and cubosomes with peptide showed longer retention within the skin | Microneedles (MNs) and cubosomes have been used successfully as a synergistic method for the delivery of vaccines via skin | [90] |
| Loaded Drug | Lipids & Stabilisers | Therapeutic Uses | References |
|---|---|---|---|
| Insulin | GMO, Pol. 407 | For treating type 1 diabetic-induced rats (insulin-dependent diabetes) | [91] |
| Ibuprofen | PYT, Pol. 407 | Non-steroidal anti-inflammatory drug with analgesic properties | [92] |
| Simvastatin | GMO, Pol. 407 | For cholesterol control in the body | [92] |
| Piperine | GMO, Pol. 407 with Tween 80 and Cremophor RH 40 | Natural alkaloids with memory-enhancing potentials used in the treatment of Alzheimer’s disease (AD) | [92] |
| Amphotericin B | PYT, Pol. 407 | For several types of fungal infections, such as histoplasmosis and Leishmaniasis | [93] |
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| MO-PEG | Monoolein—poly(ethylene glycol) |
| DDAB | Dimethyldioctadecylammonium bromide |
| GM1 | Beta-galactosidase-1 |
| POE | Polyolefin elastomers |
| PPO | Polyphenylene oxide |
| DSC | Differential scanning colorimetry |
| NMR | Nuclear magnetic resonance |
| TEM | Transmission electron microscopy |
| Pol. 407 | Polaxamer 407 |
| PEG | Polyethylene glycol |
References
- Porter, C.; Trevaskis, N.; Charman, W. Lipids and lipid-based formulations; optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Agrawal, A.K.; Jain, S.; Yadav, N.P. Novel drug delivery system: An immense hope for diabetics. Drug Deliv. 2016, 23, 2371–2390. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Habtemariam, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Maurer, N.; Wong, K.F.; Hope, M.J.; Cullis, P.R. Anomalous solubility behavior of the antibiotic ciprofloxacin encapsulated in liposomes: A 1H-NMR study. Biochim. Biophys. Acta BBA-Biomembr. 1998, 1374, 9–20. [Google Scholar] [CrossRef]
- Johnston, M.J.W.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Winters, G.; Srinivasulu, M.; Crawford, J.; Wong, M.; Amankwa, L.; Waterhouse, D.; Masin, D.; Webb, M.; Harasym, N. Development of a weak-base docetaxel derivative that can be loaded into lipid nanoparticles. J. Control Release 2010, 144, 332–340. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, N.; Yalavarthi, P.; Vadlamudi, H.; Thanniru, J.; Yaga, G.; K, H. Cubosomes as targeted drug delivery systems—A biopharmaceutical approach. Curr. Drug Discov. Technol. 2014, 11, 181–188. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumours as an example. Drug Deliv. 2010, 197, 3–53. [Google Scholar]
- He, H.; Rahimi, K.; Zhong, M.; Mourran, A.; Luebke, D.R.; Nulwala, H.B.; Möller, M.; Matyjaszewski, K. Cubosomes from hierarchical self-assembly of poly(ionic liquid) block copolymers. Nat. Commun. 2017, 8, 14057. [Google Scholar] [CrossRef]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef]
- Khedekar, P.B. Cubosomes: A vehicle for delivery of various therapeutic agents. MOJ Toxicol. 2018, 4, 19–21. [Google Scholar] [CrossRef]
- Luzzati, V.; Husson, F. The structure of the liquid-crystalline phases of lipid-water systems. J. Cell Biol. 1962, 12, 207–219. [Google Scholar] [CrossRef]
- Fontell, K.; Mandell, L.; Ekwall, P. Some isotropic mesophases in systems containing amphiphilic compounds. Acta Chem. Scand 1968, 22, 3209–3223. [Google Scholar] [CrossRef]
- Patton, J.S.; Carey, M.C. Watching fat digestion: The formation of visible product phases by pancreatic lipase is described. Science 1979, 204, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K. Two Cubic Phases in Monoolein–Water System. Nature 1983, 304, 664. [Google Scholar] [CrossRef]
- Rizwan, S.B.; Boyd, B.J. Cubosomes: Structure, preparation and use as an antigen delivery system. Subunit Vacc. Deliv. 2014, 7, 125–140. [Google Scholar]
- Gaballa, S.; El Garhy, O.; Abdelkader, H. Cubosomes: Composition, preparation, and drug delivery applications. J. Adv. Biomed. Pharm. Sci. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Salvi, S.; Sood, P.; Kulkarni, B.; Kumar, D. Cubosomes in cancer drug delivery: A review. Colloid Interface Sci. Commun. 2022, 46, 100561. [Google Scholar] [CrossRef]
- Luzzati, V.; Tardieu, A.; Gulik-Krzywicki, T.; Rivas, E.; Reiss-Husson, F. Structure of the cubic phases of lipid–water systems. Nature 1968, 220, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Tiberg, F. Self-assembled lipid superstructures: Beyond vesicles and liposomes. Nano Lett. 2005, 5, 1615–1619. [Google Scholar] [CrossRef]
- Spicer, P. Cubosome processing industrial nanoparticle technology development. Chem. Eng. Res. Des. 2005, 83, 1283–1286. [Google Scholar] [CrossRef]
- Siekmann, B.; Bunjes, H.; Koch, M.H.J.; Westesen, K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride–water phases. Int. J. Pharm. 2002, 244, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Landau, E.M.; Rosenbusch, J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar] [CrossRef] [PubMed]
- Caboi, F.; Amico, G.S.; Pitzalis, P.; Monduzzi, M.; Nylander, T.; Larsson, K. Addition of hydrophilic and lipophilic compounds of biological relevance to the monoolein/water system phase behavior. Chem. Phys. Lipids 2001, 109, 47–62. [Google Scholar] [CrossRef]
- Seddon, J.; Squires, A.; Conn, C.; Ces, O.; Heron, A.; Mulet, X.; Shearman, G.; Templer, R. Pressure-jump x-ray studies of liquid crystal transitions in lipids. Philos. Transact. A Math. Phys. Eng. Sci. 2006, 364, 2635–2655. [Google Scholar] [CrossRef] [PubMed]
- Montis, C.; Castroflorio, B.; Mendozza, M.; Salvatore, A.; Berti, D.; Baglioni, P. Magneto-cubosomes for the delivery and controlled release of therapeutics. J. Colloid Interface Sci. 2015, 449, 317–326. [Google Scholar] [CrossRef]
- Murgia, S.; Falchi, A.M.; Meli, V.; Schillén, K.; Lippolis, V.; Monduzzi, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; Bizzarri, R.; et al. cubosome formulations stabilized by a dansyl-conjugated block copolymer for possible nanomedicine applications. Colloids Surf. B Biointerfaces 2015, 129, 87–94. [Google Scholar] [CrossRef]
- Muller, F.; Salonen, A.; Glatter, O. Phase behavior of phytantriol/water bicontinuous cubic pn3m cubosomes stabilized by laponite disc-like particles. J. Colloid Interface Sci. 2010, 342, 392–398. [Google Scholar] [CrossRef]
- Lutton, E.S. Phase Behavior of Aqueous Systems of Monoglycerides. J. Am. Oil Chem. Soc. 1965, 42, 1068–1070. [Google Scholar] [CrossRef]
- Esposito, E.; Eblovi, N.; Rasi, S.; Drechsler, M.; Di Gregorio, G.M.; Menegatti, E.; Cortesi, R. Lipid-Based Supramolecular Systems for Topical Application: A Preformulatory Study. AAPS PharmSciTech 2003, 5, 62–76. [Google Scholar] [CrossRef]
- Lars, L.; Sandra, W.; Ajay Vikram, S.; Peter, L.; Andreas, L. Nanoparticle induced barrier function assessment at liquid–liquid and air–liquid interface in novel human lung epithelia cell lines. Toxicol. Res. 2019, 8, 1016–1027. [Google Scholar]
- Shah, J.; Sadhale, Y.; Chilukuri, D. Cubic phase gels as drug delivery systems. Adv. Drug. Deliver. Rev. 2001, 47, 229–250. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Joabsson, F. Cubic phase nanoparticles (cubosome): Principles for controlling size, structure, and stability. Langmuir 2005, 21, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Janakiraman, K.; Krishnaswami, V.; Sethuraman, V.; Rajendran, V.; Kandasamy, R. Development of methotrexate-loaded cubosomes with improved skin permeation for the topical treatment of rheumatoid arthritis. Appl. Nanosci. 2019, 9, 1781–1796. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today. 2019, 24, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, T.; Konovalov, O. Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research. Materials 2020, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Angelova, A.; Drechsler, M.; Garamus, V.M.; Mutafchieva, R.; Lesieur, S. Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging. Soft Matter. 2015, 11, 3686–3692. [Google Scholar] [CrossRef]
- Hafez, I.M.; Ansell, S.; Cullis, P.R. Tunable pH-sensitive liposomes composed of mixtures of cationic and anionic lipids. Biophys. J. 2000, 79, 1438–1446. [Google Scholar] [CrossRef]
- Ajay Vikram, S.; Aaron, K.; Romi Singh, M.; Ashish, K.; Gadicherla, B.; Martin Heinrich, R.; Jan, H.; Pablo del, P.; Peter, L.; Andreas, L. Coronavirus-mimicking nanoparticles (CorNPs) in artificial saliva droplets and nanoaerosols: Influence of shape and environmental factors on particokinetics/particle aerodynamics. Sci. Total Environ. 2023, 860, 160503. [Google Scholar]
- Muir, B.W.; Zhen, G.; Gunatillake, P.; Hartley, P.G. Salt induced lamellar to bicontinuous cubic phase transitions in cationic nanoparticles. J. Phys. Chem. B 2012, 116, 3551–3556. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Contado, C.; Stellin, E.; Menegatti, E.; Bonina, F.; Puglia, C. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm. Res. 2005, 22, 2163–2173. [Google Scholar] [CrossRef]
- Rizwan, S.B.; Dong, Y.-D.; Boyd, B.J.; Rades, T.; Hook, S. Characterisation of bicontinuous cubic liquid crystalline systems of phytantriol and water using cryo field emission scanning electron microscopy (Cryo FESEM). Micron 2007, 38, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles. Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zhang, W.; Wu, X.; Wang, X.; Ding, X.; Hao, Q. Biodegradable methoxy poly (ethylene glycol)-poly (lactide) nano-particles for controlled delivery of dacarbazine: Preparation, characterization and anticancer activity evaluation. Afr. J. Pharm. Pharmacol. 2011, 5, 1369–1377. [Google Scholar] [CrossRef]
- Amar-Yuli, I.; Libster, D.; Aserin, A.; Garti, N. Solubilization of food bioactives within lyotropic liquid crystalline mesophases. Curr. Opin. Colloid Interface Sci. 2009, 14, 21–32. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Gustafsson, J. cryotransmission electron microscopy of thin vitrified samples. Curr. Opin. Colloid Interface Sci. 1996, 1, 270–278. [Google Scholar] [CrossRef]
- Boyd, B.J.; Rizwan, S.B.; Dong, Y.-D.; Hook, S.; Rades, T. Self-assembled geometric liquid-crystalline nanoparticles imaged in three dimensions: Hexosomes are not necessarily flat hexagonal prisms. Langmuir 2007, 23, 12461–12464. [Google Scholar] [CrossRef] [PubMed]
- Pawley, J. The development of field-emission scanning electron microscopy for imaging biological surfaces. Scanning 1997, 19, 324–336. [Google Scholar]
- Krauel, K.; Girvan, L.; Hook, S.; Rades, T. Characterisation of colloidal drug delivery systems from the naked eye to cryo-FESEM. Micron 2007, 38, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-cell-targeted theranostic cubosomes. Langmuir 2014, 30, 6228–6236. [Google Scholar] [CrossRef]
- Murgia, S.; Bonacchi, S.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Talmon, Y.; et al. Drug-loaded fluorescent cubosomes: Versatile nanoparticles for potential theranostic applications. Langmuir 2013, 29, 6673–6679. [Google Scholar] [CrossRef]
- Norlén, L. Skin barrier structure and function: The single gel phase model. J. Investig. Dermatol. 2001, 117, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jiang, C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm. Sin. B 2020, 10, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Szlezak, M.; Nieciecka, D.; Joniec, A.; Pękała, M.; Gorecka, E.; Emo, M.; Stébé, M.J.; Krysiński, P.; Bilewicz, R. Monoolein cubic phase gels and cubosomes doped with magnetic nanoparticles–hybrid materials for controlled drug release. ACS Appl. Mater. Interfaces 2017, 9, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Han, S.; Shen, J.; Zhu, J.; Zhu, C.; Zhang, X.; Gan, Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010, 396, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Sharma, P.; Warsi, M. Fabrication and evaluation of ketorolac loaded cubosome for ocular drug delivery. J. Appl. Pharm. Sci. 2016, 9, 204–208. [Google Scholar] [CrossRef]
- Maheshwari, R.; Chaturvedi, S.; Jain, N. Novel application of hydrotropic solubilization in the analysis of some NSAIDs and their solid dosage forms. Indian J. Pharm. Sci. 2007, 69, 101. [Google Scholar] [CrossRef]
- Verma, P.; Ahuja, M. Cubic liquid crystalline nanoparticles: Optimization and evaluation for ocular delivery of tropicamide. Drug Deliv. 2016, 23, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Shen, J.; Gan, Y.; Geng, H.; Zhang, X.; Zhu, C.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef]
- Bouwstra, J. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Rattanapak, T.; Birchall, J.; Young, K.; Ishii, M.; Meglinski, I.; Rades, T.; Hook, S. transcutaneous immunization using microneedles and cubosomes: Mechanistic investigations using optical coherence tomography and two-photon microscopy. J. Control Release 2013, 172, 894–903. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A review of the state of the art. J. Mater. Chem. B. 2022, 10, 2781–2819. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M.; Barauskas, J.; Tiberg, F. Cubic phases and cubic phase dispersions in a phospholipid-based system. J. Am. Chem. Soc. 2005, 127, 1076–1077. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Devel. Ther. 2015, 9, 4209. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: Development and in vitro/in vivo characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef]
- Boyd, B.; Khoo, S.; Whittaker, D.; Davey, G.; Porter, C. A lipid-based Liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water-soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60. [Google Scholar] [CrossRef]
- Longer, M.; Tyle, P.; Mauger, J.W. A cubic-phase oral drug delivery system for controlled release of AG337. Drug Dev. Ind. Pharm. 1996, 22, 603–608. [Google Scholar] [CrossRef]
- Chung, H.; Kim, J.; Um, J.Y.; Kwon, I.C.; Jeong, S.Y. Self-assembled “Nanocubicle” as a carrier for peroral insulin delivery. Diabetologia 2002, 45, 448–451. [Google Scholar] [CrossRef]
- Dian, L.; Yang, Z.; Li, F.; Wang, Z.; Pan, X.; Peng, X.; Huang, X.; Guo, Z.; Quan, G.; Shi, X.; et al. Cubic phase nano-particles for sustained release of ibuprofen formulation characterization and enhanced bioavailability study. Int. J. Nanomed. 2013, 8, 845–854. [Google Scholar]
- Lai, J.; Chen, J.; Lu, Y.; Sun, J.; Hu, F.; Yin, Z.; Wu, W. Glyceryl monooleate/poloxamer 407 cubic nano-particles as oral drug delivery systems: In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. AAPS PharmSciTech 2009, 10, 960. [Google Scholar] [CrossRef]
- Cheng, M.R. Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects. World J. Gastroenterol. 2012, 18, 6076. [Google Scholar] [CrossRef]
- Thomson, A.B.R.; Schoeller, C.; Keelan, M.; Smith, L.; Clandinin, M.T. Lipid absorptions passing through the unstirred layers, brush-border membrane, and beyond. Can. J. Physiol. Pharmacol. 1993, 71, 531–555. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.; Etman, S.; Abdelmonsif, D.; Abdallah, O. Novel piperine-loaded tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef]
- Durgaramani, S.; Muhammad, H.S.; Osama, M.; Yosif, A.; Neelaveni, T. Polymeric lipid hybrid nanoparticles (PLNs) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar]
- Surabhi, S.; Krishnananda, K.; Shabaraya, A.R. Cubosomes and its applications—A review. Eur. J. Biomed. Pharm. Sci. 2022, 9, 111–116. [Google Scholar]
- Kajal, C.; Devender, S. Cubosomes—A potential drug delivery system. Asian J. Pharma. Res. Dev. 2021, 9, 93–101. [Google Scholar]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.M.; Squires, A.M. Phytantriol based smart nano-carriers for drug delivery applica tions. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Fornasier, M.; Biffi, S.; Bortot, B.; Macor, P.; Manhart, A.; Wurm, F.R.; Murgia, S. Cubosomes stabilized by a polyphosphoester analog of Pluronic F127 with reduced cytotoxicity. J. Colloid Interface Sci. 2020, 580, 286–297. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, X.; Tan, Y.; Chen, M.; Zhu, X.; Feng, M.; Xu, Y.; Wu, C. Optimization of the preparation process for an oral phytantriol-based amphotericin B cubosomes. J. Nanomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- de Kruijff, B.; Cullis, P.R.; Verkleij, A.J. Non-bilayer lipid structures in model and biological membranes. Trends Biochem. Sci. 1980, 5, 79–81. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Osmani, R.A.M.; Harkare, B.R.; Ghodake, P.P. Cubosomes: The inimitable nanoparticulate drug carriers. Sch. Acad. J. Pharm. 2013, 2, 481–486. [Google Scholar]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Mezzenga, R.; Meyer, C.; Servais, C.; Romoscanu, A.I.; Sagalowicz, L.; Hayward, R.C. Shear rheology of lyotropic liquid crystals: A case study. Langmuir 2005, 21, 3322–3333. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.S.; Hosny, K.M. Development and optimization of ocular in situ gels loaded with ciprofloxacin cubic liquid crystalline nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101710. [Google Scholar] [CrossRef]
- Lian, R.; Lu, Y.; Qi, J.; Tan, Y.; Niu, M.; Guan, P.; Hu, F.; Wu, W. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: Physical characterization and enhanced oral bioavailability. AAPS PharmSciTech 2011, 12, 1234–1240. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, X.; Liu, M.; Deng, Y.; He, N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics 2014, 4, 240–255. [Google Scholar] [CrossRef]
- Shanmugam, T.; Banerjee, R. Nanostructured self-assembled lipid materials for drug delivery and tissue engineering. Ther. Deliv. 2011, 2, 1485–1516. [Google Scholar] [CrossRef]
- Lee, K.; Nguyen, T.; Hanley, T.; Boyd, B. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Paradkar, A. Cubic liquid crystalline glyceryl monooleate matrices for oral delivery of enzyme. Int. J. Pharm. 2005, 294, 161–171. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Guo, Q.; Wang, Z.; Wang, H.; Yang, Y.; Huang, Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials 2012, 33, 6155–6161. [Google Scholar] [CrossRef]
- Al-mahallawi, A.M.; Abdelbary, A.A.; El-Zahaby, S.A. Norfloxacin loaded nano-cubosomes for enhanced management of otitis externa: In vitro and in vivo evaluation. Int. J. Pharm. 2021, 600, 120490. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Ke, H.; Xinsheng, P.; Zhiwen, Y.; Lingzhen, Q.; Chune, Z.; Xintian, H.; Xuan, S.; Linghui, D.; Ming, L.; et al. Nanostructured cubosomes as advanced drug delivery system. Curr. Pharm. Design 2013, 19, 6290–6297. [Google Scholar]

| Advantages | Disadvantages |
|---|---|
| Cubosomes are biocompatible, biodegradable, non-irritating and thermodynamically stable | Water-soluble drugs are less likely to be entrapped since they contain a large amount of water |
| Lipophilic, hydrophilic, and amphiphilic medicines can all be loaded into cubosomes | Large-scale production of cubosomes is challenging due to their high viscosity |
| They have a high drug-loading capacity due to their large interior surface area | Potential to leak during the storage or in vivo transmission |
| With 3-D nanostructures with both hydrophilic and hydrophobic domains, cubic liquid-crystalline phases are being employed as drug delivery systems in medical therapeutics | Phase change is possible if cubosomes are exposed to the external environment |
| While lipid ingredients are biocompatible, bioadhesive, and digestible, the huge interfacial surface can provide a complex diffusion pathway for the continuous release of entrapped drug molecules | Possibility of particle growth if the particles are left alone for a long time |
| The preparation process is simple. They are good solubilisers when compared to other lipid-based carriers. Increase the bioavailability of water-soluble peptides |
| Loaded Active Compound | Formulation |
|---|---|
| siRNA | Monoolein, DOTAP |
| siRNA | MO, DOTAP, MO-PEG |
| siRNA | Phy, DOTAP, F127 stabiliser |
| siRNA | DOTAP or DDAB, F127 stabiliser |
| Salmon sperm DNA | MO/PEG-15 Cocopolyamine |
| Thermomyces lanuginosus lipase | Monoolein, F127 stabiliser |
| Beta casein | MO/Phy, F127 stabiliser |
| Nerve growth factor | MO, beta casein stabiliser |
| Dopamine D2Lreceptor (membrane protein) | Ni(II) chelated EDTA amphiphiles |
| Cholera toxin B subunit | Phy, GM1, F127 stabiliser |
| Ovalbumin | MO/Phy, F127 stabiliser |
| Outer membrane protein F (OmpF) | Monolinolein, octyl-POE stabiliser |
| Human recombinant brain-derived neurotrophic factor (BDNF) | MO, eicosapentaenoic acid, PEG stabiliser |
| Stabiliser | Lipids |
|---|---|
| Monoolein | Laponite, Pluronic F 127, Modified starch |
| Monoelaidin | Pluronic F127 |
| Monoolein or phytantriol | Pluronic F108 |
| Sodium octyl sulphate (SCS) | Arginine-based cationic surfactant |
| Phytantriol | Myrj 59 |
| β-XP (1-O-phytanyl-β-d-xyloside) | Pluronic F127 |
| Drugs | Objective of Study | Outcome of Study | References |
|---|---|---|---|
| Antimicrobial peptide LL-37 | The antimicrobial potential of cubosomal LL-37 was evaluated using in vitro and ex-vivo skin irritation models. | The formulation provides superior protection to LL-37 against enzymatic degradation and significant bactericidal effects. | [94] |
| Erythromycin | Topical delivery of erythromycin for the treatment and prevention of acne. | The prepared cubosomes were effective in the topical delivery of erythromycin in a non-invasive and sustained manner. | [94] |
| Ketorolac | Monoolein and poloxamer cubic nanoparticles for ocular delivery of ketorolac. | Formulated cubosomes loaded with Ketorolac provided trans-corneal permeation and retention. | [95] |
| Simvastatin | Enhanced bioavailability of simvastatin to lower bad cholesterol and fats. | Prepared cubosomes enhanced the bioavailability of the lipophilic simvastatin when administered orally. | [95] |
| Indomethacin | Evaluation of Indomethacin-fabricated cubosomes for anti-inflammatory activity. | Homogenised monoolein and poloxamer-containing cubosomes prolonged the delivery of the drug. | [96] |
| Piperine | Evaluation of the memory-enhancing potentials (used in Alzheimer’s disease). | Prepared cubosomes were found to be safe with superior effects over free drugs and were effectively restoring cognitive functions. | [96] |
| Flurbiprofen (FB) | A non-steroidal anti-inflammatory drug used for the treatment of ocular inflammation. | The formulation showed less ocular irritation and enhanced transcorneal permeation of FB. | [18] |
| Insulin | Tested against the C-Type-1-diabetic-induced rat (insulin-dependent diabetes). | Cubosomes protected the loaded insulin against proteolysis. It was found to be stable at normal temperatures and controlled hyperglycaemia in a reproducible manner. | [18] |
| Dacarbazine | To reduce the side effects of melanoma. | Dacarbazine delivered through cubosomes decreases the side effects of intravenous delivery. It also enhanced the drug efficacy, safety, and shelf life. | [97] |
| 20(S) protopanaxadiol | To evaluate the anticancer activity. | Cubosomes improved the oral bioavailability of the drug as a result of enhanced absorption. | [98] |
| Timolol | Synthesis of timolol-loaded cubosomes and their evaluation. | The prepared cubosomes showed increased corneal penetration, prolonged precorneal retention time and enhanced intraocular pressure lowering effect than the commercially available eye drops. | [99] |
| Docetaxel | Synthesis and evaluation of controlled release cubosomes incorporated with docetaxel as a thermosensitive depot. | The depot showed gradual drug release, preparation was free flowing at room temperature. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivadasan, D.; Sultan, M.H.; Alqahtani, S.S.; Javed, S. Cubosomes in Drug Delivery—A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications. Biomedicines 2023, 11, 1114. https://doi.org/10.3390/biomedicines11041114
Sivadasan D, Sultan MH, Alqahtani SS, Javed S. Cubosomes in Drug Delivery—A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications. Biomedicines. 2023; 11(4):1114. https://doi.org/10.3390/biomedicines11041114
Chicago/Turabian StyleSivadasan, Durgaramani, Muhammad H. Sultan, Saad S. Alqahtani, and Shamama Javed. 2023. "Cubosomes in Drug Delivery—A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications" Biomedicines 11, no. 4: 1114. https://doi.org/10.3390/biomedicines11041114
APA StyleSivadasan, D., Sultan, M. H., Alqahtani, S. S., & Javed, S. (2023). Cubosomes in Drug Delivery—A Comprehensive Review on Its Structural Components, Preparation Techniques and Therapeutic Applications. Biomedicines, 11(4), 1114. https://doi.org/10.3390/biomedicines11041114







